Efficient Suzuki–Miyaura C-C Cross-Couplings Induced by Novel Heterodinuclear Pd-bpydc-Ln Scaffolds
Abstract
:1. Introduction
2. Experimental Section
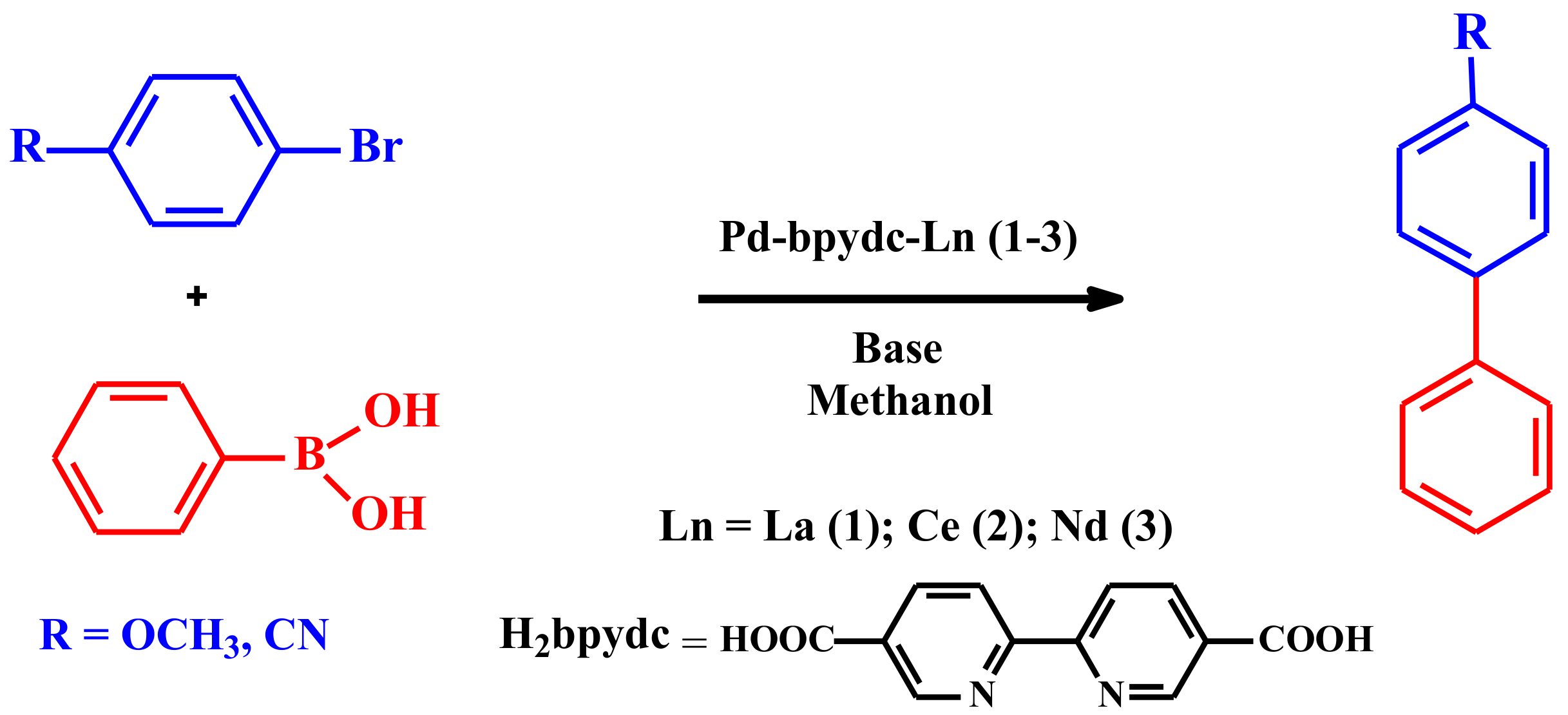
2.1. Materials and Synthesis Methodology
2.2. Synthesis and Characterization of Pd-bpydc-Ln (1–3).
2.3. General Procedure for Suzuki–Miyaura Reaction
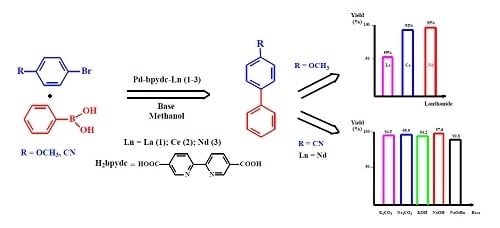
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Trusova, M.E.; Rodriguez-Zubiri, M.; Kutonova, K.V.; Jung, N.; Bräse, S.; Felpin, F.-X.; Postnikov, P.S. Ultra-fast Suzuki and Heck reactions for the synthesis of styrenes and stilbenes using arenediazonium salts as super-electrophiles. Org. Chem. Front. 2018, 5, 41–45. [Google Scholar] [CrossRef] [Green Version]
- Kostas, I.D. (Ed.) Suzuki–Miyaura Cross-Coupling Reaction and Potential Applications; MDPI: Basel, Switzerland, 2017. [Google Scholar]
- Martina, K.; Manzoli, M.; Calcio Gaudino, E.; Cravotto, G. Eco-Friendly Physical Activation Methods for Suzuki–Miyaura Reactions. Catalysts 2017, 7, 98. [Google Scholar] [CrossRef]
- Colacot, T. (Ed.) New Trends in Cross-Coupling: Theory and Applications; RSC Publishing: Cambridge, UK, 2015. [Google Scholar]
- De Meijere, A.; Bräse, S.; Oestreich, M. (Eds.) Metal-Catalyzed Cross-Coupling Reactions and More; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2014; pp. 1–1511. [Google Scholar]
- Johansson Seechurn, C.C.C.; Kitching, M.O.; Colacot, T.J.; Snieckus, V. Palladium-catalyzed cross-coupling: A historical contextual perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 2012, 51, 5062–5085. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A. Cross-coupling reactions of organoboranes: An easy way to construct C-C bonds (Nobel Lecture). Angew. Chem. Int. Ed. 2011, 50, 6722–6737. [Google Scholar] [CrossRef] [PubMed]
- Molnar, A. Efficient, Selective, and Recyclable Palladium Catalysts in Carbon−Carbon Coupling Reactions. Chem. Rev. 2011, 111, 2251–2320. [Google Scholar] [CrossRef] [PubMed]
- Rivera, R.P.; Ehlers, P.; Ohlendorf, L.; Rodriguez, E.T.; Villinger, A.; Langer, P. Chemoselective Synthesis of Arylpyridines through Suzuki–Miyaura Cross-Coupling Reactions. Eur. J. Org. Chem. 2018, 8, 990–1003. [Google Scholar] [CrossRef]
- Liu, N.; Liu, C.; Jin, Z. Green synthesis of fluorinated biaryl derivatives via thermoregulated ligand/palladium-catalyzed Suzuki reaction. J. Organomet. Chem. 2011, 696, 2641–2647. [Google Scholar] [CrossRef]
- Lopušanskaja, E.; Paju, A.; Järving, I.; Lopp, M. Synthesis of Cyclic 3-Aryl-Substituted 1,2-Dicarbonyl Compounds via Suzuki Cross-Coupling Reactions. Synthesis 2018, 50, 1883–1890. [Google Scholar] [CrossRef]
- Dai, J.J.; Liu, J.H.; Luo, D.F. Pd-catalysed decarboxylative Suzuki reactions and orthogonal Cu-based O-arylation of aromatic carboxylic acids. Chem. Commun. 2011, 47, 677–679. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, V.; Rani, M.J.; Reddy, N.D. A Zwitterionic Palladium(II) Complex as a Precatalyst for Neat Water Mediated Cross-Coupling Reactions of Heteroaryl, Benzyl and Aryl Acid Chlorides with Organoboron Reagents. Eur. J. Org. Chem. 2017, 48, 7238–7255. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Decottignies, A.; Len, C.; Fihri, A. Suzuki–Miyaura Cross-Coupling Reactions in Aqueous Media: Green and Sustainable Syntheses of Biaryls. ChemSusChem 2010, 3, 502–522. [Google Scholar] [CrossRef] [PubMed]
- Drozdov, F.V.; Cherkaev, G.V.; Muzafarov, A.M. The Suzuki modification of functional polydimethylsiloxanes. Mendeleev. Commun. 2017, 27, 570–571. [Google Scholar] [CrossRef]
- Wu, W.B.; Wang, C.; Zhong, C.; Ye, C.; Qiu, G.; Qin, J.; Li, Z. Changing the shape of chromophores from “H-type” to “star-type”: Increasing the macroscopic NLO effects by a large degree. Polym. Chem. 2013, 4, 378–396. [Google Scholar] [CrossRef]
- Liu, M.; Su, S.-J.; Jung, M.-C.; Qi, Y.B.; Zhao, W.-M.; Kido, J. Hybrid Heterocycle-Containing Electron-Transport Materials Synthesized by Regioselective Suzuki Cross-Coupling Reactions for Highly Efficient Phosphorescent OLEDs with Unprecedented Low Operating Voltage. Chem. Mater. 2012, 24, 3817–3827. [Google Scholar] [CrossRef]
- Soloducho, J.; Cabaj, J.; Olech, K.; Data, P.; Lapkowski, M. Advanced Heterocyclic Branched Semiconducting Units—Highly Efficient Synthesis and Physicochemical Characteristic. Curr. Org. Chem. 2013, 17, 283–295. [Google Scholar] [CrossRef]
- Martinez-Amezaga, M.; Delpiccolo, C.M.L.; Mata, E.G. Immobilized boronic acid for Suzuki–Miyaura coupling: Application to the generation of pharmacologically relevant molecules. RSC Adv. 2017, 7, 34994–35003. [Google Scholar] [CrossRef]
- Magano, J.; Dunetz, J.R. Large-scale applications of transition metal-catalyzed couplings for the synthesis of pharmaceuticals. Chem. Rev. 2011, 111, 2177–2250. [Google Scholar] [CrossRef] [PubMed]
- Torborg, C.; Beller, M. Recent Applications of Palladium-Catalyzed Coupling Reactions in the Pharmaceutical, Agrochemical, and Fine Chemical Industries. Adv. Synth. Catal. 2009, 351, 3027–3043. [Google Scholar] [CrossRef]
- De Vries, J.G. Palladium-Catalysed Coupling Reactions. In Organometallics as Catalysts in the Fine Chemical Industry; Beller, M., Blaser, H.-U., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 2012; pp. 1–34. [Google Scholar]
- Kitney, S.P.; Cheng, F.; Khan, S.; Hope, C.N.; McNab, W.; Kelly, S.M. Synthesis of liquid crystals using Suzuki–Miyaura coupling reactions using mesoporous, ligand-free Pd/Si3N4 catalysts in aqueous media. Liq. Cryst. 2011, 38, 1027–1033. [Google Scholar] [CrossRef]
- Kal Koshvandi, A.T.; Heravi, M.M.; Momeni, T. Current Applications of Suzuki–Miyaura Coupling Reaction in the Total Synthesis of Natural Products: An update. Appl. Organomet. Chem. 2018, 32, e4210. [Google Scholar] [CrossRef]
- Zheng, S.; Laraia, L.; O′Connor, C.J.; Sorrell, D.; Tan, Y.S.; Xu, Z.; Venkitaraman, A.R.; Wu, W.; Spring, D.R. Synthesis and biological profiling of tellimagrandin I and analogues reveals that the medium ring can significantly modulate biological activity. Org. Biomol. Chem. 2012, 10, 2590–2593. [Google Scholar] [CrossRef] [PubMed]
- Lopopolo, G.; Fiorella, F.; de Candia, M.; Nicolotti, O.; Martel, S.; Carrupt, P.A.; Altomare, C. Biarylmethoxy isonipecotanilides as potent and selective inhibitors of blood coagulation factor Xa. Eur. J. Pharm. Sci. 2011, 42, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Okano, A.; Pierce, J.G.; James, R.C.; Stamm, S.; Crane, C.M.; Boger, D.L. Total Synthesis of [Ψ[C(═S)NH]Tpg4]Vancomycin Aglycon, [Ψ[C(═NH)NH]Tpg4]Vancomycin Aglycon, and Related Key Compounds: Reengineering Vancomycin for Dual d-Ala-d-Ala and d-Ala-d-Lac Binding. J. Am. Chem. Soc. 2012, 134, 1284–1297. [Google Scholar] [CrossRef] [PubMed]
- Edwankar, C.R.; Edwankar, R.V.; Deschamps, J.R.; Cook, J.M. Nature-inspired stereospecific total synthesis of P-(+)-dispegatrine and four other monomeric sarpagine indole alkaloids. Angew. Chem. Int. Ed. 2012, 51, 11762–11765. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, K.; Tanaka, K. Ligand: Self-Assembly of Palladium Nanoparticles and Catalytic Reactivity on C–C. Bond Formation. Synthsis 2017. [Google Scholar] [CrossRef]
- Deraedt, C.; Astruc, D. “Homeopathic” palladium nanoparticle catalysis of cross carbon-carbon coupling reactions. Acc. Chem. Res. 2014, 47, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Sydnes, M.O. Use of nanoparticles as catalysts in organic synthesis—Cross-coupling reactions. Curr. Org. Chem. 2014, 18, 312–326. [Google Scholar] [CrossRef]
- Heinrich, F.; Keßler, M.T.; Dohmen, S.; Singh, M.; Prechtl, M.H.G.; Mathur, S. Molecular Palladium Precursors for Pd Nanoparticle Preparation by Microwave Irradiation: Synthesis, Structural Characterization and Catalytic Activity. Eur. J. Inorg. Chem. 2012, 36, 6027–6033. [Google Scholar] [CrossRef]
- Zhi, J.; Song, D.; Li, Z. Palladium nanoparticles in carbon thin film-lined SBA-15 nanoreactors: Efficient heterogeneous catalysts for Suzuki–Miyaura cross coupling reaction in aqueous media. Chem. Commun. 2011, 47, 10707–10709. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, P.; Santhanalakshmi, J. Synthesis, characterization and catalytic activity of trimetallic nanoparticles in the Suzuki C–C coupling reaction. J. Mol. Catal. A-Chem. 2010, 326, 99–106. [Google Scholar] [CrossRef]
- Fujiki, K.; Tanaka, K. Bis[N,N′-(2-indanolyl)]-1,5-diazacyclooctane as Unique Metal Ligand: Self-Assembly of Palladium Nanoparticles and Catalytic Reactivity on C–C Bond Formation. Synthesis 2017, 49, 1097–1104. [Google Scholar]
- Pourjavadi, A.; Habibi, Z. Palladium nanoparticle-decorated magnetic pomegranate peel-derived porous carbon nanocomposite as an excellent catalyst for Suzuki–Miyaura and Sonogashira cross-coupling reactions. Appl. Organomet. Chem. 2018, e4480. [Google Scholar] [CrossRef]
- Baran, T.; Sargin, I.; Kaya, M.; Menteş, A.; Ceter, T. Design and application of sporopollenin microcapsule supported palladium catalyst: Remarkably high turnover frequency and reusability in catalysis of biaryls. J. Colloid Interface Sci. 2017, 486, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.-X.; Hu, Z.-L.; Yu, S.-C.; Zhao, Z.-X.; Wei, D.-C.; Li, H.-L. NHC Pd(II) and Ag(I) Complexes: Synthesis, Structure, and Catalytic Activity in Three Types of C−C. Coupling Reactions. ACS Omega 2018, 3, 4035–4047. [Google Scholar] [CrossRef]
- Akkoç, M.; İmik, F.; Yaşar, S.; Dorcet, V.; Roisnel, T.; Bruneau, C.; Özdemir, I. An Efficient Protocol for Palladium N-Heterocyclic Carbene-Catalysed Suzuki-Miyaura Reaction at room temperature. Chem. Sel. 2017, 2, 5729–5734. [Google Scholar] [CrossRef]
- Chartoire, A.; Nolan, S.P. Chapter. 4: Advances in C-C and C-X Coupling Using Palladium N-Heterocyclic Carbene (Pd-NHC) Complexes. In New Trends in Cross-Coupling: Theory and Applications; Colacot, T., Ed.; RSC Publishing: Cambridge, UK, 2015. [Google Scholar]
- Izquierdo, F.; Corpet, M.M.A.E.; Nolan, S.P. The Suzuki–Miyaura Reaction Performed Using a Palladium–N-Heterocyclic Carbene Catalyst and a Weak Inorganic Base. Eur. J. Org. Chem. 2015, 9, 1920–1924. [Google Scholar] [CrossRef]
- Guisado-Barrios, G.; Hiller, J.; Peris, E. Pyracene-Linked Bis-Imidazolylidene Complexes of Palladium and Some Catalytic Benefits Produced by Bimetallic Catalysts. Chem.-Eur. J. 2013, 19, 10405–10411. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.; Xu, Q.; Shi, M. Synthesis of Novel N-Heterocyclic Carbene-Oxazoline Palladium Complexes and Their Applications in Suzuki–Miyaura Cross-Coupling Reaction. Synlett 2013, 24, 1255–1259. [Google Scholar] [CrossRef]
- Szulmanowicz, M.S.; Gniewek, A.; Gil, W.; Trzeciak, A.M. Palladium(II) Complexes with Small N-Heterocyclic Carbene Ligands as Highly Active Catalysts for the Suzuki–Miyaura Cross-Coupling Reaction. ChemCatChem. 2013, 5, 1152–1160. [Google Scholar] [CrossRef]
- DeAngelis, A.; Colacot, T.J. Chapter. 2: Prominent Ligand Types in Modern Cross-Coupling Reactions. In New Trends in Cross-Coupling: Theory and Applications; Colacot, T., Ed.; RSC Publishing: Cambridge, UK, 2015. [Google Scholar]
- Stradiotto, M. Chapter. 5: Ancillary Ligand Design in the Development of Palladium Catalysts for Challenging Selective Monoarylation Reactions. In New Trends in Cross-Coupling: Theory and Applications; Colacot, T., Ed.; RSC Publishing: Cambridge, UK, 2015. [Google Scholar]
- Gogoi, G.; Bora, U.; Borah, G.; Gogoi, P.K. Nanosilica-anchored Pd(II)-Schiff base complex as efficient heterogeneous catalyst for activation of aryl halides in Suzuki–Miyaura cross-coupling reaction in water. Appl. Organomet. Chem. 2017, 31, e3686. [Google Scholar] [CrossRef]
- Sobhani, S.; Mesbah Falatooni, Z.M.; Asadi, S.; Honarmand, M. Palladium-Schiff Base Complex Immobilized Covalently on Magnetic Nanoparticles as an Efficient and Recyclable Catalyst for Heck and Suzuki Cross-Coupling Reactions. Catal. Lett. 2016, 146, 255–268. [Google Scholar] [CrossRef]
- Liu, N.; Liu, C.; Jin, Z. Poly(ethylene glycol)-functionalized imidazolium salts–palladium-catalyzed Suzuki reaction in water. Green Chem. 2012, 14, 592–597. [Google Scholar] [CrossRef]
- Chen, W.; Lu, X.-Y.; Xu, B.-H.; Yu, W.-G.; Zhou, Z.-N.; Hu, Y. The Rational Design and Synthesis of Water-Soluble Thiourea Ligands for Recoverable Pd-Catalyzed Aerobic Aqueous Suzuki–Miyaura Reactions at Room Temperature. Synthesis 2018, 50, 1499–1510. [Google Scholar] [CrossRef]
- Johansson Seechurn, C.C.C.; Li, H.; Colacot, T.J. Chapter. 3: Pd-Phosphine Precatalysts for Modern Cross-Coupling Reactions. In New Trends in Cross-Coupling: Theory and Applications; Colacot, T., Ed.; RSC Publishing: Cambridge, UK, 2015. [Google Scholar]
- Li, H.B.; Johansson Seechurn, C.C.C.; Colacot, T.J. Development of Preformed Pd Catalysts for Cross-Coupling Reactions, Beyond the 2010 Nobel Prize. ACS Catal. 2012, 2, 1147–1164. [Google Scholar] [CrossRef]
- Kumar, S.; Ahmed, N. A facile approach for the synthesis of novel 1-oxa- and 1-aza-flavonyl-4-methyl-1H-benzo[d][1,3]oxazin-2(4H)-ones by microwave enhanced Suzuki–Miyaura coupling using bidentate chromen-4-one-based Pd(II)–diimine complex as catalyst. RSC Adv. 2015, 5, 77075–77087. [Google Scholar] [CrossRef]
- Zhou, Z.G.; Shi, J.C.; Hu, Q.S.; Xie, Y.R.; Du, Z.Y.; Zhang, S.Y. Suzuki cross-coupling reactions of aryl chlorides using [Cl2Pd(COD)]/piperazine derivative under microwave conditions. Appl. Organomet. Chem. 2011, 25, 616–619. [Google Scholar] [CrossRef]
- Kong, S.; Malik, A.U.; Qian, X.; Shu, M.; Xiao, W. C-C Coupling Reactions in Water Catalyzed by Palladium(II) Supported on Microporous Organic Polymer. Chin. J. Org. Chem. 2018, 38, 432–442. [Google Scholar] [CrossRef]
- Fareghi-Alamdari, R.; Golestanzadeh, M.; Bagheri, O. An efficient and recoverable palladium organocatalyst for Suzuki reaction in aqueous media. Appl. Organomet. Chem. 2017, 31, e3698. [Google Scholar] [CrossRef]
- Aktaş, A.; Celepci, D.B.; Gök, Y.; Aygün, M. 2-Hydroxyethyl-Substituted Pd-PEPPSI Complexes: Synthesis, Characterization and the Catalytic Activity in the Suzuki-Miyaura Reaction for Aryl Chlorides in Aqueous Media. ChemistrySelect 2018, 3, 9974–9980. [Google Scholar] [CrossRef]
- Chatterjee, A.; Ward, T.R. Recent advances in the palladium catalyzed Suzuki–Miyaura cross-coupling reaction in water. Catal. Lett. 2016, 146, 820–840. [Google Scholar] [CrossRef]
- Liang, Q.B.; Xing, P.; Huang, Z.G.; Dong, J.J.; Sharpless, K.B.; Li, X.X.; Jiang, B. Palladium-Catalyzed Ligand-Free Suzuki Reaction In Water Using Aryl Fluorosulfates. Org. Lett. 2015, 17, 1942–1945. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Pöthig, A.; Feng, Y.; Riener, K.; Herrmann, W.A.; Kühn, F.E. Facile-prepared sulfonated water-soluble PEPPSI-Pd-NHC catalysts for aerobic aqueous Suzuki–Miyaura cross-coupling reactions. Green Chem. 2014, 16, 4955–4962. [Google Scholar] [CrossRef] [Green Version]
- Mao, S.L.; Sun, Y.; Yu, G.A.; Zhao, C.; Han, Z.J.; Yuan, J.; Zhu, X.; Yang, Q.; Liu, S.H. A highly active catalytic system for Suzuki–Miyaura cross-coupling reactions of aryl and heteroaryl chlorides in water. Org. Biomol. Chem. 2012, 10, 9410–9417. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, J.; Zhou, C.; Wang, R.; Hong, M. pH-Responsive chelating N-heterocyclic dicarbene palladium(II) complexes: Recoverable precatalysts for Suzuki–Miyaura reaction in pure water. Green Chem. 2011, 13, 2071–2077. [Google Scholar] [CrossRef]
- Wu, X.F.; Neumann, H.; Beller, M. Suzuki Coupling of Benzyl Halides with Potassium Aryltrifluoroborates in Aqueous Media. Adv. Synth. Catal. 2011, 353, 788–792. [Google Scholar] [CrossRef]
- Crisóstomo-Lucas, C.; Toscano, R.A. Synthesis and characterization of new potentially hydrosoluble pincer ligands and their application in Suzuki–Miyaura cross-coupling reactions in water. Tetrahedron Lett. 2013, 54, 3116–3119. [Google Scholar]
- Conelly-Espinosa, P.; Morales-Morales, D. [Pd(HQS)2] (HQS = 8-hydroxyquinoline-5-sulfonic acid) a highly efficient catalyst for Suzuki-Miyaura cross couplings in water. Inorg. Chim. Acta 2010, 363, 1311–1315. [Google Scholar] [CrossRef]
- Basauri-Molina, M.; Hernández-Ortega, S.; Morales-Morales, D. Microwave-assisted C-C and C-S couplings catalysed by organometallic Pd-SCS or coordination Ni-SNS pincer complexes. Eur. J. Inorg. Chem. 2014, 2014, 4619–4625. [Google Scholar] [CrossRef]
- Arvela, R.K.; Leadbeater, N.E. Suzuki coupling of aryl chlorides with phenylboronic acid in water, using microwave heating with simultaneous cooling. Org. Lett. 2005, 7, 2101–2104. [Google Scholar] [CrossRef] [PubMed]
- Lipshutz, B.H.; Taft, B.R.; Abela, A.R.; Ghorai, S.; Krasovskiy, A.; Duplais, C. Catalysis in the Service of Green Chemistry: Nobel Prize-Winning Palladium-Catalysed Cross-Couplings, Run in Water at Room Temperature: Heck, Suzuki–Miyaura and Negishi Reactions Carried Out in the Absence of Organic Solvents, Enabled by Micellar Catalysis. Platin. Met. Rev. 2012, 56, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ni, Q.; Bao, F.; Qiu, J. A simple and efficient protocol for a palladium-catalyzed ligand-free Suzuki reaction at room temperature in aqueous DMF. Green Chem. 2011, 13, 1260–1266. [Google Scholar] [CrossRef]
- Karimi, B.; Zamani, A. SBA-15-functionalized palladium complex partially confined with ionic liquid: An efficient and reusable catalyst system for aqueous-phase Suzuki reaction. Org. Biomol. Chem. 2012, 10, 4531–4536. [Google Scholar] [CrossRef] [PubMed]
- Malineni, J.; Jezorek, R.L.; Zhang, N.; Percec, V. NiIICl(1-Naphthyl)(PCy3)2, An Air-Stable σ-NiII Precatalyst for Quantitative Cross-Coupling of Aryl C–O Electrophiles with Aryl Neopentylglycolboronates. Synthesis 2016, 48, 2808–2815. [Google Scholar] [CrossRef]
- Malineni, J.; Jezorek, R.L.; Zhang, N.; Percec, V. An Indefinitely Air-Stable σ-NiII Precatalyst for Quantitative Cross-Coupling of Unreactive Aryl Halides and Mesylates with Aryl Neopentylglycolboronates. Synthesis 2016, 48, 2795–2807. [Google Scholar] [CrossRef]
- Jezorek, R.L.; Zhang, N.; Leowanawat, P.; Brunner, M.H.; Gutsche, N.; Pesti, A.K.R.; Olsen, J.T.; Percec, V. Air-Stable Nickel Precatalysts for Fast and Quantitative Cross-Coupling of Aryl Sulfamates with Aryl Neopentylglycolboronates at Room Temperature. Org. Lett. 2014, 16, 6326–6329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Hoffman, D.J.; Gutsche, N.; Gupta, J.; Percec, V. Comparison of Arylboron-Based Nucleophiles in Ni-Catalyzed Suzuki–Miyaura Cross-Coupling with Aryl Mesylates and Sulfamates. J. Org. Chem. 2012, 77, 5956–5964. [Google Scholar] [CrossRef] [PubMed]
- Leowanawat, P.; Zhang, N.; Percec, V. Nickel Catalyzed Cross-Coupling of Aryl C–O Based Electrophiles with Aryl Neopentylglycolboronates. J. Org. Chem. 2012, 77, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Baghbanzadeh, M.; Pilger, C.; Kappe, C.O. Rapid Nickel-Catalyzed Suzuki−Miyaura Cross-Couplings of Aryl Carbamates and Sulfamates Utilizing Microwave Heating. J. Org. Chem. 2011, 76, 1507–1510. [Google Scholar] [CrossRef] [PubMed]
- Hanley, P.S.; Ober, M.S.; Krasovskiy, A.L.; Whiteker, G.T.; Kruper, W.J. Nickel- and Palladium-Catalyzed Coupling of Aryl Fluorosulfonates with Aryl Boronic Acids Enabled by Sulfuryl Fluoride. ACS Catal. 2015, 5, 5041–5046. [Google Scholar] [CrossRef]
- You, L.-X.; Liu, H.-J.; Cui, L.-X.; Ding, F.; Xiong, G.; Wang, S.-J.; Ren, B.-Y.; Dragutan, I.; Dragutan, V.; Sun, Y.-G. The synergistic effect of cobalt on a Pd/Co catalyzed Suzuki–Miyaura cross-coupling in water. Dalton Trans. 2016, 45, 18455–18458. [Google Scholar] [CrossRef] [PubMed]
- Kamijo, S.; Yamamoto, Y. A New Pd0–CuI Bimetallic Catalyst for the Synthesis of Indoles from Isocyanates and Allyl Carbonates. Angew. Chem. Int. Ed. 2002, 41, 3230–3233. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, G.K.; Kumar, S.; Singh, A.K. Organosulphur and related ligands in Suzuki–Miyaura C–C coupling. Dalton Trans. 2013, 42, 5200–5223. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.N.; Joshi, H.; Sharma, A.K.; Prakash, O.; Singh, A.K. Selenium-Containing N-Heterocyclic Carbenes and Their First Palladium(II) Complexes: Synthesis, Structure, and Pendent Alkyl Chain Length Dependent Catalytic Activity for Suzuki–Miyaura Coupling. Organometallics 2013, 32, 2443–2451. [Google Scholar] [CrossRef]
- He, K.; Song, F.; Sun, H.; Huang, Y. Transition-Metal-Free Suzuki–Type Cross-Coupling Reaction of Benzyl Halides and Boronic Acids via 1,2-Metalate Shift. J. Am. Chem. Soc. 2018, 140, 2693–2699. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.L.; Jia, A.Q.; Jin, G.X. Pd(diimine)Cl2 embedded heterometallic compounds with porous structures as efficient heterogeneous catalysts. Chem. Commun. 2013, 49, 2403–2405. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Zong, W.; Xiong, G.; Ding, F.; Wang, S.; Ren, B.; Dragutan, I.; Dragutan, V.; Sun, Y.-G. Cooperative effects of lanthanides when associated with palladium in novel, 3D Pd/Ln coordination polymers. Sustainable applications as water-stable, heterogeneous catalysts in carbon–carbon cross-coupling reactions. Appl. Catal. A Gen 2016, 511, 1–10. [Google Scholar] [CrossRef]
- You, L.; Zhu, W.; Wang, S.; Xiong, G.; Ding, F.; Ren, B.; Dragutan, I.; Dragutan, V.; Sun, Y.-G. High Catalytic Activity in Aqueous Heck and Suzuki–Miyaura Reactions Catalyzed by Novel Pd/Ln Coordination Polymers Based on 2,2′-Bipyridine-4,4′-dicarboxylic Acid as a Heteroleptic Ligand. Polyhedron 2016, 115, 47–53. [Google Scholar] [CrossRef]
- Wu, X.-R.; Yao, S.-Y.; Wei, L.-Q.; Li, L.-P.; Ye, B.-H. Construction of Heterometallic M2Pd3 Supramolecular Cages via a Metalloligand Strategy as Heterogeneous Catalyst for Suzuki–Miyaura Coupling Reaction. Inorg. Chim. Acta 2018, 482, 605–611. [Google Scholar] [CrossRef]
- Xiong, G.; Chen, X.-L.; You, L.-X.; Ren, B.-Y.; Ding, F.; Dragutan, I.; Dragutan, V.; Sun, Y.-G. La-Metal–Organic Framework Incorporating Fe3O4 Nanoparticles, Post-Synthetically Modified with Schiff Base and Pd. A Highly Active, Magnetically Recoverable, Recyclable Catalyst for C–C Cross-Couplings at Low Pd Loadings. J. Catal. 2018, 361, 116–125. [Google Scholar] [CrossRef]
- You, L.-X.; Cui, L.-X.; Zhao, B.-B.; Xiong, G.; Ding, F.; Ren, B.-Y.; Shi, Z.-L.; Dragutan, I.; Dragutan, V.; Sun, Y.-G. Tailoring the structure, pH sensitivity and catalytic performance in Suzuki–Miyaura cross-couplings of Ln/Pd MOFs based on the 1,1′-di(p-carboxybenzyl)-2,2′-diimidazole linker. Dalton Trans. 2018, 47, 8755–8763. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-G.; Li, J.; You, J.; Guo, Y.; Xiong, G.; Ren, B.Y.; You, L.-X.; Ding, F.; Wang, S.-J.; Verpoort, F.; et al. Bis-imidazole coordination polymers controlled by oxalate as an auxiliary ligand. J. Coord. Chem. 2015, 68, 1199–1212. [Google Scholar] [CrossRef]
- Sun, Y.-N.; Xiong, G.; Dragutan, V.; Dragutan, I.; Ding, F.; Sun, Y.-G. Novel luminescent heterobimetallic Ln-Cu(I) 3D coordination polymers based on 5-(4-pyridyl) isophthalic acid as heteroleptic ligand. Synthesis and structural characterization. Inorg. Chem. Commun. 2015, 62, 103–106. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 1997; pp. 1230–1242. ISBN 0-08-037941-9. [Google Scholar]
- Cotton, S. Lanthanide and Actinide Chemistry; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2006; ISBN 9780470010051. [Google Scholar]
- Amatore, C.; LeDuc, G.; Jutand, A. Mechanism of Palladium-Catalyzed Suzuki–Miyaura Reactions: Multiple and Antagonistic Roles of Anionic “Bases” and Their Countercations. Chem. Eur. J. 2013, 19, 10082–10093. [Google Scholar] [CrossRef] [PubMed]
- Amatore, C.; Jutand, A.; Le Duc, G. Mechanistic Origin of Antagonist Effects of Usual Anionic Bases (OH−, CO32−) as Modulated by their Countercations (Na+, Cs+, K+) in Palladium-Catalyzed Suzuki–Miyaura Reactions. Chem. Eur. J. 2012, 18, 6616–6625. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.F.R.A.C.; Rodrigues, A.S.M.C.; Silva, V.L.M.; Silva, A.M.S.; Santos, M.N.B.F. Role of the Base and Control of Selectivity in the Suzuki-Miyaura Cross-Coupling Reaction. ChemCatChem 2014, 6, 1291–1302. [Google Scholar] [CrossRef]
Sample Availability: No samples of the compounds are available from the authors. |



| Entry | Catalyst | T (°C) | Time (h) | Yield (%) a,b,c |
|---|---|---|---|---|
| 1 | Pd-bpydc-La (1) | 30 | 8 | 35.0 a |
| 2 | 30 | 8 | 15.0 b | |
| 3 | 60 | 4 | 55.0 a | |
| 4 | 60 | 4 | 14.9 b | |
| 5 | Pd-bpydc-Ce (2) | 30 | 8 | 25.0 a |
| 6 | 30 | 8 | 25.9 b | |
| 7 | 60 | 4 | 91.9 a | |
| 8 | 60 | 4 | 88.9 b | |
| 9 | Pd-bpydc-Nd (3) | 30 | 8 | 95.0 a |
| 10 | 30 | 8 | 93.4 b | |
| 11 | 60 | 4 | 95.0 a | |
| 12 | 60 | 4 | 94.9 b |
| Lanthanide | La | Ce | Nd |
|---|---|---|---|
| Ln3+ radius (pm) a | 103 | 102 | 98.3 |
| Ln3+ electron configuration b | 4f0 | 4f1 | 4f3 |
| Entry | Catalyst | Base | Yield (%) b |
|---|---|---|---|
| 1 | Pd-bpydc-Nd (3) | KOH | 95.0 |
| 2 | K2CO3 | 91.9 | |
| 3 | NaOH | 92.0 | |
| 4 | Na2CO3 | 92.0 | |
| 5 | NaOtBu | 95.0 |
| Entry | Catalyst | Base | Yield (%) b |
|---|---|---|---|
| 1 | Pd-bpydc-Nd (3) | KOH | 94,2 |
| 2 | K2CO3 | 94.5 | |
| 3 | NaOH | 97.6 | |
| 4 | Na2CO3 | 95.9 | |
| 5 | NaOtBu | 90.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, F.; Li, Y.; Yan, P.; Deng, Y.; Wang, D.; Zhang, Y.; Dragutan, I.; Dragutan, V.; Wang, K. Efficient Suzuki–Miyaura C-C Cross-Couplings Induced by Novel Heterodinuclear Pd-bpydc-Ln Scaffolds. Molecules 2018, 23, 2435. https://doi.org/10.3390/molecules23102435
Ding F, Li Y, Yan P, Deng Y, Wang D, Zhang Y, Dragutan I, Dragutan V, Wang K. Efficient Suzuki–Miyaura C-C Cross-Couplings Induced by Novel Heterodinuclear Pd-bpydc-Ln Scaffolds. Molecules. 2018; 23(10):2435. https://doi.org/10.3390/molecules23102435
Chicago/Turabian StyleDing, Fu, Yanli Li, Pingxuan Yan, Yan Deng, Dongping Wang, Yajing Zhang, Ileana Dragutan, Valerian Dragutan, and Kangjun Wang. 2018. "Efficient Suzuki–Miyaura C-C Cross-Couplings Induced by Novel Heterodinuclear Pd-bpydc-Ln Scaffolds" Molecules 23, no. 10: 2435. https://doi.org/10.3390/molecules23102435
APA StyleDing, F., Li, Y., Yan, P., Deng, Y., Wang, D., Zhang, Y., Dragutan, I., Dragutan, V., & Wang, K. (2018). Efficient Suzuki–Miyaura C-C Cross-Couplings Induced by Novel Heterodinuclear Pd-bpydc-Ln Scaffolds. Molecules, 23(10), 2435. https://doi.org/10.3390/molecules23102435