Pharmacokinetic, Antiproliferative and Apoptotic Effects of Phenolic Acids in Human Colon Adenocarcinoma Cells Using In Vitro and In Silico Approaches
Abstract
:1. Introduction
2. Results
2.1. Antioxidant Activity and In Silico Approach of 3,4-DHPAA, p-CoA, VA and FA
2.2. Effect of 3,4-DHPAA, p-CoA, VA and FA in Cell Viability
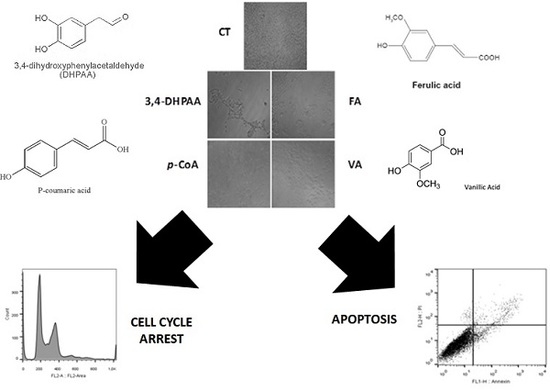
2.3. Effect of 3,4-DHPAA, p-CoA, VA and FA in Cell Cycle Progression
2.4. Effect of 3,4-DHPAA, p-CoA, VA and FA in Apoptosis
3. Discussion
4. Materials and Methods
4.1. Standards and Chemicals
4.2. Antioxidant Activity Analyses
4.2.1. Oxygen-Radical Absorbance Capacity Assay (ORAC)
4.2.2. Ferric Reducing Ability (FRAP)
4.2.3. DPPH Assay
4.2.4. Trolox Equivalent Antioxidant Capacity (ABTS/TEAC)
4.3. Cell Culture and Treatment Protocol
4.4. Cell Viability Assays
4.4.1. MTT Assay
4.4.2. Cell Counting with Neubauer Chamber
4.5. Cell Cycle Analysis
4.6. Apoptosis
4.7. In Silico Approach
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Availability of Data and Materials
References
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics. Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.H.P.; Sarkar, S.; Yang, S.Y.; Seifalian, A.M.; Winslet, M.C. In vivo models for early development of colorectal liver metastasis. Int. J. Exp. Pathol. 2008, 89, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Olthof, M.R.; Hollman, P.C.; Katan, M.B. Chlorogenic acid and caffeic acid are absorbed in humans. J. Nutr. 2001, 131, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Latifah, S.Y.; Armania, N.; Tze, T.H.; Azhar, Y.; Nordiana, A.H.; Norazalina, S. Germinated brown rice (GBR) reduces the incidence of aberrant crypt foci with the involvement of β-catenin and COX−2 in azoxymethane-induced colon cancer in rats. Nutr. J. 2010, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, S.; Venkatachalam, K.; Jeyavel, K.; Namasivayam, N. Protective effect of p-methoxycinnamic acid, an active phenolic acid against 1,2-dimethylhydrazine-induced colon carcinogenesis: Modulating biotransforming bacterial enzymes and xenobiotic metabolizing enzymes. Mol. Cell. Biochem. 2014, 394, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar] [CrossRef] [PubMed]
- Senawong, T.; Khaopha, S.; Misuna, S.; Komaikul, J.; Senawong, G.; Wongphakham, P.; Yunchalard, S. Phenolic acid composition and anticancer activity against human cancer cell lines of the commercially available fermentation products of Houttuynia cordata. Sci. Asia 2014, 40, 420–427. [Google Scholar] [CrossRef]
- Janicke, B.; Hegardt, C.; Krogh, M.; Önning, G.; Åkesson, B.; Cirenajwis, H.M.; Oredsson, S.M. The Antiproliferative Effect of Dietary Fiber Phenolic Compounds Ferulic Acid and p-Coumaric Acid on the Cell Cycle of Caco-2 Cells. Nutr. Cancer 2011, 63, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, S.K.; Supriyanto, E.; Mandal, M. Events associated with apoptotic effect of p-Coumaric acid in HCT-15 colon cancer cells. World J. Gastroenterol. 2013, 19, 7726–7734. [Google Scholar] [CrossRef] [PubMed]
- Swizdor, A.; Panek, A.; Milecka-Tronina, N.; Kołek, T. Biotransformations utilizing β-oxidation cycle reactions in the synthesis of natural compounds and medicines. Int. J. Mol. Sci. 2012, 13, 16514–16543. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Kim, M.-C.; Um, J.-Y.; Hong, S.-H. The Beneficial Effect of Vanillic Acid on Ulcerative Colitis. Molecules 2010, 15, 7208–7217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, H.; Xie, W.; Jiang, Z.; Wang, M.; Wang, J.; Zhao, H.; Zhang, X. 3,4-Dihydroxyphenylacetic acid, a microbiota-derived metabolite of quercetin, attenuates acetaminophen (APAP)-induced liver injury through activation of Nrf-2. Xenobiotica 2016, 46, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Raneva, V.; Shimasaki, H.; Ishida, Y.; Ueta, N.; Niki, E. Antioxidative activity of 3,4-dihydroxyphenylacetic acid and caffeic acid in rat plasma. Lipids 2001, 36, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.S.; Silva, N.J.A.; Soares, N.C.P.; Monteiro, M.C.; Teodoro, A.J. Anticancer Properties of Phenolic Acids in Colon Cancer–A Review. J. Nutr. Food Sci. 2016, 06, 1–7. [Google Scholar] [CrossRef]
- Fiuza, S.M.; Gomes, C.; Teixeira, L.J.; Girão Da Cruz, M.T.; Cordeiro, M.N.D.S.; Milhazes, N.; Borges, F.; Marques, M.P.M. Phenolic acid derivatives with potential anticancer properties-a structure-activity relationship study. Part 1: Methyl, propyl and octyl esters of caffeic and gallic acids. Bioorg. Med. Chem. 2004, 12, 3581–3589. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Khan, N.; Andrés-Lacueva, C.; Urpí-Sardá, M.; Vázquez-Agell, M.; Lamuela-Raventós, R.M.; Estruch, R. Dihydroxylated phenolic acids derived from microbial metabolism reduce lipopolysaccharide-stimulated cytokine secretion by human peripheral blood mononuclear cells. Br. J. Nutr. 2009, 102, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Tai, A.; Sawano, T.; Ito, H. Antioxidative Properties of Vanillic Acid Esters in Multiple Antioxidant Assays. Biosci. Biotechnol. Biochem. 2012, 76, 314–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiliç, I.; Yesiloglu, Y. Spectroscopic studies on the antioxidant activity of p-coumaric acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 115, 719–724. [Google Scholar] [CrossRef]
- Gao, K.; Xu, A.; Krul, C.; Venema, K.; Liu, Y.; Niu, Y.; Lu, J.; Bensoussan, L.; Seeram, N.P.; Heber, D.; et al. Of the Major Phenolic Acids Formed during Human Microbial Fermentation of Tea, Citrus, and Soy Flavonoid Supplements, Only 3,4-dihydroxyphenylacetic Acid Has Antiproliferative Activity. J. Nutr. 2006, 136, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Manthey, J.A.; Guthrie, N. Antiproliferative activities of citrus flavonoids against six human cancer cell lines. J. Agric. Food Chem. 2002, 50, 5837–5843. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.M.; Wang, P.; Abgaryan, N.; Vicinanza, R.; De Oliveira, D.M.; Zhang, Y.; Lee, R.; Carpenter, C.L.; William, J. Phenolic acid concentrations in plasma and urine from men consuming green or black tea and potential chemopreventive properties for colon cancer. Mol. Nutr. Food Res. 2013, 57, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Kawabata, K.; Yoshimi, N.; Tanaka, T.; Murakami, T.; Okada, T.; Murai, H. Chemopreventive effects of ferulic acid on oral and rice germ on large bowel carcinogenesis. Anticancer Res. 1999, 19, 3775–3778. [Google Scholar] [PubMed]
- Hudson, E.A.; Dinh, P.A.; Kokubun, T.; Simmonds, M.S.; Gescher, A. Characterization of potentially chemopreventive phenols in extracts of brown rice that inhibit the growth of human breast and colon cancer cells. Cancer Epidemiol. Biomark. Prev. 2000, 9, 1163–1170. [Google Scholar]
- Andreasen, M.F.; Kroon, P.A.; Williamson, G.; Garcia-Conesa, M.-T. Intestinal release and uptake of phenolic antioxidant diferulic acids. Free Radic. Biol. Med. 2001, 31, 304–314. [Google Scholar] [CrossRef]
- Janicke, B.; Onning, G.; Oredsson, S.M. Differential effects of ferulic acid and p-coumaric acid on S phase distribution and length of S phase in the human colonic cell line Caco-2. J. Agric. Food Chem. 2005, 53, 6658–6665. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.R.; Zhu, S.; Harris, P.J. Antioxidant and antigenotoxic effects of plant cell wall hydroxycinnamic acids in cultured HT-29 cells. Mol. Nutr. Food Res. 2005, 49, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.; Yazan, L.S.; Ismail, N.; Ismail, M. Apoptosis and cell cycle arrest of human colorectal cancer cell line HT-29 induced by vanillin. Cancer Epidemiol. 2009, 33, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Czerwonka, A.; Kawka, K.; Cykier, K.; Lemieszek, M.; Rzeski, W. Evaluation of anticancer activity of water and juice extracts of young Hordeum vulgare in human cancer cell lines HT-29 and A549. Ann. Agric. Environ. Med. 2017, 24, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.P.; Jaganathan, S.K.; Mandal, M.; Supriyanto, E.; Muhamad, I.I. Gallic acid induced apoptotic events in HCT-15 colon cancer cells. World J. Gastroenterol. 2016, 22, 3952–3961. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.I.; Nakayama, K. Ubiquitin ligases: Cell-cycle control and cancer. Nat. Rev. Cancer 2006, 6, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Gloria, N.F.; Soares, N.; Brand, C.; Oliveira, F.L.; Borojevic, R.; Teodoro, A.J. Lycopene and β-carotene induce cell-cycle arrest and apoptosis in human breast cancer cell lines. Anticancer Res. 2014, 34, 1377–1386. [Google Scholar] [PubMed]
- Soares, N.C.P.; Machado, C.L.; Trindade, B.B.; Lima, I.C.C.; Gimba, E.R.P.; Teodoro, A.J.; Takiya, C.; Borojevic, R. Lycopene extracts from different tomato-based food products induce apoptosis in cultured human primary prostate cancer cells and regulate TP53, Bax and Bcl-2 transcript expression. Asian Pac. J. Cancer Prev. 2017, 18, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.P.B.S.; Ammani, K.; Azhar, M.; Jaya, G. Vanillic acid induces oxidative stress and apoptosis in nonsmall lung cancer cell line. Int. J. Recent Sci. Res. 2013, 4, 1077–1083. [Google Scholar]
- Zhang, X.; Lin, D.; Jiang, R.; Li, H.; Wan, J.; Li, H. Ferulic acid exerts antitumor activity and inhibits metastasis in breast cancer cells by regulating epithelial to mesenchymal transition. Oncol. Rep. 2016, 36, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Gong, X.; Jiang, R.; Li, H.; Du, W.; Kuang, G. Ferulic acid inhibits proliferation and promotes apoptosis via blockage of PI3K/Akt pathway in osteosarcoma cell. Am. J. Transl. Res. 2016, 8, 968–980. [Google Scholar] [PubMed]
- Nunes, C.; Almeida, L.; Laranjinha, J. 3,4-dihydroxyphenylacetic acid (DOPAC) modulates the toxicity induced by nitric oxide in PC-12 cells via mitochondrial dysfunctioning. Neurotoxicology 2008, 29, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.-S.; Jeong, C.-H.; Choi, J.-S.; Kim, K.-J.; Jeong, J.-W. Antiangiogenic Effects of p-Coumaric Acid in Human Endothelial Cells. Phytother. Res. 2012, 199, 317–323. [Google Scholar] [CrossRef]
- Femia, A.P.; Caderni, G.; Vignali, F.; Salvadori, M.; Giannini, A.; Biggeri, A.; Gee, J.; Przybylska, K.; Cheynier, V.; Dolara, P. Effect of polyphenolic extracts from red wine and 4-OH-coumaric acid on 1,2-dimethylhydrazine-induced colon carcinogenesis in rats. Eur. J. Nutr. 2005, 44, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Singletary, K.W.; Stansbury, M.J.; Giusti, M.; van Breemen, R.B.; Wallig, M.; Rimando, A. Inhibition of Rat Mammary Tumorigenesis by Concord Grape Juice Constituents. J. Agric. Food Chem. 2003, 51, 7280–7286. [Google Scholar] [CrossRef] [PubMed]
- Bhilare, N.V.; Dhaneshwar, S.S.; Mahadik, K.R. Phenolic acid-tethered isoniazid for abrogation of drug-induced hepatotoxicity: Design, synthesis, kinetics and pharmacological evaluation. Drug Deliv. Transl. Res. 2018, 8, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, C.; Santangelo, R. Ferulic acid: Pharmacological and toxicological aspects. Food Chem. Toxicol. 2014, 65, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Tada, Y.; Tayama, K.; Aoki, N. Acute oral toxicity of ferulic acid, natural food additive, in rats. Ann. Rep. Tokyo Metr. 1999, 3, 311–313. [Google Scholar]
- Ou, S.; Kwok, K.C. Ferulic acid: Pharmaceutical functions, preparation and applications in foods. J. Sci. Food Agric. 2004, 84, 1261–1269. [Google Scholar] [CrossRef]
- Wang, B.-H.; Ou-Yang, J.-P. Pharmacological actions of sodium ferulate in cardiovascular system. Cardiovasc. Drug Rev. 2005, 23, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for Hydrophilic and Lipophilic Antioxidant Capacity (oxygen radical absorbance capacity (ORACFL)) of Plasma and Other Biological and Food Samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef] [PubMed]
- Bauer, D.; De Abreu, J.P.; Oliveira, H.S.S.; Goes-Neto, A.; Koblitz, M.G.B.; Teodoro, A.J. Antioxidant Activity and Cytotoxicity Effect of Cocoa Beans Subjected to Different Processing Conditions in Human Lung Carcinoma Cells. Oxid. Med. Cell. Longev. 2016, 2016, 7428515. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Vindelov, L.L. Flow microfluorometric analysis of nuclear DNA in cells from solid tumors and cell suspensions. A new method for rapid isolation and straining of nuclei. Virchows Arch. B Cell Pathol. 1977, 24, 227–242. [Google Scholar] [PubMed]
- Maunz, A.; Gütlein, M.; Rautenberg, M.; Vorgrimmler, D.; Gebele, D.; Helma, C. lazar: A modular predictive toxicology framework. Front. Pharmacol. 2013, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |






| Parameters | 3,4-DHPAA | p-CoA | VA | FA | Unit | |
|---|---|---|---|---|---|---|
| AMES toxicity | pkCSM | No | No | No | No | Categorical (Yes/No) |
| LAZAR | No | No | No | Yes | ||
| Hepatotoxicity | pkCSM | No | No | No | No | Categorical (Yes/No) |
| LAZAR | 0.246 | - | - | NA | ||
| Oral Rat Acute Toxicity (LD50) | pkCSM | 1.871 | 1.963 | 2.004 | 1.954 | Numeric (mol/kg) |
| LAZAR | - | - | - | - | NA | |
| Oral Rat Chronic Toxicity (LOAEL) | pkCSM | 3.045 | 2.94 | 2.827 | 2.892 | Numeric (log mg/kg_bw/day) |
| LAZAR | - | - | - | - | NA | |
| Carcinogenicity (rat) | pkCSM | - | - | - | _ | NA |
| LAZAR | No | No | No | No | Categorical (Yes/No) | |
| Carcinogenicity (mouse) | pkCSM | - | - | - | _ | NA |
| LAZAR | No | No | No | No | Categorical (Yes/No) | |
| Carcinogenicity (rodents) | pkCSM | - | - | - | _ | NA |
| LAZAR | No | No | Yes | Yes | Categorical (Yes/No) | |
| Max. tolerated dose (human) | pkCSM | 1.453 | 1.505 | 1.404 | 1.366 | Numeric (log mg/kg/day) |
| LAZAR | 1.66 | 0.145 | 5.7 | 2.94 | Numeric (mg/kg_bw/day) | |
| DPPH (25.0 µM) | 93.84 | 11.77 | 16.40 | 14.35 | DPPH reduction (%) | |
| FRAP (5.0 µM) | 970.62 | 33.06 | 240.56 | 141.39 | (μMol Ferrous Sulfate/µmol of compound) | |
| ABTS (5.0 µM) | 3044.75 | 1879.75 | 1376.00 | 2387.25 | (µMol Trolox/µmol of compound) | |
| ORAC (3.0 µM) | 12.9 | 15.0 | 11.7 | 4.73 | (meq Trolox/mol of compound) | |
| Compounds | Viable Cells (Annexin V − PI−) | Early Apoptosis (Annexin V + PI−) | Late Apoptosis (Annexin V + PI+) | Non-Apoptotic Cells (Annexin V − PI+) | |
|---|---|---|---|---|---|
| CT | 89.75 ± 0.13 a | 3.87 ± 1.33 a | 3.97 ± 0.26 a | 0.17 ± 0.02 a | |
| 3,4-DHPAA | 10 μM | 87.90 ± 1.04 b | 5.49 ± 0.28 b | 5.84 ± 1.48 a,b | 2.41 ± 2.06 b |
| 100 μM | 85.20 ± 0.57 c | 6.93 ± 1.21 b | 7.69 ± 0.80 b | 0.78 ± 0.35 c | |
| CT | 92.50 ± 0.28 a | 2.08 ± 0.23 a | 2.98 ± 0.04 a | 2.45 ± 0.47 a | |
| p-CoA | 10 μM | 76.95 ± 0.64 b | 12.75 ± 0.49 b | 9.83 ± 0.16 b | 0.48 ± 0.06 b |
| 100 μM | 89.40 ± 1.48 c | 0.17 ± 0.10 c | 1.38 ± 0.24 c | 6.01 ± 1.11 c | |
| CT | 92.50 ± 0.28 a | 2.08 ± 0.23 a | 2.98 ± 0.04 a | 2.45 ± 0.47 a | |
| VA | 10 μM | 90.00 ± 4.10 a | 0.34 ± 0.14 b | 0.93 ± 0.62 b | 8.74 ± 3.34 b |
| 100 μM | 91.10 ± 2.83 a | 0.09 ± 0.11 b | 1.00 ± 0.03 b | 7.84 ± 2.88 b | |
| CT | 92.50 ± 0.28 a | 2.08 ± 0.23 a | 2.98 ± 0.04 a | 2.45 ± 0.47 a | |
| FA | 10 μM | 82.70 ± 1.27 b | 8.28 ± 1.53 b | 7.52 ± 1.68 b | 1.51 ± 1.10 b |
| 100 μM | 89.90 ± 1.84 c | 0.48 ± 0.07 a | 1.70 ± 0.18 a | 8.20 ± 2.28 c |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosa, L.D.S.; Jordão, N.A.; Da Costa Pereira Soares, N.; DeMesquita, J.F.; Monteiro, M.; Teodoro, A.J. Pharmacokinetic, Antiproliferative and Apoptotic Effects of Phenolic Acids in Human Colon Adenocarcinoma Cells Using In Vitro and In Silico Approaches. Molecules 2018, 23, 2569. https://doi.org/10.3390/molecules23102569
Rosa LDS, Jordão NA, Da Costa Pereira Soares N, DeMesquita JF, Monteiro M, Teodoro AJ. Pharmacokinetic, Antiproliferative and Apoptotic Effects of Phenolic Acids in Human Colon Adenocarcinoma Cells Using In Vitro and In Silico Approaches. Molecules. 2018; 23(10):2569. https://doi.org/10.3390/molecules23102569
Chicago/Turabian StyleRosa, Lana De Souza, Nathállia Araújo Jordão, Nathália Da Costa Pereira Soares, Joelma Freire DeMesquita, Mariana Monteiro, and Anderson Junger Teodoro. 2018. "Pharmacokinetic, Antiproliferative and Apoptotic Effects of Phenolic Acids in Human Colon Adenocarcinoma Cells Using In Vitro and In Silico Approaches" Molecules 23, no. 10: 2569. https://doi.org/10.3390/molecules23102569
APA StyleRosa, L. D. S., Jordão, N. A., Da Costa Pereira Soares, N., DeMesquita, J. F., Monteiro, M., & Teodoro, A. J. (2018). Pharmacokinetic, Antiproliferative and Apoptotic Effects of Phenolic Acids in Human Colon Adenocarcinoma Cells Using In Vitro and In Silico Approaches. Molecules, 23(10), 2569. https://doi.org/10.3390/molecules23102569