Self-Assembly and Applications of Amphiphilic Hybrid POSS Copolymers
Abstract
:1. Introduction
2. Mechanisms of Self-Assembly of POSS-Based Amphiphilic Copolymers
2.1. Stimuli-Responsive Micelles
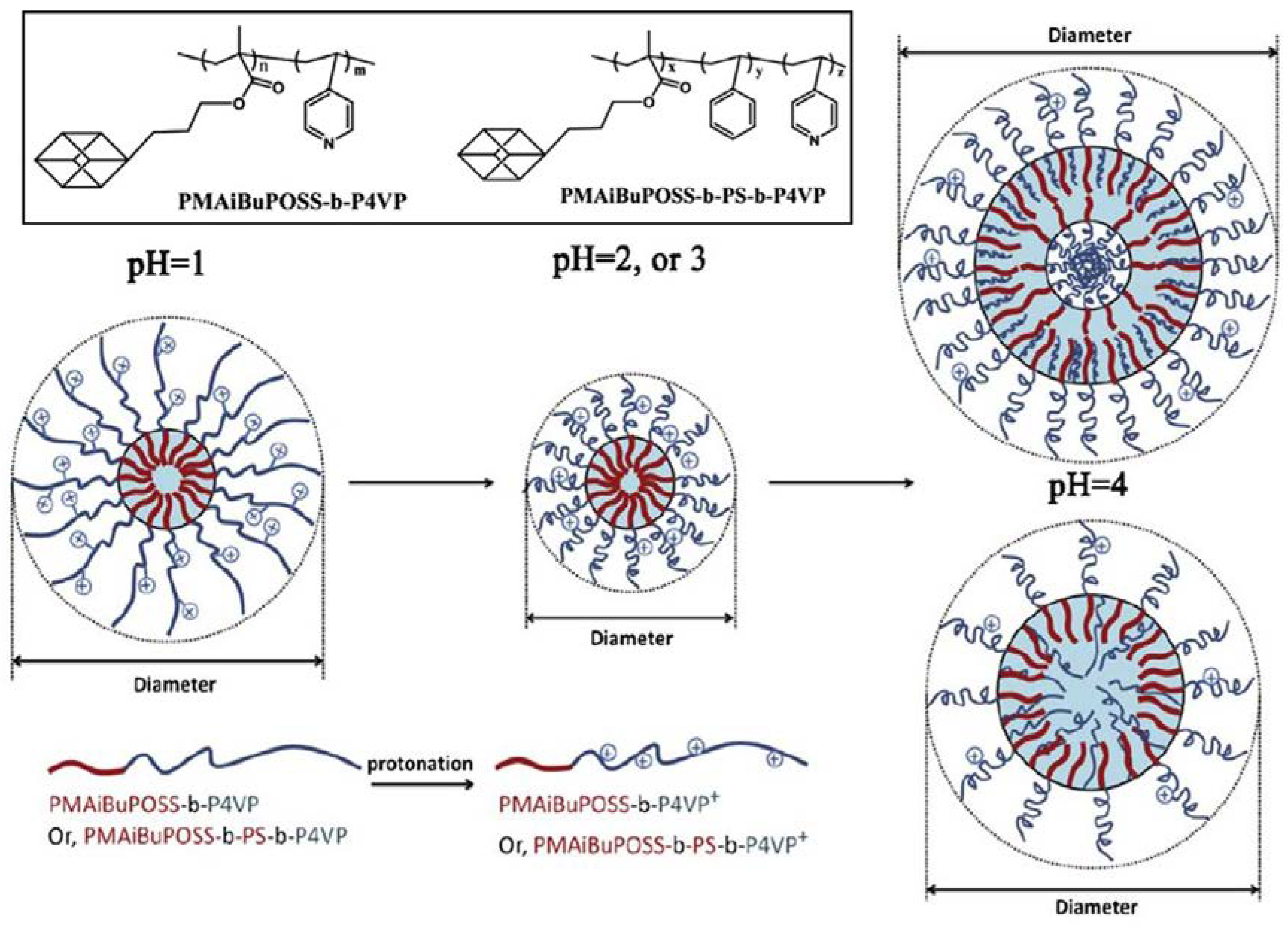
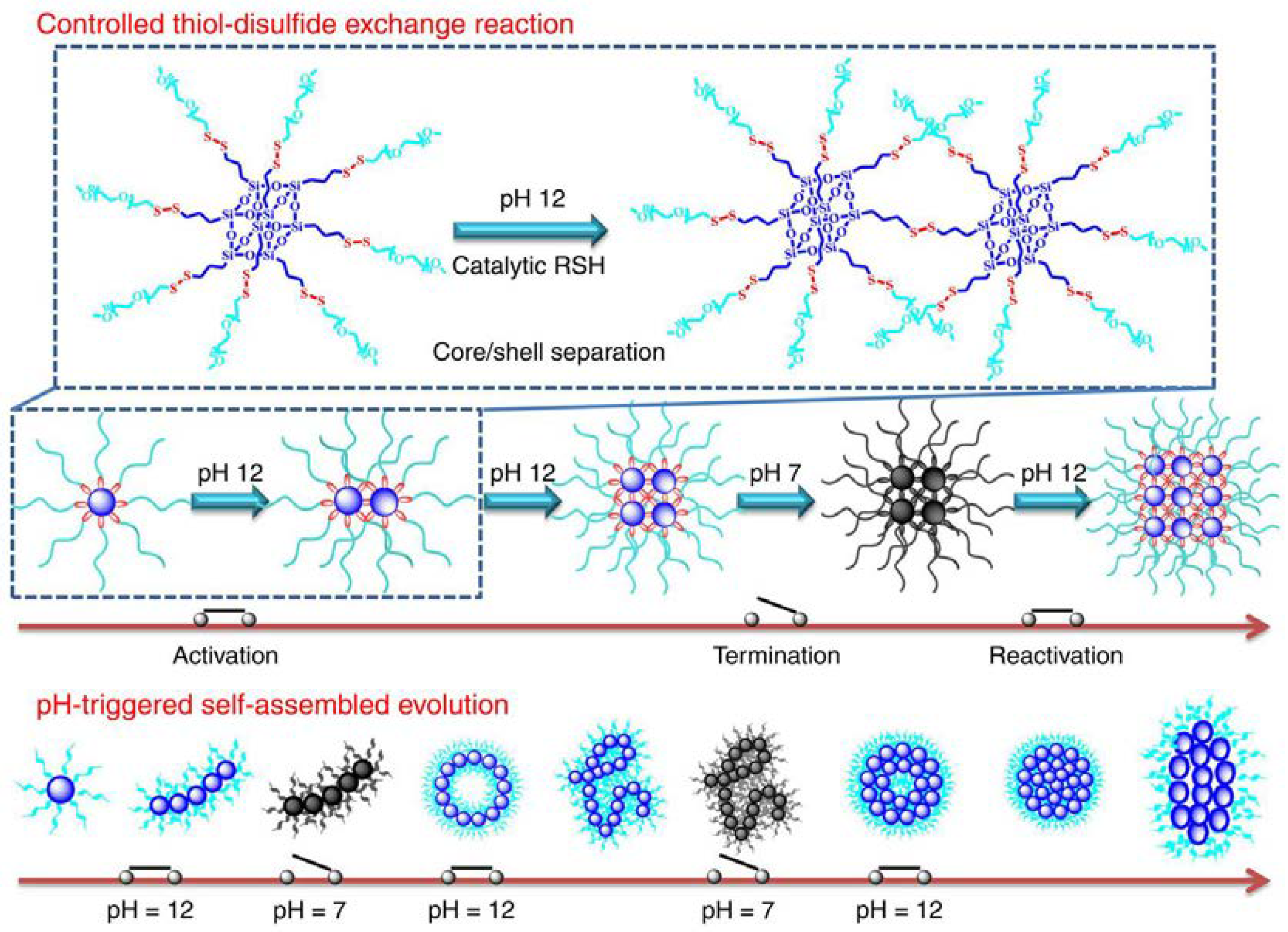
2.1.1. pH-Sensitive Micelles
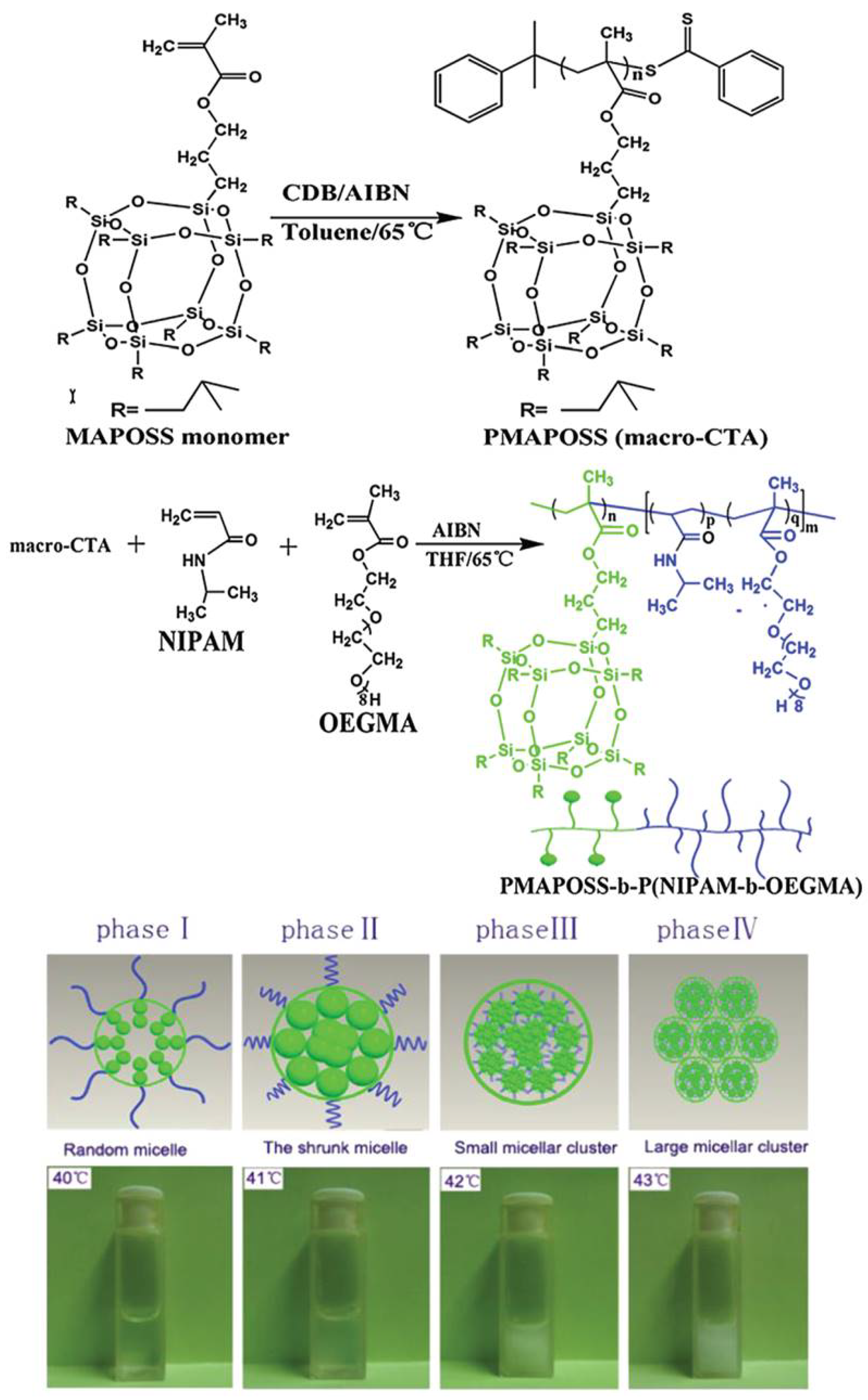
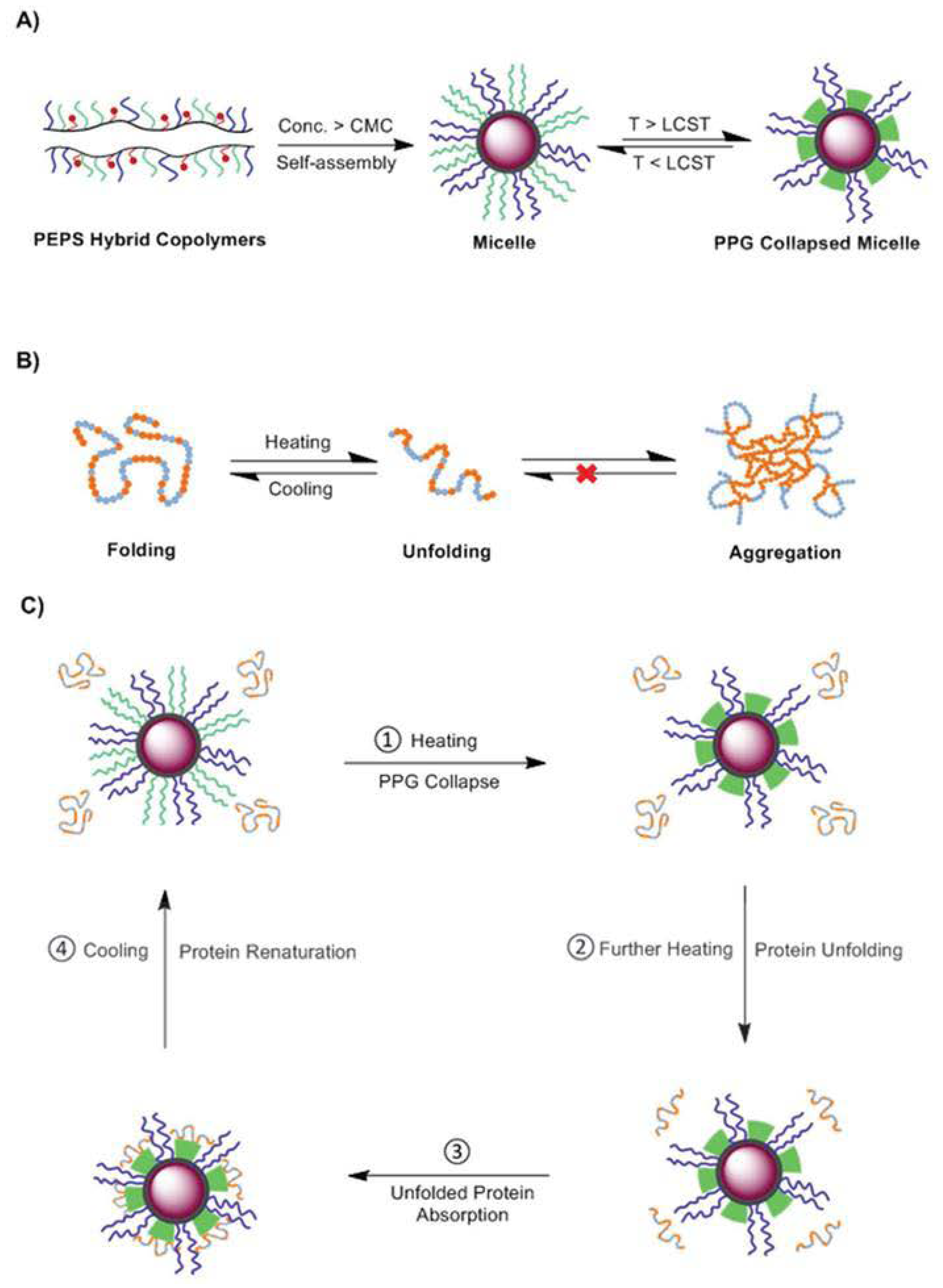
2.1.2. Thermosensitive Micelles
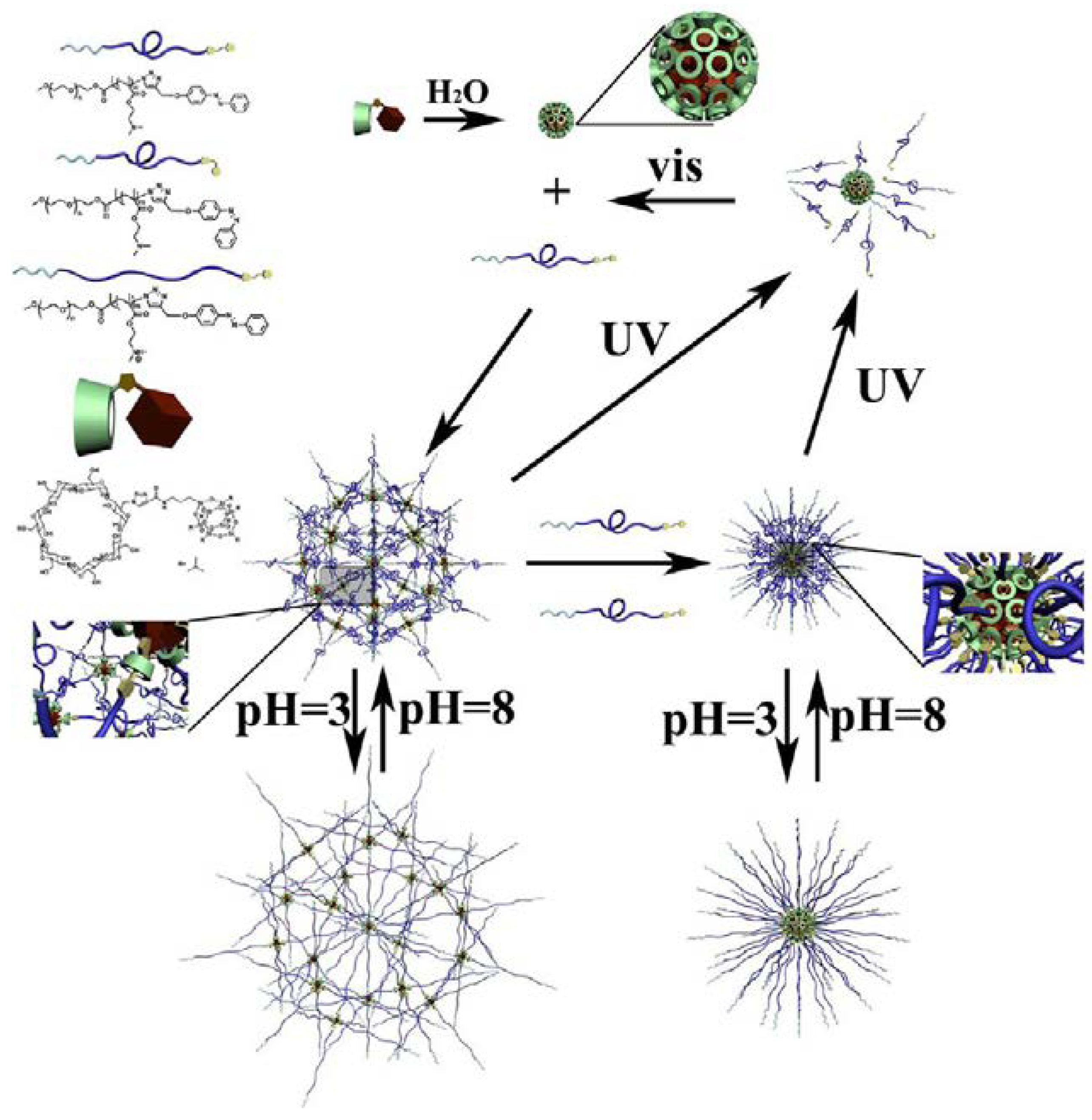
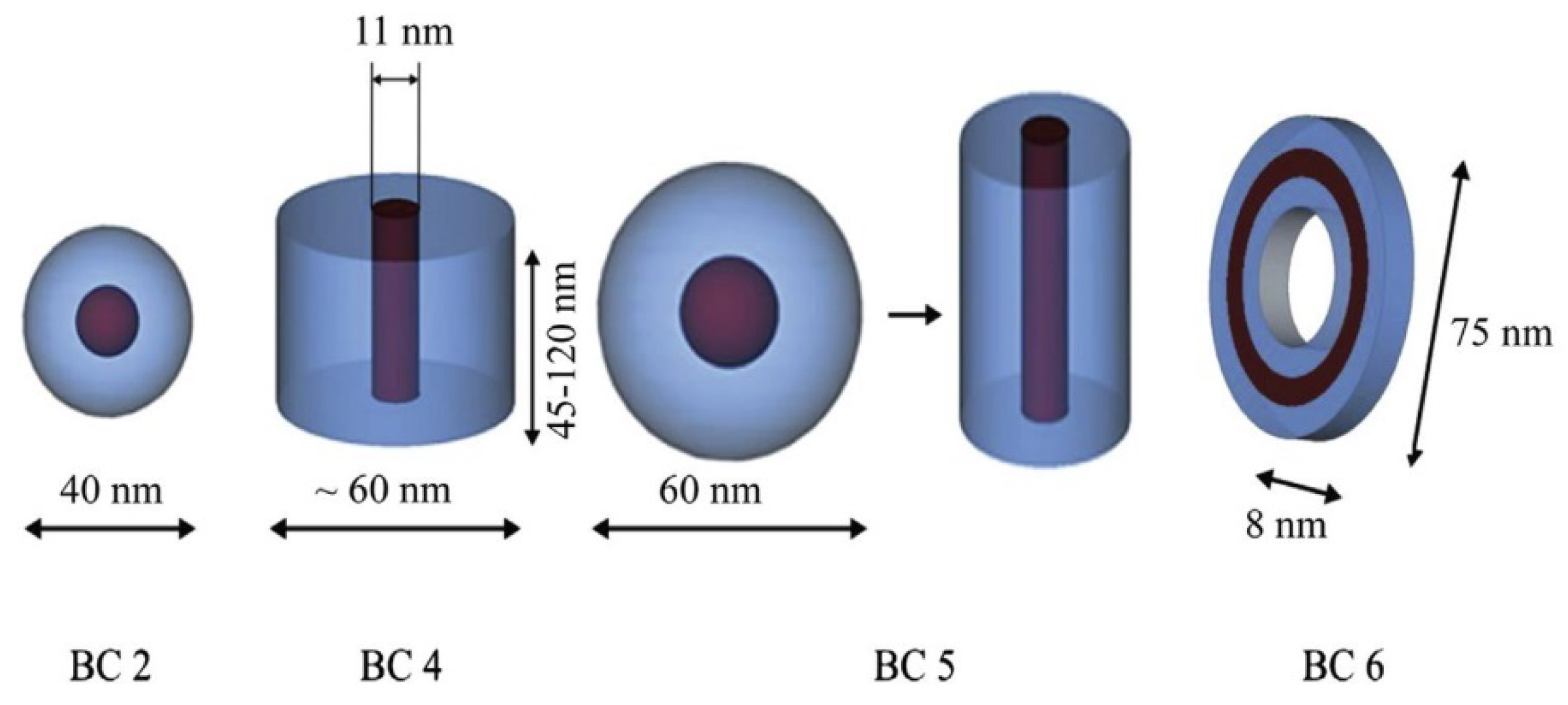
2.1.3. Photoactive Micelles
2.2. Other Mechanisms in Different Assemblies
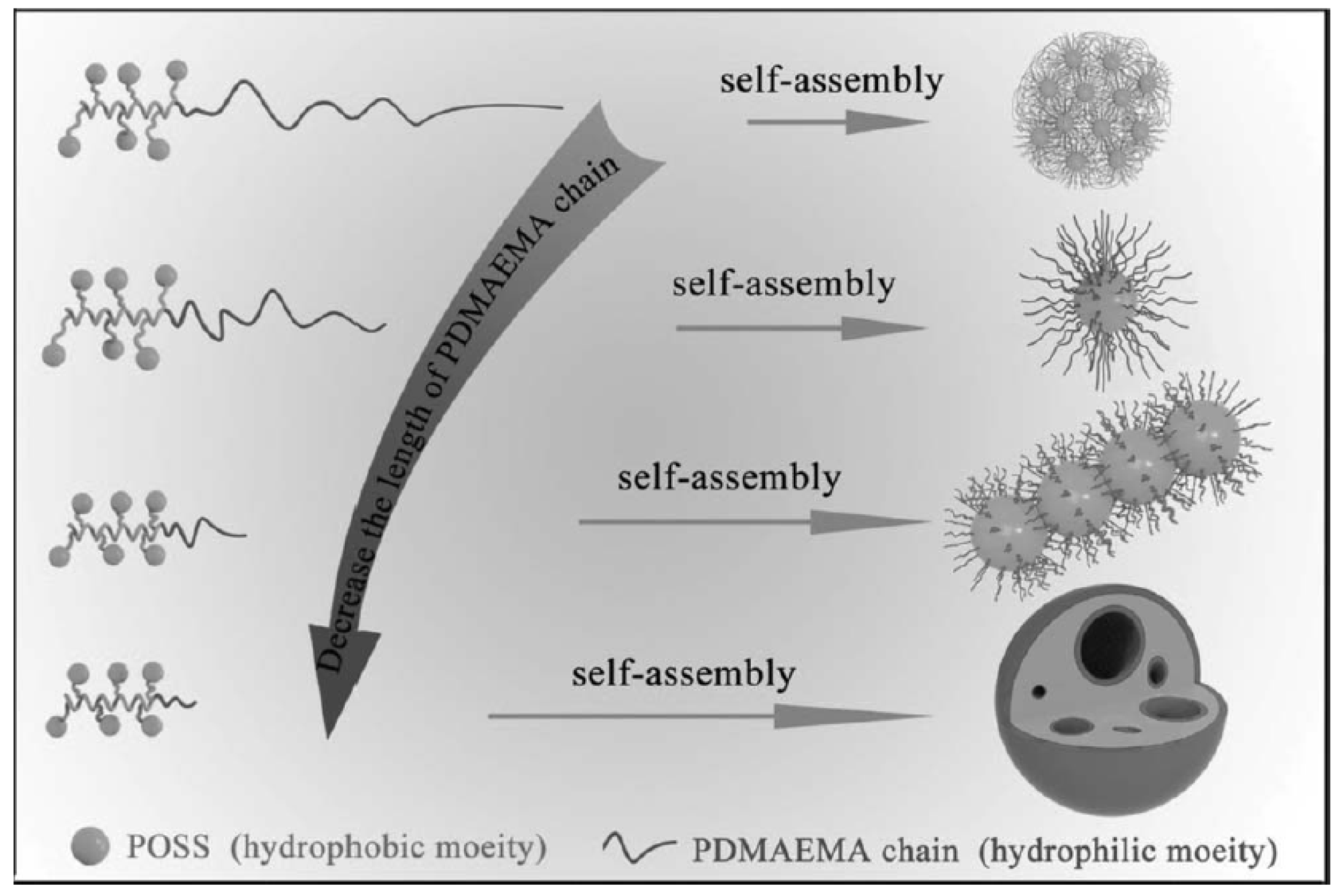
2.2.1. Micelles
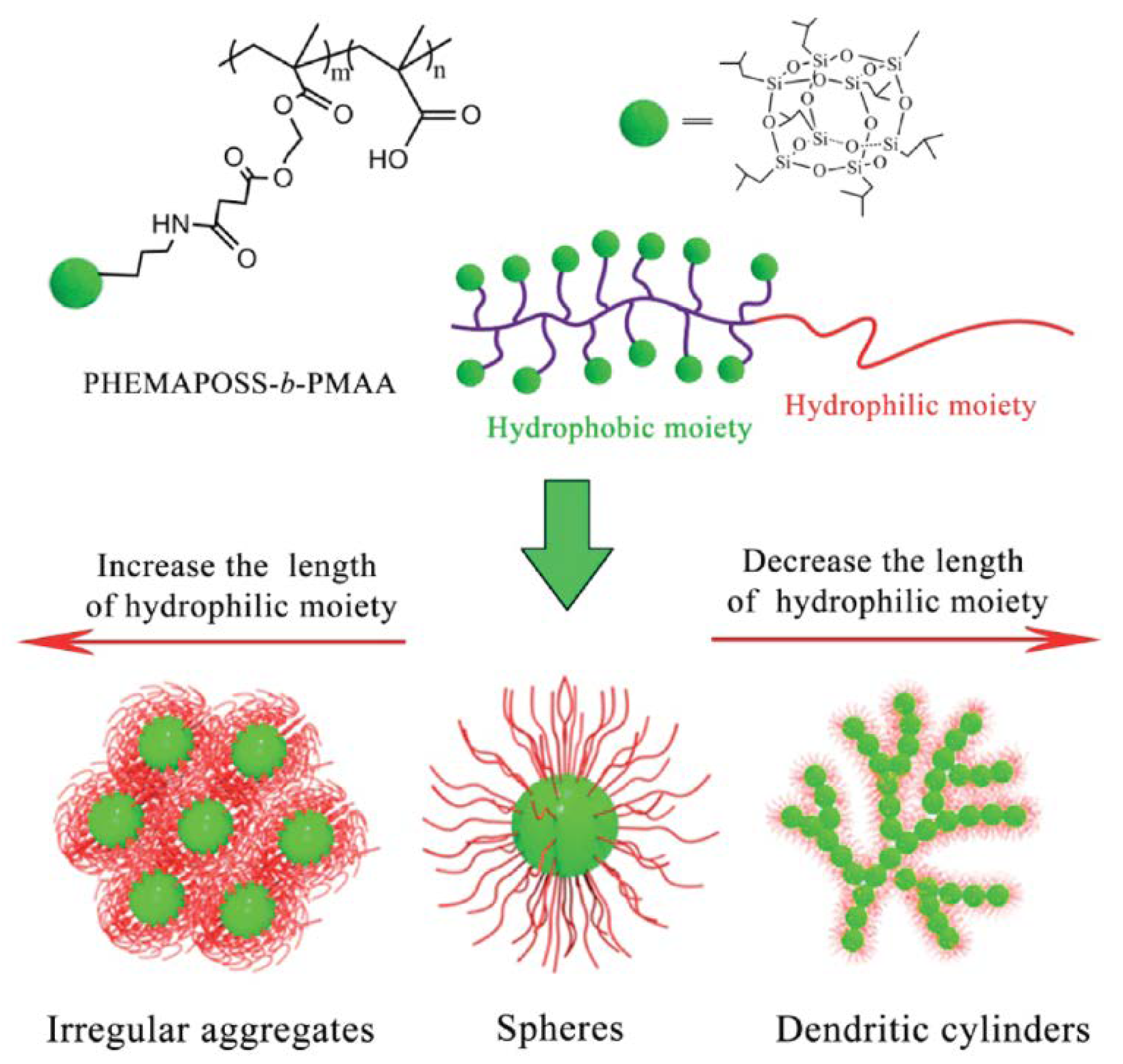
2.2.2. Spheres
2.2.3. Sheets
3. Applications
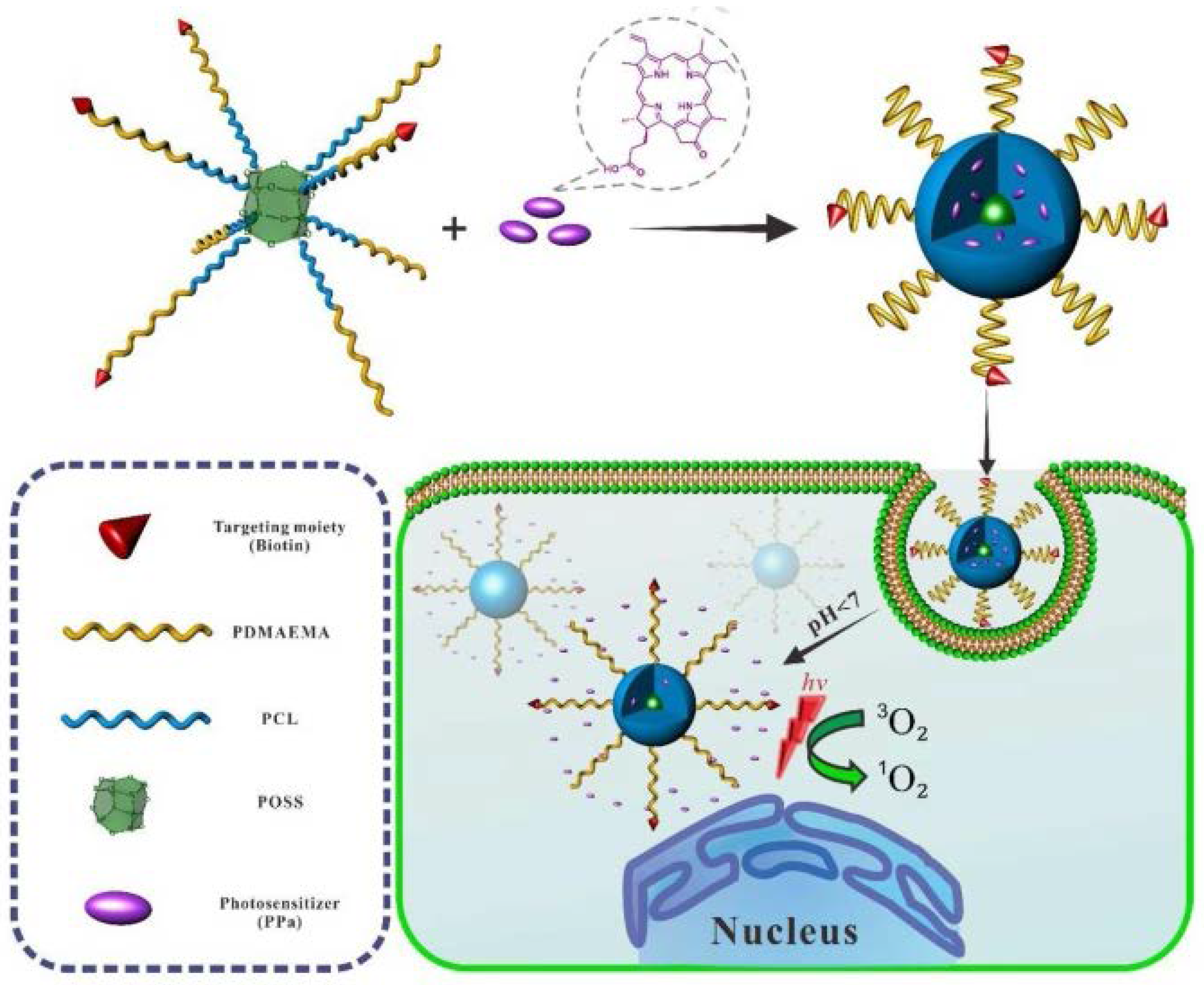
3.1. Drug Delivery
3.2. Photodynamic Therapy
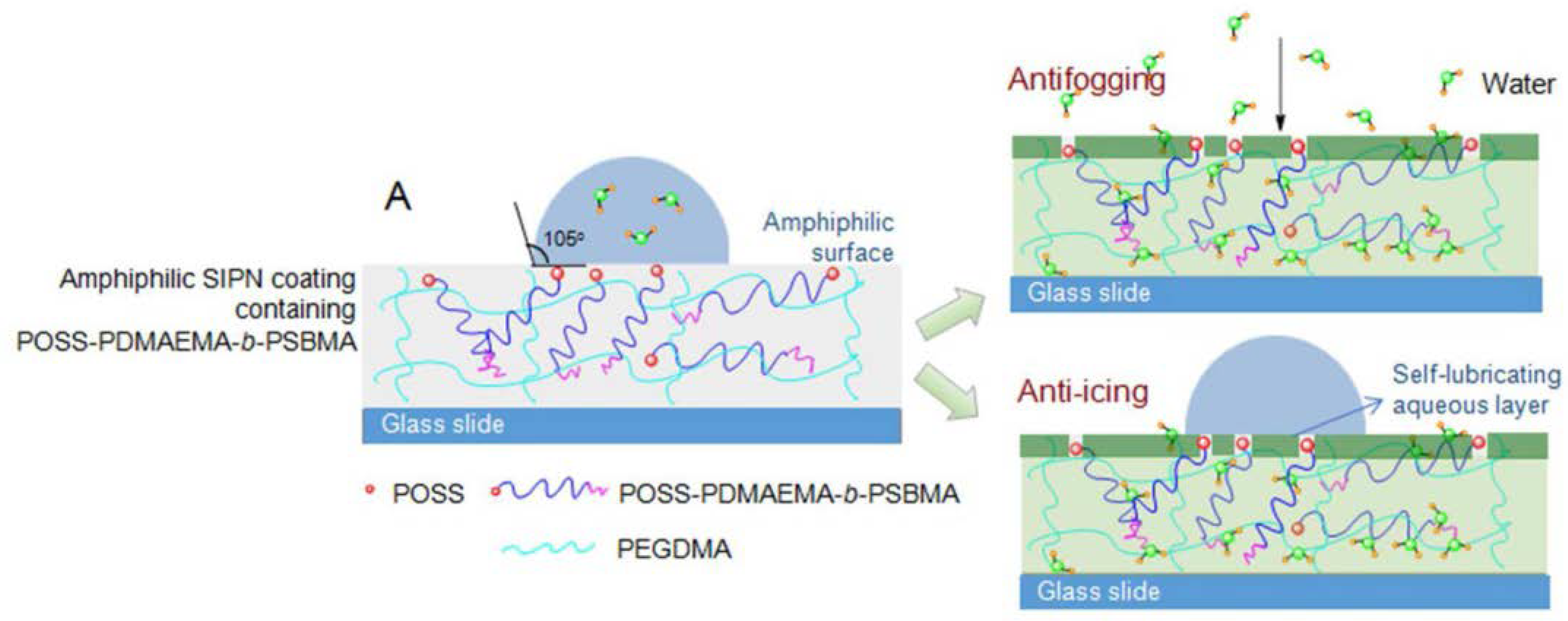
3.3. Coating
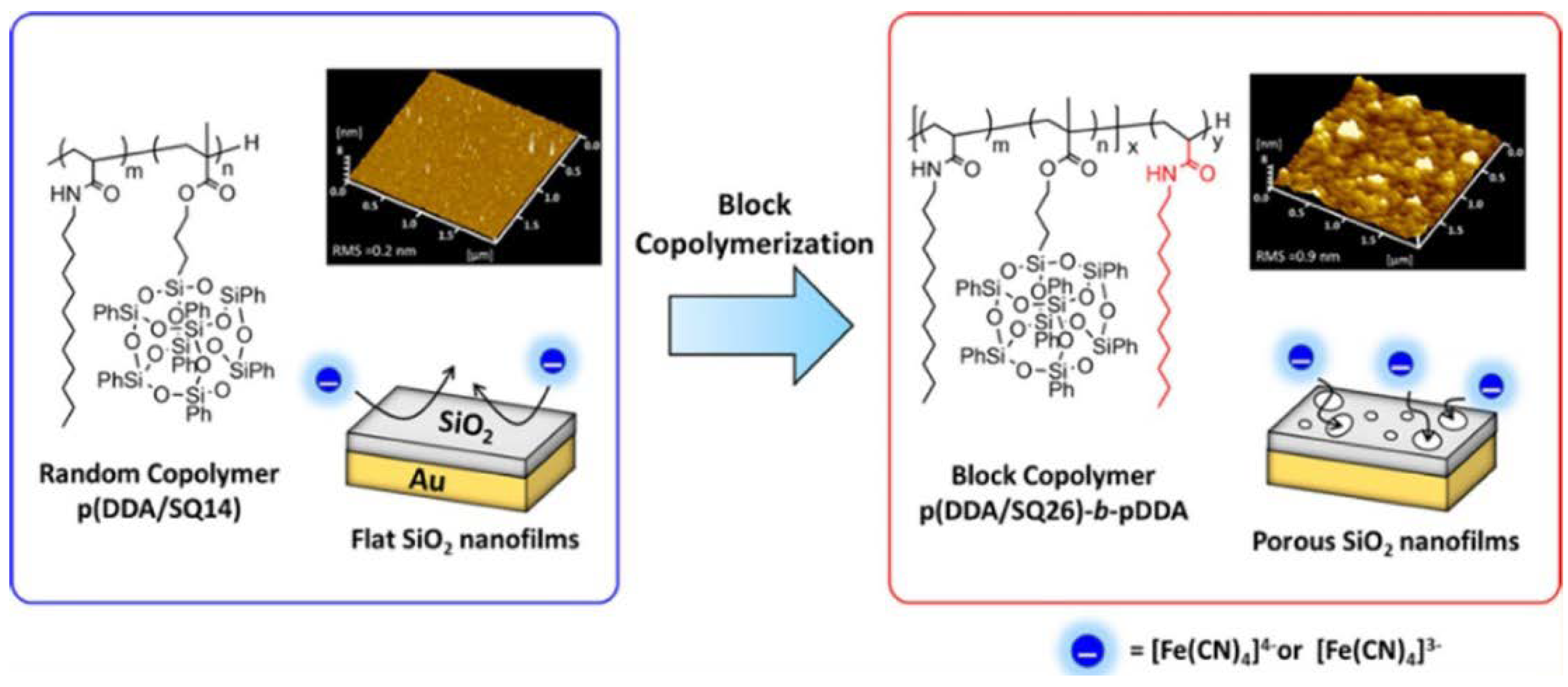
3.4. LB Films
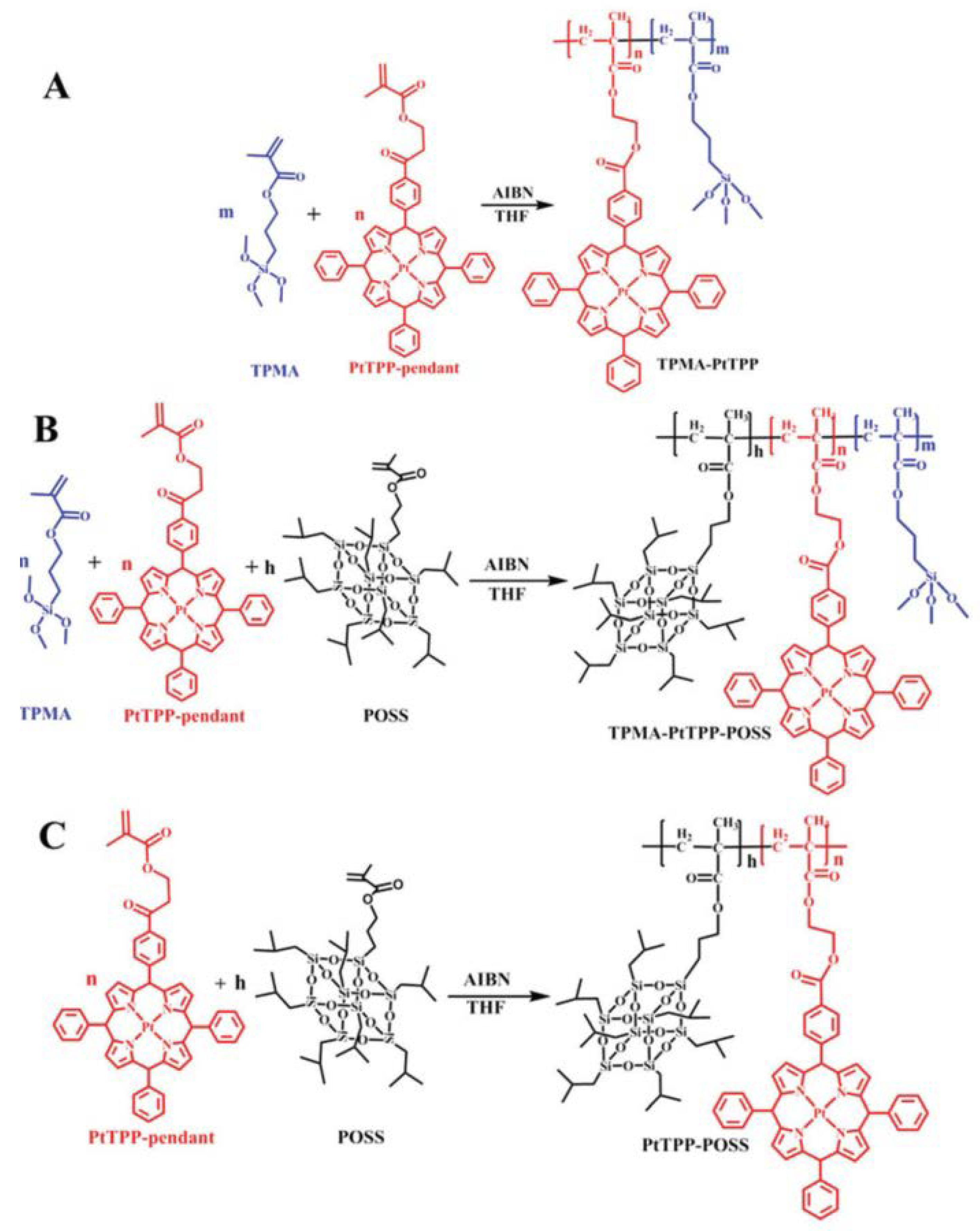
3.5. Optical Sensors
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Hendricks, M.P.; Sato, K.; Palmer, L.C.; Stupp, S.I. Supramolecular assembly of peptide amphiphiles. Acc. Chem. Res. 2017, 50, 2440–2448. [Google Scholar] [CrossRef] [PubMed]
- Krieg, E.; Bastings, M.M.C.; Besenius, P.; Rybtchinski, B. Supramolecular polymers in aqueous media. Chem. Rev. 2016, 116, 2414–2477. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Li, W.; Shin, S.; Lee, M. Development of toroidal nanostructures by self-assembly: Rational designs and applications. Acc. Chem. Res. 2013, 46, 2888–2897. [Google Scholar] [CrossRef] [PubMed]
- Rösler, A.; Vandermeulen, G.W.M.; Klok, H.-A. Advanced drug delivery devices via self-assembly of amphiphilic block copolymers. Adv. Drug Deliv. Rev. 2012, 64, 270–279. [Google Scholar] [CrossRef]
- Tyrrell, Z.L.; Shen, Y.; Radosz, M. Fabrication of micellar nanoparticles for drug delivery through the self-assembly of block copolymers. Prog. Polym. Sci. 2010, 35, 1128–1143. [Google Scholar] [CrossRef]
- Mai, Y.; Eisenberg, A. Self-assembly of block copolymers. Chem. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, G.; Park, H.; Kim, J.; Park, D.; Pramanick, S.; Kim, D.H.; Kim, W.J. Miktoarm amphiphilic block copolymer with singlet oxygen-labile stereospecific β-aminoacrylate junction: Synthesis, self-assembly, and photodynamically triggered drug release. Biomacromolecules 2018, 19, 2202–2213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Hong, C.Y.; Pan, C.Y. Polymerization-Induced Self-Assembly of Functionalized Block Copolymer Nanoparticles and Their Application in Drug Delivery. Macromol. Rapid Commun. 2018, 1800279. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.-W.; Zhou, H.; Zhou, S.-T.; Zhang, H.-J.; Feng, A.-C.; Jian, C.-M.; Hu, J.; Gao, W.-P.; Yuan, J.-Y. Synthesis and self-assembly of CO2-temperature dual stimuli-responsive triblock copolymers. Macromolecules 2014, 47, 2938–2946. [Google Scholar] [CrossRef]
- Xie, C.; Zhang, Z.; Wang, D.; Guan, G.; Gao, D.; Liu, J. Surface molecular self-assembly strategy for TNT imprinting of polymer nanowire/nanotube arrays. Anal. Chem. 2006, 78, 8339–8346. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Zhen, X.; Lei, Q.; Ni, R.; Pu, K. Self-Assembly of Semiconducting Polymer Amphiphiles for In Vivo Photoacoustic Imaging. Adv. Funct. Mater. 2017, 27, 1605397. [Google Scholar] [CrossRef]
- Yu, G.; Zhao, R.; Wu, D.; Zhang, F.; Shao, L.; Zhou, J.; Yang, J.; Tang, G.; Chen, X.; Huang, F. Pillar[5]arene-based amphiphilic supramolecular brush copolymers: Fabrication, controllable self-assembly and application in self-imaging targeted drug delivery. Polym. Chem. 2016, 7, 6178–6188. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Dong, R.; Chen, J.; Chen, F.; Jiang, W.; Zhou, Y.; Zhu, X.; Yan, D. Synthesis and self-assembly of amphiphilic aptamer-functionalized hyperbranched multiarm copolymers for targeted cancer imaging. Biomacromolecules 2014, 15, 1828–1836. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Jang, C.-J.; Ryu, J.-H. Supramolecular Reactor from Self-Assembly of Rod-Coil Molecule in Aqueous Environment. J. Am. Chem. Soc. 2004, 126, 8082–8083. [Google Scholar] [CrossRef] [PubMed]
- Shchukin, D.G.; Sukhorukov, G.B. Nanoparticle synthesis in engineered organic nanoscale reactors. Adv. Mater. 2004, 16, 671–682. [Google Scholar] [CrossRef]
- Yamada, Y.M.A.; Sarkar, S.M.; Uozumi, Y. Amphiphilic self-assembled polymeric copper catalyst to parts per million levels: Click chemistry. J. Am. Chem. Soc. 2012, 134, 9285–9290. [Google Scholar] [CrossRef] [PubMed]
- Souzy, R.; Suau, J.-M.; Kensicher, Y.; Guerret, O. Cosmetic Formulation Containing a Non Water-Soluble Amphiphilic Copolymer as Thickening Agent. U.S. Patents US8790622B2, 29 July 2014. [Google Scholar]
- Kaji, M.; Takeyama, Y.; Nioh, A.; Tsuyuki, M.; Akatsuka, H.; Fujiwara, S.; Sakai, K.; Sakai, H. Surface Morphology of Cosmetic Film Consisting of PEG-Diisostearate Amphiphilic Random Copolymer, Xanthan Gum, and Solvents. J. Oleo Sci. 2017, 66, 1239–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mondet, J.; Lion, B.; Candau, D.; Simon, P. Oily Cosmetic Composition Containing, as a Thickener, an Association of Two Copzolymers and Optionally Containing an Amphiphilic Rheology Corrector. U.S. Patents US5519063A, 21 May 1996. [Google Scholar]
- Huang, J.; Xu, J.; Wang, D.; Li, L.; Guo, X. Effects of amphiphilic copolymer dispersants on rheology and stability of coal water slurry. Ind. Eng. Chem. Res. 2013, 52, 8427–8435. [Google Scholar] [CrossRef]
- Shin, H.i.; Min, B.G.; Jeong, W.; Park, C. Amphiphilic block copolymer micelles: New dispersant for single wall carbon nanotubes. Macromol. Rapid Commun. 2005, 26, 1451–1457. [Google Scholar] [CrossRef]
- Yazdi, A.V.; Lepilleur, C.; Singley, E.J.; Liu, W.; Adamsky, F.A.; Enick, R.M.; Beckman, E.J. Highly carbon dioxide soluble surfactants, dispersants and chelating agents. Fluid Ph. Equilibria 1996, 117, 297–303. [Google Scholar] [CrossRef]
- Ullah, A.; Ullah, S.; Khan, G.S.; Shah, S.M.; Hussain, Z.; Muhammad, S.; Siddiq, M.; Hussain, H. Water soluble polyhedral oligomeric silsesquioxane based amphiphilic hybrid polymers: Synthesis, self-assembly, and applications. Eur. Polym. J. 2016, 75, 67–92. [Google Scholar] [CrossRef]
- Uner, A.; Doganci, E.; Tasdelen, M.A.; Yilmaz, F.; Gürek, A.G. Synthesis, characterization and surface properties of star-shaped polymeric surfactants with polyhedral oligomeric silsesquioxane core. Polym. Int. 2017, 66, 1610–1616. [Google Scholar] [CrossRef]
- Yu, X.; Zhong, S.; Li, X.; Tu, Y.; Yang, S.; Van Horn, R.M.; Ni, C.; Pochan, D.J.; Quirk, R.P.; Wesdemiotis, C. A giant surfactant of polystyrene-(carboxylic acid-functionalized polyhedral oligomeric silsesquioxane) amphiphile with highly stretched polystyrene tails in micellar assemblies. J. Am. Chem. Soc. 2010, 132, 16741–16744. [Google Scholar] [CrossRef] [PubMed]
- Ni, B.; Huang, M.; Chen, Z.; Chen, Y.; Hsu, C.-H.; Li, Y.; Pochan, D.; Zhang, W.-B.; Cheng, S.Z.D.; Dong, X.-H. Pathway toward large two-dimensional hexagonally patterned colloidal nanosheets in solution. J. Am. Chem. Soc. 2015, 137, 1392–1395. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, Y.; Gao, P.; Yang, F.; Shen, H.; Guo, H.; Wu, D. Synthesis, self-assembly, and photoresponsive behavior of tadpole-shaped azobenzene polymers. ACS Macro Lett. 2015, 4, 1321–1326. [Google Scholar] [CrossRef]
- Pu, Y.; Zhang, L.; Zheng, H.; He, B.; Gu, Z. Drug release of pH-sensitive poly (l-aspartate)-b-poly (ethylene glycol) micelles with POSS cores. Polym. Chem. 2014, 5, 463–470. [Google Scholar] [CrossRef]
- Yang, Y.-Y.; Wang, X.; Hu, Y.; Hu, H.; Wu, D.-C.; Xu, F.-J. Bioreducible POSS-cored star-shaped polycation for efficient gene delivery. ACS Appl. Mater. Interfaces 2013, 6, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Cao, M.; Zhang, X.; Li, Z. Synthesis of star-like hybrid POSS-(PDMAEMA-b-PDLA) 8 copolymer and its stereocomplex properties with PLLA. Mater. Sci. Eng. C 2017, 76, 211–216. [Google Scholar] [CrossRef]
- Weng, J.-T.; Yeh, T.-F.; Samuel, A.Z.; Huang, Y.-F.; Sie, J.-H.; Wu, K.-Y.; Peng, C.-H.; Hamaguchi, H.-O.; Wang, C.-L. Cylindrical micelles of a POSS amphiphilic dendrimer as nano-reactors for polymerization. Nanoscale 2018, 10, 3509–3517. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Chen, S.; Tong, X.; An, Z.; Ma, M.; Wang, X.; Wang, X. Self-Assembly of a Strong Polyhedral Oligomeric Silsesquioxane Core-Based Aspartate Derivative Dendrimer Supramolecular Gelator in Different Polarity Solvents. Langmuir 2017, 33, 13332–13342. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Inafuku, K.; Naka, K.; Chujo, Y. Enhancement of entrapping ability of dendrimers by a cubic silsesquioxane core. Org. Biomol. Chem. 2008, 6, 3899–3901. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Fan, X.; He, C. Hybrid Starlike Block Copolymer POSS–(PDMAEMA-b-PNIPAm)8: Thermal Gelation and Its Blends with Poly (vinyl alcohol). Macromolecules 2016, 49, 4236–4244. [Google Scholar] [CrossRef]
- Hong, L.; Zhang, Z.; Zhang, W. Synthesis of Organic/Inorganic Polyhedral Oligomeric Silsesquioxane-Containing Block Copolymers via Reversible Addition-Fragmentation Chain Transfer Polymerization and Their Self-Assembly in Aqueous Solution. Ind. Eng. Chem. Res. 2014, 53, 10673–10680. [Google Scholar] [CrossRef]
- Chi, H.; Lim, S.L.; Wang, F.; Wang, X.; He, C.; Chin, W.S. Pure Blue-Light Emissive Poly(oligofluorenes) with Bifunctional POSS in the Main Chain. Macromol. Rapid Commun. 2014, 35, 801–806. [Google Scholar] [CrossRef] [PubMed]
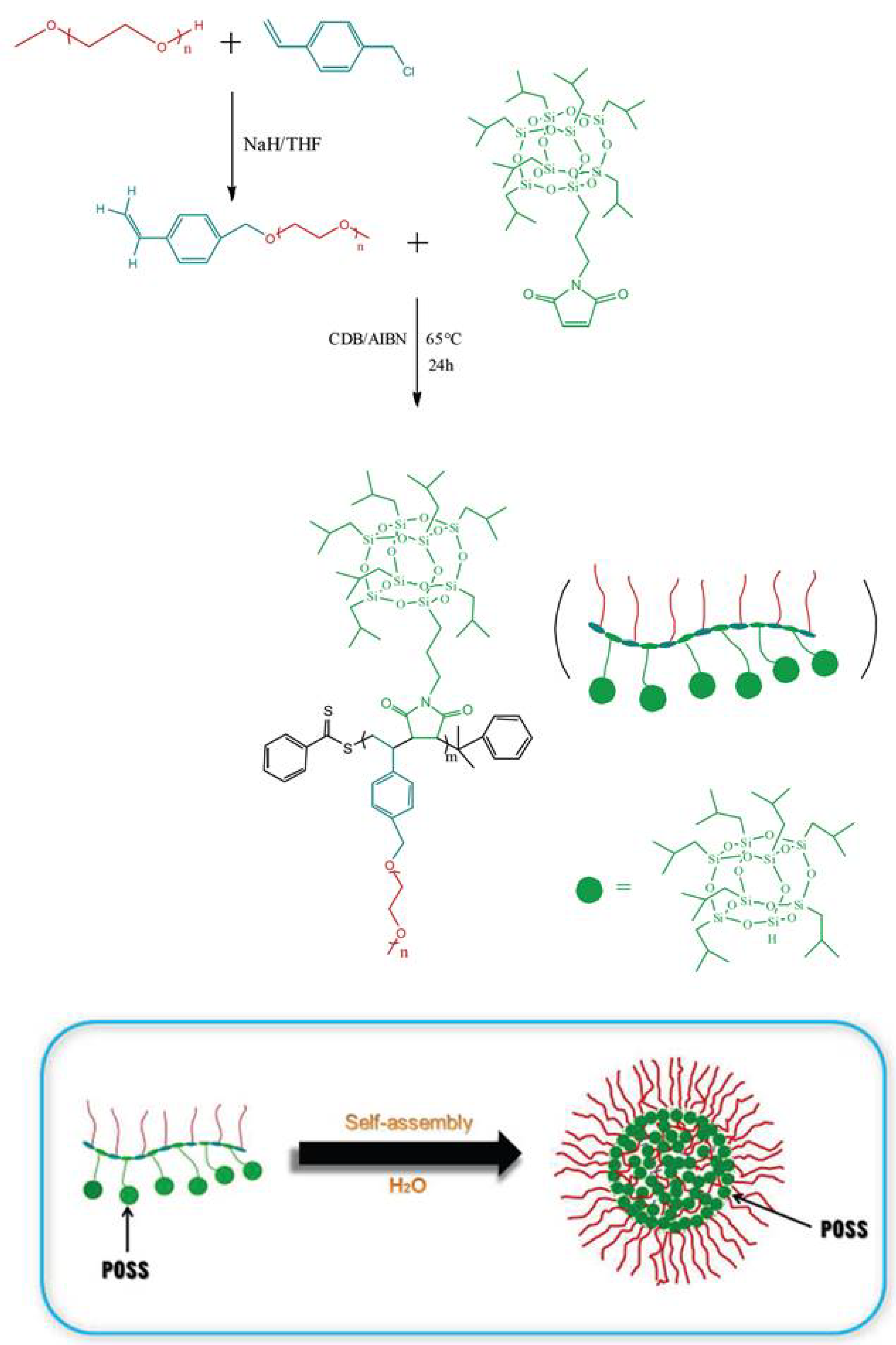
- Zhang, Z.; Hong, L.; Li, J.; Liu, F.; Cai, H.; Gao, Y.; Zhang, W. One-pot synthesis of well-defined amphiphilic alternating copolymer brushes based on POSS and their self-assembly in aqueous solution. RSC Adv. 2015, 5, 21580–21587. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Hsu, K.-C.; Hong, J.-L.; Kuo, S.-W. Unexpected fluorescence from maleimide-containing polyhedral oligomeric silsesquioxanes: Nanoparticle and sequence distribution analyses of polystyrene-based alternating copolymers. Polym. Chem. 2016, 7, 135–145. [Google Scholar] [CrossRef]
- Han, Y.; Wang, F.; Lim, C.Y.; Chi, H.; Chen, D.; Wang, F.; Jiao, X. High-Performance Nano-Photoinitiators with Improved Safety for 3D Printing. ACS Appl. Mater. Interfaces 2017, 9, 32418–32423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chi, H.; Li, T.; Wang, F.; Chin, W.S.; Xu, J. Energy transfer along a sequence controlled hybrid polymer. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 1225–1233. [Google Scholar] [CrossRef]
- Almutary, A.; Sanderson, B. Toxicity of four novel Polyhedral Oligomeric Silsesquioxane (POSS) particles used in anti-cancer drug delivery. J. Appl. Pharm. Sci. 2017, 7, 101–105. [Google Scholar] [CrossRef]
- Yang, D.P.; Oo, M.N.N.L.; Deen, G.R.; Li, Z.; Loh, X.J. Nano-Star-Shaped Polymers for Drug Delivery Applications. Macromol. Rapid Commun. 2017, 38, 1700410. [Google Scholar] [CrossRef] [PubMed]
- Pramudya, I.; Rico, C.G.; Lee, C.; Chung, H. POSS-containing bioinspired adhesives with enhanced mechanical and optical properties for biomedical applications. Biomacromolecules 2016, 17, 3853–3861. [Google Scholar] [CrossRef] [PubMed]
- Yahyaei, H.; Mohseni, M.; Ghanbari, H.; Messori, M. Synthesis and characterization of polyhedral oligomeric titanized silsesquioxane: A new biocompatible cage like molecule for biomedical application. Mater. Sci. Eng. C 2016, 61, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Falk, A.; Dreimann, J.M.; Vogt, D. POSS-modification of Metathesis Catalysts-Improved Recycling and Life-Time in Membrane Separation. ACS Sustain. Chem. Eng. 2018, 6, 7221–7226. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, G.; Lu, C.; Nie, J.; Chen, Z.; Ren, J. POSS supported C2-symmetric bisprolinamide as a recyclable chiral catalyst for asymmetric Aldol reaction. Catal. Commun. 2016, 75, 23–27. [Google Scholar] [CrossRef]
- Zhang, W.; Camino, G.; Yang, R. Polymer/polyhedral oligomeric silsesquioxane (POSS) nanocomposites: An overview of fire retardance. Prog. Polym. Sci. 2017, 67, 77–125. [Google Scholar] [CrossRef]
- Lin, H.K.; Liu, Y.L. Reactive Hybrid of Polyhedral Oligomeric Silsesquioxane (POSS) and Sulfur as a Building Block for Self-Healing Materials. Macromol. Rapid Commun. 2017, 38, 1700051. [Google Scholar] [CrossRef] [PubMed]
- Shang, D.; Fu, J.; Lu, Q.; Chen, L.; Yin, J.; Dong, X.; Xu, Y.; Jia, R.; Yuan, S.; Chen, Y. A novel polyhedral oligomeric silsesquioxane based ionic liquids (POSS-ILs) polymer electrolytes for lithium ion batteries. Solid State Ion. 2018, 319, 247–255. [Google Scholar] [CrossRef]
- Li, Z.; Kong, J.; Wang, F.; He, C. Polyhedral oligomeric silsesquioxanes (POSSs): An important building block for organic optoelectronic materials. J. Mater. Chem. C 2017, 5, 5283–5298. [Google Scholar] [CrossRef]
- Wang, F.; Lu, X.; He, C. Some recent developments of polyhedral oligomeric silsesquioxane (POSS)-based polymeric materials. J. Mater. Chem. 2011, 21, 2775–2782. [Google Scholar] [CrossRef]
- Ye, Q.; Zhou, H.; Xu, J. Cubic polyhedral oligomeric silsesquioxane based functional materials: Synthesis, assembly, and applications. Chem. A Eur. J. 2016, 11, 1322–1337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Müller, A.H.E. Architecture, self-assembly and properties of well-defined hybrid polymers based on polyhedral oligomeric silsequioxane (POSS). Prog. Polym. Sci. 2013, 38, 1121–1162. [Google Scholar] [CrossRef]
- Hirai, T.; Leolukman, M.; Liu, C.C.; Han, E.; Kim, Y.J.; Ishida, Y.; Hayakawa, T.; Kakimoto, M.a.; Nealey, P.F.; Gopalan, P. One-Step Direct-Patterning Template Utilizing Self-Assembly of POSS-Containing Block Copolymers. Adv. Mater. 2009, 21, 4334–4338. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Hong, S.; Cardoen, G.; Burgaz, E.; Gido, S.P.; Coughlin, E.B. Polymer nanocomposites through controlled self-assembly of cubic silsesquioxane scaffolds. Macromolecules 2004, 37, 8606–8611. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, C.; Jin, H.; Jiang, B.; Zhu, X.; Zhou, Y.; Lu, Z.; Yan, D. A supramolecular Janus hyperbranched polymer and its photoresponsive self-assembly of vesicles with narrow size distribution. J. Am. Chem. Soc. 2013, 135, 4765–4770. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hong, L.; McGowan, P.C. A Giant Capsule from the Self-Assembly of a Penta-Telechelic Hybrid Poly(acrylic acid) Based on Polyhedral Oligomeric Silsesquioxane. Macromol. Chem. Phys. 2014, 215, 900–905. [Google Scholar] [CrossRef]
- Raghunath, J.; Zhang, H.; Edirisinghe, M.J.; Darbyshire, A.; Butler, P.E.; Seifalian, A.M. A new biodegradable nanocomposite based on polyhedral oligomeric silsesquioxane nanocages: Cytocompatibility and investigation into electrohydrodynamic jet fabrication techniques for tissue-engineered scaffolds. Biotechnol. Appl. Biochem. 2009, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lin, Q.; Shen, C.; Han, Y.; Tang, J.; Chen, H. Synthesis of MA POSS–PMMA as an intraocular lens material with high light transmittance and good cytocompatibility. RSC Adv. 2014, 4, 52959–52966. [Google Scholar] [CrossRef]
- Kannan, R.Y.; Salacinski, H.J.; Edirisinghe, M.J.; Hamilton, G.; Seifalian, A.M. Polyhedral oligomeric silsequioxane-polyurethane nanocomposite microvessels for an artificial capillary bed. Biomaterials 2006, 27, 4618–4626. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.Y.; Salacinski, H.J.; De Groot, J.; Clatworthy, I.; Bozec, L.; Horton, M.; Butler, P.E.; Seifalian, A.M. The antithrombogenic potential of a polyhedral oligomeric silsesquioxane (POSS) nanocomposite. Biomacromolecules 2006, 7, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Ata, S.; Banerjee, S.L.; Singha, N.K. Polymer nano-hybrid material based on graphene oxide/POSS via surface initiated atom transfer radical polymerization (SI-ATRP): Its application in specialty hydrogel system. Polymer 2016, 103, 46–56. [Google Scholar] [CrossRef]
- Suenaga, K.; Tanaka, K.; Chujo, Y. Heat-Resistant Mechanoluminescent Chromism of the Hybrid Molecule Based on Boron Ketoiminate Modified Octasubstituted Polyhedral Oligomeric Silsesquioxane. Chem. Eur. J. 2017, 23, 1409–1414. [Google Scholar] [CrossRef]
- Tanaka, K.; Kozuka, H.; Ueda, K.; Jeon, J.H.; Chujo, Y. POSS-based molecular fillers for simultaneously enhancing thermal and viscoelasticity of poly (methyl methacrylate) films. Mater. Lett. 2017, 203, 62–67. [Google Scholar] [CrossRef]
- Liu, L.; Tian, M.; Zhang, W.; Zhang, L.; Mark, J.E. Crystallization and morphology study of polyhedral oligomeric silsesquioxane (POSS)/polysiloxane elastomer composites prepared by melt blending. Polymer 2007, 48, 3201–3212. [Google Scholar] [CrossRef]
- Liu, H.; Zheng, S. Polyurethane networks nanoreinforced by polyhedral oligomeric silsesquioxane. Macromol. Rapid Commun. 2005, 26, 196–200. [Google Scholar] [CrossRef]
- Li, Z.; Tan, B.H.; Jin, G.; Li, K.; He, C. Design of polyhedral oligomeric silsesquioxane (POSS) based thermo-responsive amphiphilic hybrid copolymers for thermally denatured protein protection applications. Polym. Chem. 2014, 5, 6740–6753. [Google Scholar] [CrossRef]
- Li, Y.; Xu, B.; Bai, T.; Liu, W. Co-delivery of doxorubicin and tumor-suppressing p53 gene using a POSS-based star-shaped polymer for cancer therapy. Biomaterials 2015, 55, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Huang, J.; Li, Y.; Wang, M.; Cao, Y.; Yuan, C.; Zeng, B.; Dai, L. A novel hybrid polyhedral oligomeric silsesquioxane-based copolymer with zwitterion: Synthesis, characterization, self-assembly behavior and pH responsive property. Macromol. Res. 2017, 25, 817–825. [Google Scholar] [CrossRef]
- Ueda, K.; Tanaka, K.; Chujo, Y. Remarkably high miscibility of octa-substituted POSS with commodity conjugated polymers and molecular fillers for the improvement of homogeneities of polymer matrices. Polym. J. 2016, 48, 1133–1139. [Google Scholar] [CrossRef]
- Fan, X.; Wang, Z.; He, C. “Breathing” unimolecular micelles based on a novel star-like amphiphilic hybrid copolymer. J. Mater. Chem. B 2015, 3, 4715–4722. [Google Scholar] [CrossRef]
- Fan, X.; Hu, Z.; Wang, G. Synthesis and unimolecular micelles of amphiphilic copolymer with dendritic poly (l-lactide) core and poly (ethylene oxide) shell for drug delivery. RSC Adv. 2015, 5, 100816–100823. [Google Scholar] [CrossRef]
- Rizvi, S.B.; Yang, S.Y.; Green, M.; Keshtgar, M.; Seifalian, A.M. Novel POSS–PCU nanocomposite material as a biocompatible coating for quantum dots. Bioconjug. Chem. 2015, 26, 2384–2396. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M.; Grzybowski, B. Self-assembly at all scales. Science 2002, 295, 2418–2421. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Li, Z. A review of drug release mechanisms from nanocarrier systems. Mater. Sci. Eng. C 2017, 76, 1440–1453. [Google Scholar] [CrossRef]
- Li, C.; Bai, S.; Li, X.; Zhao, Y.; Ren, L.; Zhu, K.; Yuan, X. Amphiphilic Copolymers Containing POSS and SBMA with N-Vinylcaprolactam and N-Vinylpyrrolidone for THF Hydrate Inhibition. ACS Omega 2018, 3, 7371–7379. [Google Scholar] [CrossRef]
- Ishizaki, Y.; Yamamoto, S.; Miyashita, T.; Mitsuishi, M. Synthesis and Porous SiO2 Nanofilm Formation of the Silsesquioxane-Containing Amphiphilic Block Copolymer. Langmuir 2018, 34, 8007–8014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chu, Y.; Mu, G.; Eghtesadi, S.A.; Liu, Y.; Zhou, Z.; Lu, X.; Kashfipour, M.A.; Lillard, R.S.; Yue, K. Rationally controlling the self-assembly behavior of triarmed POSS–organic hybrid macromolecules: From giant surfactants to macroions. Macromolecules 2017, 50, 5042–5050. [Google Scholar] [CrossRef]
- Zhu, H.; Akkus, B.; Gao, Y.; Liu, Y.; Yamamoto, S.; Matsui, J.; Miyashita, T.; Mitsuishi, M. Regioselective Synthesis of Eight-Armed Cyclosiloxane Amphiphile for Functional 2D and 3D Assembly Motifs. ACS Appl. Mater. Interfaces 2017, 9, 28144–28150. [Google Scholar] [CrossRef] [PubMed]
- Israelachvili, J.N.; Mitchell, D.J.; Ninham, B.W. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. J. Chem. Soc. Faraday Trans. 2 1976, 72, 1525–1568. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.-B.; Hsieh, I.F.; Zhang, G.; Cao, Y.; Li, X.; Wesdemiotis, C.; Lotz, B.; Xiong, H.; Cheng, S.Z.D. Breaking symmetry toward nonspherical Janus particles based on polyhedral oligomeric silsesquioxanes: Molecular design, “click” synthesis, and hierarchical structure. J. Am. Chem. Soc. 2011, 133, 10712–10715. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, W.-B.; Yue, K.; Li, X.; Liu, H.; Xin, Y.; Wang, C.-L.; Wesdemiotis, C.; Cheng, S.Z.D. Giant molecular shape amphiphiles based on polystyrene–hydrophilic [60] fullerene conjugates: Click synthesis, solution self-assembly, and phase behavior. J. Am. Chem. Soc. 2012, 134, 7780–7787. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Yue, K.; Huang, J.; Liu, C.; Zhou, Z.; Wang, J.; Wu, K.; Shan, W.; Shi, A.-C.; Cheng, S.Z.D. Highly Asymmetric Phase Behaviors of Polyhedral Oligomeric Silsesquioxane-Based Multiheaded Giant Surfactants. ACS Nano 2018, 12, 1868–1877. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, S.; Guo, Q.; Lu, X.; Liu, Y.; Mao, J.; Wesdemiotis, C.; Li, T.; Li, Y.; Cheng, S.Z.D. Multilevel Manipulation of Supramolecular Structures of Giant Molecules via Macromolecular Composition and Sequence. ACS Macro Lett. 2018, 7, 635–640. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, X.; Mao, J.; Hsu, C.H.; Mu, G.; Huang, M.; Guo, Q.; Liu, H.; Wesdemiotis, C.; Li, T. Sequence-Mandated, Distinct Assembly of Giant Molecules. Angew. Chem. Int. Ed. 2017, 129, 15210–15215. [Google Scholar] [CrossRef]
- Li, Y.; Dong, X.-H.; Zou, Y.; Wang, Z.; Yue, K.; Huang, M.; Liu, H.; Feng, X.; Lin, Z.; Zhang, W. Polyhedral oligomeric silsesquioxane meets “click” chemistry: Rational design and facile preparation of functional hybrid materials. Polymer 2017, 125, 303–329. [Google Scholar] [CrossRef]
- Niu, H.; Zhang, L.; Gao, M.; Chen, Y. Amphiphilic ABC triblock copolymer-assisted synthesis of core/shell structured CdTe nanowires. Langmuir 2005, 21, 4205–4210. [Google Scholar] [CrossRef] [PubMed]
- Schilli, C.M.; Zhang, M.; Rizzardo, E.; Thang, S.H.; Chong, Y.K.; Edwards, K.; Karlsson, G.; Müller, A.H.E. A New Double-Responsive Block Copolymer Synthesized via RAFT Polymerization: Poly (N-isopropylacrylamide)-b lock-poly (acrylic acid). Macromolecules 2004, 37, 7861–7866. [Google Scholar] [CrossRef]
- Wu, W.; Wang, W.; Li, S.; Wang, J.; Zhang, Q.; Li, X.; Luo, X.; Li, J. Physiological pH-triggered morphological transition of amphiphilic block copolymer self-assembly. J. Polym. Res. 2014, 21, 494. [Google Scholar] [CrossRef]
- Zhang, B.-Y.; He, W.-D.; Li, W.-T.; Li, L.-Y.; Zhang, K.-R.; Zhang, H. Preparation of block-brush PEG-bP (NIPAM-g-DMAEMA) and its dual stimulus-response. Polymer 2010, 51, 3039–3046. [Google Scholar] [CrossRef]
- Pietsch, C.; Mansfeld, U.; Guerrero-Sanchez, C.; Hoeppener, S.; Vollrath, A.; Wagner, M.; Hoogenboom, R.; Saubern, S.; Thang, S.H.; Becer, C.R. Thermo-induced self-assembly of responsive poly (DMAEMA-b-DEGMA) block copolymers into multi-and unilamellar vesicles. Macromolecules 2012, 45, 9292–9302. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, M.; Xie, J.; Li, C.; Yang, C.; Deng, Y.; Yuan, C.; Chang, F.-C.; Dai, L. Synthesis, characterization and self-assembly of hybrid pH-sensitive block copolymer containing polyhedral oligomeric silsesquioxane (POSS). React. Funct. Polym. 2013, 73, 1646–1655. [Google Scholar] [CrossRef]
- Wang, X.; Gao, P.; Yang, Y.; Guo, H.; Wu, D. Dynamic and programmable morphology and size evolution via a living hierarchical self-assembly strategy. Nat. Commun. 2018, 9, 2772. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Lin, L.; Yan, Z.; Yu, Y. Dual responsive block copolymer micelles functionalized by NIPAM and azobenzene. Macromol. Rapid Commun. 2010, 31, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Thakur, N.; Ranganath, A.S.; Sopiha, K.; Baji, A. Thermoresponsive Cellulose Acetate-Poly(N-isopropylacrylamide) Core-Shell Fibers for Controlled Capture and Release of Moisture. ACS Appl. Mater. Interfaces 2017, 9, 29224–29233. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Wang, X.; Cao, M.; Wang, C.; Hu, Z.; Wu, Y.L.; Li, Z.; Loh, X.J. “Y”-shape armed amphiphilic star-like copolymers: Design, synthesis and dual-responsive unimolecular micelle formation for controlled drug delivery. Polym. Chem. 2017, 8, 5611–5620. [Google Scholar] [CrossRef]
- Miladinovic, Z.R.; Micic, M.; Mrakovic, A.; Suljovrujic, E. Smart hydrogels with ethylene glycol propylene glycol pendant chains. J. Polym. Res. 2018, 25, 1. [Google Scholar] [CrossRef]
- Li, L.; Lu, B.; Wu, J.; Fan, Q.; Guo, X.; Liu, Z. Synthesis and self-assembly behavior of thermo-responsive star-shaped POSS-(PCL-P (MEO 2 MA-co-PEGMA)) 16 inorganic/organic hybrid block copolymers with tunable lower critical solution temperature. New J. Chem. 2016, 40, 4761–4768. [Google Scholar] [CrossRef]
- Washington, K.E.; Kularatne, R.N.; Du, J.; Ren, Y.; Gillings, M.J.; Geng, C.X.; Biewer, M.C.; Stefan, M.C. Thermoresponsive star-like γ-substituted poly (caprolactone) s for micellar drug delivery. J. Mater. Chem. B 2017, 5, 5632–5640. [Google Scholar] [CrossRef]
- Truong, T.T.; Thai, S.H.; Nguyen, H.T.; Nguyen, T.H.; Nguyen, L.-T.T. Poly (ε-caprolactone) networks with tunable thermoresponsive shape memory via a facile photo-initiated thiol–ene pathway. J. Mater. Sci. 2018, 53, 2236–2252. [Google Scholar] [CrossRef]
- Fang, Q.; Chen, T.; Zhong, Q.; Wang, J. Thermoresponsive polymers based on oligo (ethylene glycol) methyl ether methacrylate and modified substrates with thermosensitivity. Macromol. Res. 2017, 25, 206–213. [Google Scholar] [CrossRef]
- Alejo, T.; Prieto, M.; García-Juan, H.; Andreu, V.; Mendoza, G.; Sebastián, V.; Arruebo, M. A facile method for the controlled polymerization of biocompatible and thermoresponsive oligo (ethylene glycol) methyl ether methacrylate copolymers. Polym. J. 2018, 50, 203–211. [Google Scholar] [CrossRef]
- Topuzogullari, M.; Elalmis, Y.B.; Isoglu, S.D. Thermo-Responsive Complexes of c-Myc Antisense Oligonucleotide with Block Copolymer of Poly (OEGMA) and Quaternized Poly (4-Vinylpyridine). Macromol. Biosci. 2017, 17, 1600263. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xie, J.; Chen, L.; Gao, H.; Yuan, C.; Li, C.; Luo, W.; Zeng, B.; Dai, L. Synthesis, characterization, and temperature-responsive behaviors of novel hybrid amphiphilic block copolymers containing polyhedral oligomeric silsesquioxane. Polym. Adv. Technol. 2014, 25, 613–623. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Ji, S.; Zhou, Z.; Li, Q. Synthesis and self-assembly behavior of thermoresponsive poly(oligo(ethylene glycol) methyl ether methacrylate)-POSS with tunable lower critical solution temperature. Colloid Polym. Sci. 2014, 292, 2993–3001. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Z.; Ma, L.; Chen, G.; Li, Q. Hierarchical assembly of amphiphilic POSS-cyclodextrin molecules and azobenzene end-capped polymers. Macromolecules 2014, 47, 5739–5748. [Google Scholar] [CrossRef]
- Shao, Y.; Aizhao, P.; Ling, H. POSS end-capped diblock copolymers: Synthesis, micelle self-assembly and properties. J. Colloid. Interface Sci. 2014, 425, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tan, B.H.; Hussain, H.; He, C. pH-responsive amphiphilic hybrid random-type copolymers of poly(acrylic acid) and poly(acrylate-POSS): Synthesis by ATRP and self-assembly in aqueous solution. Colloid Polym. Sci. 2013, 291, 1803–1815. [Google Scholar] [CrossRef]
- Matějka, L.; Janata, M.; Pleštil, J.; Zhigunov, A.; Šlouf, M. Self-assembly of POSS-containing block copolymers: Fixing the hierarchical structure in networks. Polymer 2014, 55, 126–136. [Google Scholar] [CrossRef]
- Wu, J.; Song, X.; Zeng, L.; Xing, J. Synthesis and assembly of polyhedral oligomeric silsesquioxane end-capped amphiphilic polymer to enhance the fluorescent intensity of tetraphenylethene. Colloid Polym. Sci. 2016, 294, 1315–1324. [Google Scholar] [CrossRef]
- Xu, Y.; Cao, Y.; Xie, J.; Li, Q.; Chen, X.; Kuo, S.-W.; Dai, L. Mixed micelles from synergistic self-assembly of hybrid copolymers with charge difference electrostatic interaction induced re-organization of micelles from hybrid copolymers. J. Mater. Res. 2016, 31, 2046–2057. [Google Scholar] [CrossRef]
- Peng, W.; Xu, S.; Li, L.; Zhang, C.; Zheng, S. Organic-inorganic nanocomposites via self-assembly of an amphiphilic triblock copolymer bearing a Poly (butadiene-g-POSS) subchain in epoxy thermosets: Morphologies, surface hydrophobicity, and dielectric properties. J. Phys. Chem. B 2016, 120, 12003–12014. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.-S.; Yan, Y.-K.; Wang, W. Multi-POSS cluster-wrapped polymers and their block copolymers with a PEO bottlebrush polymer: Synthesis and aggregation. Polym. Chem. 2017, 8, 6824–6833. [Google Scholar] [CrossRef]
- Hong, L.; Zhang, Z.; Zhang, Y.; Zhang, W. Synthesis and self-assembly of stimuli-responsive amphiphilic block copolymers based on polyhedral oligomeric silsesquioxane. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 2669–2683. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, J.; Cui, X.; Wang, Y.; Gao, Y.; Sun, P.; Liu, F.; Shimanoe, K.; Yamazoe, N.; Lu, G. Enhanced gas sensing properties to acetone vapor achieved by α-Fe2O3 particles ameliorated with reduced graphene oxide sheets. Sens. Actuator B-Chem. 2017, 241, 904–914. [Google Scholar] [CrossRef]
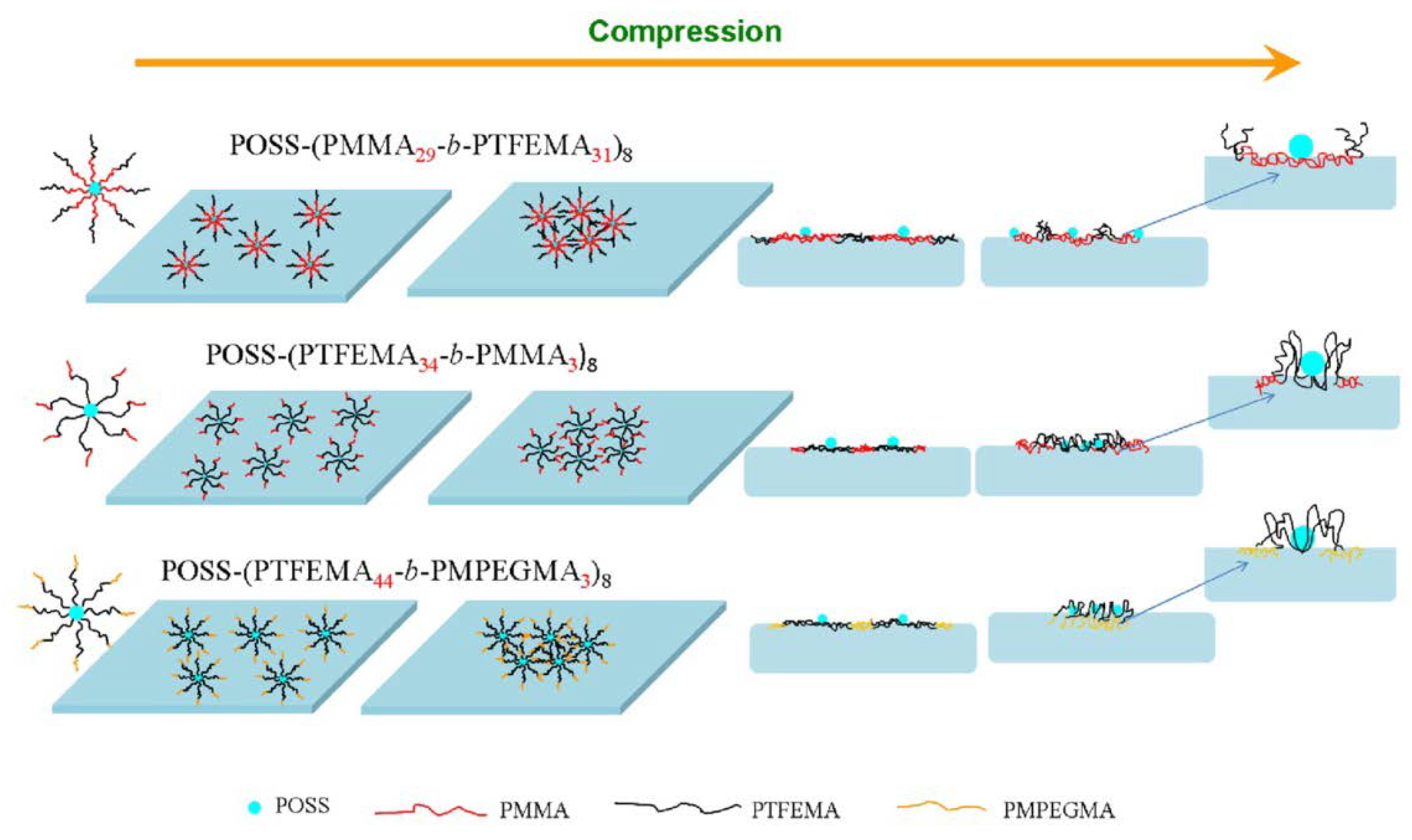
- Li, Z.; Ma, X.; Guan, X.; Qiang, X.; Zang, D.; Chen, F. Aggregation behavior of star-shaped fluoropolymers containing polyhedral oligomeric silsesquioxane (POSS) at the air-water interface. Colloid Polym. Sci. 2016, 295, 157–170. [Google Scholar] [CrossRef]
- Gaucher, G.; Dufresne, M.-H.; Sant, V.P.; Kang, N.; Maysinger, D.; Leroux, J.-C. Block copolymer micelles: Preparation, characterization and application in drug delivery. J. Control. Release 2005, 109, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Kumari, P.; Lakhani, P.M.; Ghosh, B. Recent advances in polymeric micelles for anti-cancer drug delivery. Eur. J. Pharm. Sci. 2016, 83, 184–202. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ouyang, J.; Liu, H.; Chen, M.; Zeng, K.; Sheng, J.; Liu, Z.; Han, Y.; Wang, L.; Li, J. Black Phosphorus Nanosheet-Based Drug Delivery System for Synergistic Photodynamic/Photothermal/Chemotherapy of Cancer. Adv. Mater. 2017, 29, 1603864. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Saw, P.E.; Tao, W.; Li, Y.; Ji, X.; Bhasin, S.; Liu, Y.; Ayyash, D.; Rasmussen, J.; Huo, M. ROS-responsive polyprodrug nanoparticles for triggered drug delivery and effective cancer therapy. Adv. Mater. 2017, 29, 1700141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, H.; Dai, Y. Interlayer-crosslinked micelles prepared from star-shaped copolymers via click chemistry for sustained drug release. Nanotechnology 2017, 28, 205601. [Google Scholar] [CrossRef] [PubMed]
- Jaskula-Sztul, R.; Xu, W.; Chen, G.; Harrison, A.; Dammalapati, A.; Nair, R.; Cheng, Y.; Gong, S.; Chen, H. Thailandepsin A-loaded and octreotide-functionalized unimolecular micelles for targeted neuroendocrine cancer therapy. Biomaterials 2016, 91, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Ye, E.; Lakshminarayanan, R.; Loh, X.J. Recent advances of using hybrid nanocarriers in remotely controlled therapeutic delivery. Small 2016, 12, 4782–4806. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, L.; Sun, W.; Zhou, Z.; Huang, Y. Dual stimuli-responsive hybrid polymeric nanoparticles self-assembled from POSS-based starlike copolymer-drug conjugates for efficient intracellular delivery of hydrophobic drugs. ACS Appl. Mater. Interfaces 2016, 8, 13251–13261. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lu, B.; Fan, Q.; Wu, J.; Wei, L.; Hou, J.; Guo, X.; Liu, Z. Synthesis and self-assembly behavior of pH-responsive star-shaped POSS-(PCL-P (DMAEMA-co-PEGMA)) 16 inorganic/organic hybrid block copolymer for the controlled intracellular delivery of doxorubicin. RSC Adv. 2016, 6, 61630–61640. [Google Scholar] [CrossRef]
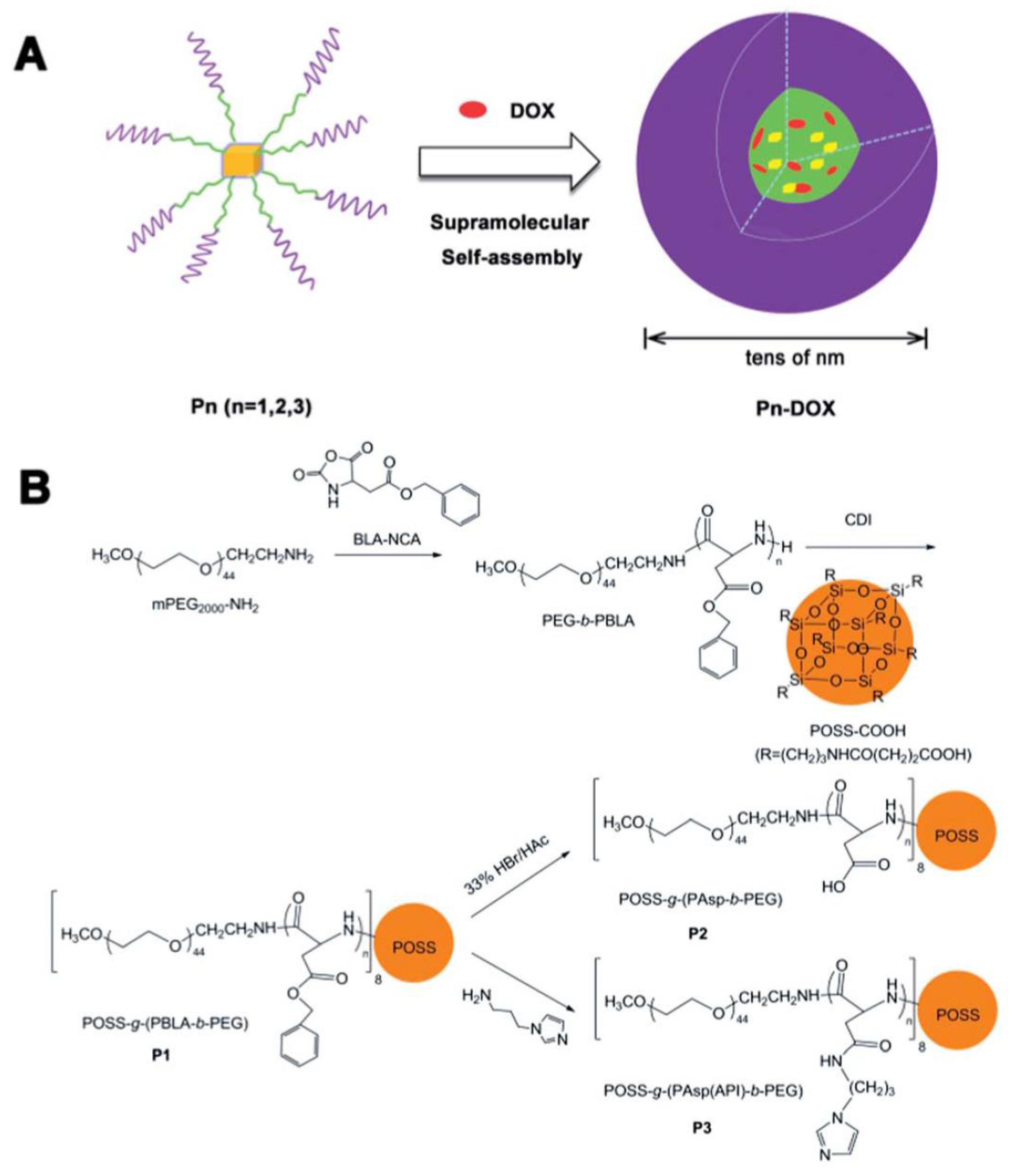
- Pu, Y.; Zhang, L.; Zheng, H.; He, B.; Gu, Z. Synthesis and Drug Release of Star-S haped Poly (benzyl l-aspartate)-block-poly (ethylene glycol) Copolymers with POSS Cores. Macromol. Biosci. 2014, 14, 289–297. [Google Scholar] [CrossRef] [PubMed]
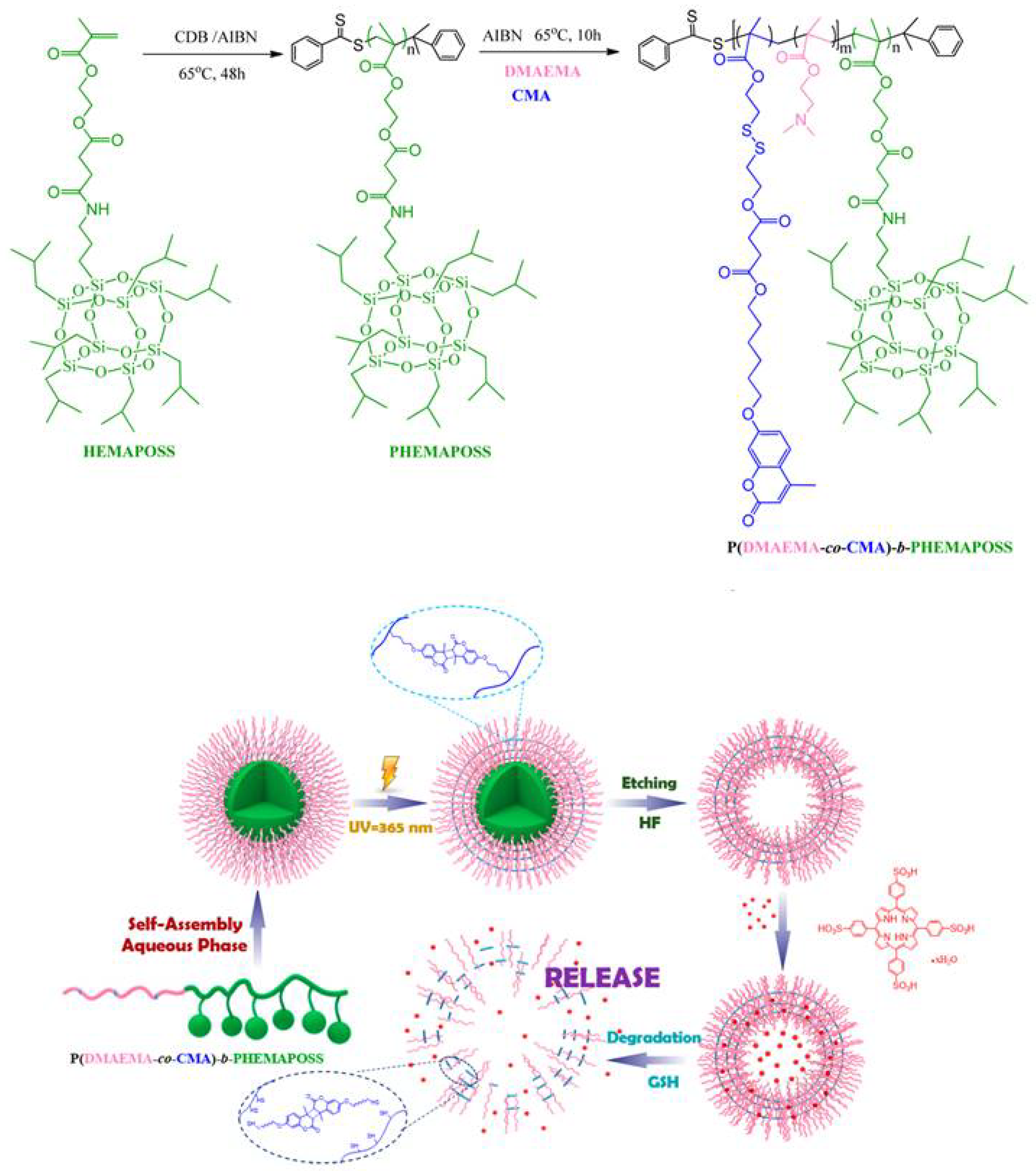
- Zhang, Z.; Xue, Y.; Zhang, P.; Müller, A.H.E.; Zhang, W. Hollow polymeric capsules from POSS-based block copolymer for photodynamic therapy. Macromolecules 2016, 49, 8440–8448. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, Z.; Jiang, X.; Rui, L.; Gao, Y.; Zhang, W. Unimolecular micelles from POSS-based star-shaped block copolymers for photodynamic therapy. Polymer 2017, 118, 268–279. [Google Scholar] [CrossRef]
- Ogieglo, W.; Wormeester, H.; Eichhorn, K.-J.; Wessling, M.; Benes, N.E. In situ ellipsometry studies on swelling of thin polymer films: A review. Prog. Polym. Sci. 2015, 42, 42–78. [Google Scholar] [CrossRef]
- Zhang, K.; Li, X.; Zhao, Y.; Zhu, K.; Li, Y.; Tao, C.; Yuan, X. UV-curable POSS-fluorinated methacrylate diblock copolymers for icephobic coatings. Prog. Org. Coat. 2016, 93, 87–96. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, K.; Tao, C.; Zhao, Y.; Li, X.; Zhu, K.; Yuan, X. Strategies for anti-icing: Low surface energy or liquid-infused? RSC Adv. 2016, 6, 70251–70260. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Tao, C.; Ren, L.; Zhao, Y.; Bai, S.; Yuan, X. Amphiphilic antifogging/anti-icing coatings containing POSS-PDMAEMA-b-PSBMA. ACS Appl. Mater. Interfaces 2017, 9, 22959–22969. [Google Scholar] [CrossRef] [PubMed]
- Zasadzinski, J.A.; Viswanathan, R.; Madsen, L.; Garnaes, J.; Schwartz, D.K. Langmuir-blodgett films. Science 1994, 263, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, G.; Bai, X.; Sun, X.; Wang, X.; Wang, E.; Dai, H. Highly conducting graphene sheets and Langmuir–Blodgett films. Nat. Nanotech. 2008, 3, 538–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hottle, J.R.; Deng, J.; Kim, H.-J.; Farmer-Creely, C.E.; Viers, B.D.; Esker, A.R. Blends of amphiphilic poly (dimethylsiloxane) and nonamphiphilic octaisobutyl-POSS at the air/water interface. Langmuir 2005, 21, 2250–2259. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Chen, F.; Ma, X.; Qiang, X.; Li, Z.; Dong, C.; Zang, D. Tadpole-shaped POSS-based copolymers and the aggregation behavior at air/water interface. Adv. Cond. Matter. Phys. 2018, 3787843. [Google Scholar] [CrossRef]
- Skrzypiec, M.; Wamke, A.; Dopierała, K.; Prochaska, K. Effect of chemical structure of fluorinated polyhedral oligomeric silsesquioxanes on formation of Langmuir monolayers and Langmuir-Blodgett films. Colloids Surf. A Physicochem. Eng. Asp. 2018, 556, 140–147. [Google Scholar] [CrossRef]
- Wamke, A.; Makowiecki, J.; Dopierała, K.; Karasiewicz, J.; Prochaska, K. Hydrophobic ultrathin films formed by fluorofunctional cage silsesquioxanes. Appl. Surf. Sci. 2018, 443, 280–290. [Google Scholar] [CrossRef]
- Mao, Y.; Zhao, Q.; Wu, J.; Pan, T.; Zhou, B.; Tian, Y. A highly sensitive and fast-responding oxygen sensor based on POSS-containing hybrid copolymer films. J. Mater. Chem. C 2017, 5, 11395–11402. [Google Scholar] [CrossRef]
- Chen, L.; Zeng, B.; Xie, J.; Yu, S.; Yuan, C.; Pan, Y.; Luo, W.; Liu, X.; He, K.; Xu, Y.; et al. A metal-sensitive organic-inorganic hybrid surfactant: POSS-capped dipicolinic acid-functionalized poly(ethylene glycol) amphiphile. React. Funct. Polym. 2013, 73, 1022–1029. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, H.; Wang, M.; Xiao, Y.; Wang, F.; K.S, J. Self-Assembly and Applications of Amphiphilic Hybrid POSS Copolymers. Molecules 2018, 23, 2481. https://doi.org/10.3390/molecules23102481
Chi H, Wang M, Xiao Y, Wang F, K.S J. Self-Assembly and Applications of Amphiphilic Hybrid POSS Copolymers. Molecules. 2018; 23(10):2481. https://doi.org/10.3390/molecules23102481
Chicago/Turabian StyleChi, Hong, Mingyue Wang, Yiting Xiao, Fuke Wang, and Joshy K.S. 2018. "Self-Assembly and Applications of Amphiphilic Hybrid POSS Copolymers" Molecules 23, no. 10: 2481. https://doi.org/10.3390/molecules23102481
APA StyleChi, H., Wang, M., Xiao, Y., Wang, F., & K.S, J. (2018). Self-Assembly and Applications of Amphiphilic Hybrid POSS Copolymers. Molecules, 23(10), 2481. https://doi.org/10.3390/molecules23102481




