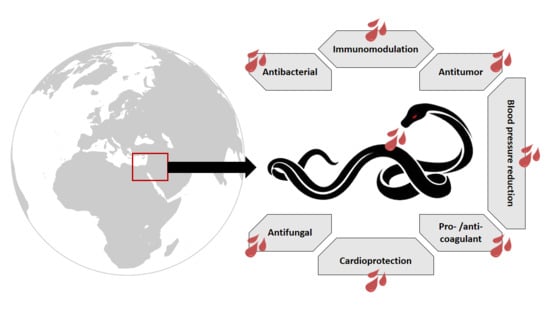
Vipers of the Middle East: A Rich Source of Bioactive Molecules
Abstract
:1. Snake Venom: An Overview
1.1. Types and Functions
1.2. Snake Venom Components
2. Snake Venom Uses
2.1. Medicinal Applications
2.2. Drugs Based on Venom Components
3. The Viperidae Snake Family
4. Montivipera bornmuelleri
4.1. Venom Composition
4.2. Venom Biological Activities
5. Macrovipera lebetina
5.1. Venom Composition
5.2. Venom Biological Activities
6. Vipera palaestinae
6.1. Venom Composition
6.2. Venom Biological Activities
7. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Utkin, Y.N. Animal venom studies: Currentbenefits and future developments. World J. Biol. Chem. 2015, 6, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Warrell, D.A. Snake bite. Lancet 2010, 375, 77–88. [Google Scholar] [CrossRef]
- Bottrall, J.L.; Madaras, F.; Biven, C.D.; Venning, M.G.; Mirtschin, P.J. Proteolytic activity of Elapid and Viperid Snake venoms and its implication to digestion. J. Venom Res. 2010, 1, 18–28. [Google Scholar] [PubMed]
- Hodges, S.J.; Agbaji, A.S.; Harvey, A.L.; Hider, R.C. Cobra cardiotoxins. purification, effects on skeletal muscle and structure/activity relationships. Eur. J. Biochem. 1987, 165, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Kumar, S. Snake venom: A potent anticancer agent. Asian Pac. J. Cancer Prev. 2012, 13, 4855–4860. [Google Scholar] [CrossRef] [PubMed]
- Gasanov, S.E.; Alsarraj, M.A.; Gasanov, N.E.; Rael, E.D. Cobra venom cytotoxin free of phospholipase A2 and its effect on model membranes and T Leukemia Cells. J. Membr. Biol. 1997, 155, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Takacs, Z.; Wilhelmsen, K.C.; Sorota, S. Cobra (Naja spp.) nicotinic acetylcholine receptor exhibits resistance to Erabu Sea snake (Laticauda semifasciata) short-chain α-Neurotoxin. J. Mol. Evol. 2004, 58, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Straight, R.; Glenn, J.L.; Snyder, C.C. Antivenom activity of rattlesnake blood plasma. Nature 1976, 261, 259–260. [Google Scholar] [CrossRef] [PubMed]
- Mackessy, S.P.; Baxter, L.M. Bioweapons synthesis and storage: The venom gland of front-fanged snakes. Zool. Anz. 2006, 245, 147–159. [Google Scholar] [CrossRef]
- Carregari, V.C.; Rosa-Fernandes, L.; Baldasso, P.; Bydlowski, S.P.; Marangoni, S.; Larsen, M.R.; Palmisano, G. Snake venom extracellular vesicles (SVEVs) reveal wide molecular and functional proteome diversity. Sci. Rep. 2018, 8, 12067. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.E.; Dennis, E.A. Phospholipase A2 biochemistry. Cardiovasc. Drugs Ther. 2009, 23, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.W.; Harris, J.B. Myotoxic activity of the toxic phospholipase, notexin, from the venom of the Australian Tiger Snake. J. Neuropathol. Exp. Neurol. 1996, 55, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Logonder, U.; Jenko-Praznikar, Z.; Scott-Davey, T.; Pungercar, J.; Krizaj, I.; Harris, J.B. Ultrastructural evidence for the uptake of a neurotoxic snake venom phospholipase A2 into mammalian motor nerve terminals. Exp. Neurol. 2009, 219, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Sudarshan, S.; Dhananjaya, B.L. Antibacterial activity of an acidic phospholipase A2 (NN-XIb-PLA2) from the venom of Naja naja (Indian Cobra). Springerplus 2016, 5, 112. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.F.P.; Landucci, E.C.T.; Antunes, E.; Chacur, M.; Cury, Y. Inflammatory effects of snake venom myotoxic phospholipases A2. Toxicon 2003, 42, 947–962. [Google Scholar] [CrossRef] [PubMed]
- Kamiguti, A.S.; Hay, C.R.; Theakston, R.D.; Zuzel, M. Insights into the mechanism of haemorrhage caused by snake venom metalloproteinases. Toxicon 1996, 34, 627–642. [Google Scholar] [CrossRef]
- Hati, R.; Mitra, P.; Sarker, S.; Bhattacharyya, K.K. Snake venom hemorrhagins. Crit. Rev. Toxicol. 1999, 29, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Rucavado, A. Snake venom metalloproteinases: Their role in the pathogenesis of local tissue damage. Biochimie 2000, 82, 841–850. [Google Scholar] [CrossRef]
- Araki, S.; Masuda, S.; Maeda, H.; Ying, M.J.; Hayashi, H. Involvement of specific integrins in apoptosis induced by vascular apoptosis-inducing protein 1. Toxicon 2002, 40, 535–542. [Google Scholar] [CrossRef]
- Zaganelli, G.L.; Zaganelli, M.G.; Magalhães, A.; Diniz, C.R.; de Lima, M.E. Purification and Characterization of a Fibrinogen-Clotting Enzyme from the Venom of Jararacuçu (Bothrops jararacussu). Toxicon 1996, 34, 807–819. [Google Scholar] [CrossRef]
- Guan, A.L.; Retzios, A.D.; Henderson, G.N.; Markland, F.S. Purification and characterization of a fibrinolytic enzyme from venom of the southern copperhead snake (Agkistrodon contortrix contortrix). Arch. Biochem. Biophys. 1991, 289, 197–207. [Google Scholar] [CrossRef]
- Wang, W.-J.; Shih, C.-H.; Huang, T.-F. Primary structure and antiplatelet mechanism of a snake venom metalloproteinase, acurhagin, from agkistrodon acutus venom. Biochimie 2005, 87, 1065–1077. [Google Scholar] [CrossRef] [PubMed]
- Anai, K.; Sugiki, M.; Yoshida, E.; Maruyama, M. Neutralization of a snake venom hemorrhagic metalloproteinase prevents coagulopathy after subcutaneous injection of bothrops jararaca venom in rats. Toxicon 2002, 40, 63–68. [Google Scholar] [CrossRef]
- Frobert, Y.; Créminon, C.; Cousin, X.; Rémy, M.-H.; Chatel, J.-M.; Bon, S.; Bon, C.; Grassi, J. Acetylcholinesterases from Elapidae snake venoms: Biochemical, immunological and enzymatic characterization. Biochim. Biophys. Acta 1997, 1339, 253–267. [Google Scholar] [CrossRef]
- Girish, K.S.; Jagadeesha, D.K.; Rajeev, K.B.; Kemparaju, K. Snake venom hyaluronidase: An evidence for isoforms and extracellular matrix degradation. Mol. Cell. Biochem. 2002, 240, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Yingprasertchai, S.; Bunyasrisawat, S.; Ratanabanangkoon, K. Hyaluronidase inhibitors (sodium cromoglycate and sodium auro-thiomalate) reduce the local tissue damage and prolong the survival time of mice injected with Naja kaouthia and Calloselasma rhodostoma venoms. Toxicon 2003, 42, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Nathan, I.; Dvilansky, A.; Yirmiyahu, T.; Aharon, M.; Livne, A. Impairment of platelet aggregation by Echis Colorata venom mediated by l-amino acid oxidase or H2O2. Thromb. Haemost. 1982, 48, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Yu, T.F.; Lian, E.C. Purification and characterization of l-amino acid oxidase from King Cobra (Ophiophagus Hannah) venom and its effects on human platelet aggregation. Toxicon 1994, 32, 1349–1358. [Google Scholar] [CrossRef]
- Stiles, B.G.; Sexton, F.W.; Weinstein, S.A. Antibacterial effects of different snake venoms: Purification and characterization of antibacterial proteins from Pseudechis australis (Australian King Brown or Mulga snake) venom. Toxicon 1991, 29, 1129–1141. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Wang, J.-H.; Lee, W.-H.; Wang, Q.; Liu, H.; Zheng, Y.-T.; Zhang, Y. Molecular characterization of trimeresurus stejnegeri venom l-amino acid oxidase with potential anti-HIV activity. Biochem. Biophys. Res. Commun. 2003, 309, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.R.; Menaldo, D.L.; Prinholato da Silva, C.; Sorrechia, R.; de Albuquerque, S.; Pietro, R.C.L.R.; Ghisla, S.; Antunes, L.M.G.; Sampaio, S.V. Evaluating the microbicidal, antiparasitic and antitumor effects of CR-LAAO from Calloselasma rhodostoma venom. Int. J. Biol. Macromol. 2015, 80, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Zaqueo, K.D.; Kayano, A.M.; Domingos, T.F.S.; Moura, L.A.; Fuly, A.L.; da Silva, S.L.; Acosta, G.; Oliveira, E.; Albericio, F.; Zanchi, F.B.; et al. BbrzSP-32, the first serine protease isolated from Bothrops brazili venom: Purification and characterization. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2016, 195, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.S.; Georgieva, D.; Genov, N.; Murakami, M.T.; Sinha, M.; Kumar, R.P.; Kaur, P.; Kumar, S.; Dey, S.; Sharma, S.; et al. Enzymatic toxins from snake venom: Structural characterization and mechanism of catalysis. FEBS J. 2011, 278, 4544–4576. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-Y.; Lee, H.; You, W.-K.; Chung, K.-H.; Kim, D.-S.; Song, K. The snake venom disintegrin salmosin induces apoptosis by disassembly of focal adhesions in bovine ccapillary endothelial cells. Biochem. Biophys. Res. Commun. 2003, 302, 502–508. [Google Scholar] [CrossRef]
- Smith, C.G.; Vane, J.R. The discovery of captopril: A reply. FASEB J. 2004, 18, 935. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, B.; Zhu, S.; Rong, R. Hypotensive peptides from snake venoms: Structure, function and mechanism. Curr. Top. Med. Chem. 2015, 15, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, Y.; Mizuguchi, J.; Iwanaga, S.; Kini, R.M. Hemextin AB complex, a unique anticoagulant protein complex from Hemachatus haemachatus (African ringhals cobra) venom that inhibits clot initiation and factor VIIa activity. J. Biol. Chem. 2005, 280, 42601–42611. [Google Scholar] [CrossRef] [PubMed]
- Perumal Samy, R.; Gopalakrishnakone, P.; Thwin, M.M.; Chow, T.K.V.; Bow, H.; Yap, E.H.; Thong, T.W.J. Antibacterial activity of snake, scorpion and bee venoms: A comparison with purified venom phospholipase A2 enzymes. J. Appl. Microbiol. 2007, 102, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Oguiura, N.; Boni-Mitake, M.; Affonso, R.; Zhang, G. In vitro antibacterial and hemolytic activities of crotamine, a small basic myotoxin from rattlesnake crotalus durissus. J. Antibiot. (Tokyo) 2011, 64, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.; Hung, D.Y.; Siebert, G.A.; Bridle, K.; Roberts, M.S. Therapeutic effects and possible mechanisms of a snake venom preparation in the fibrotic rat liver. Dig. Dis. Sci. 2005, 50, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.C.; Wong, P.T.; Gopalakrishnakone, P. A novel analgesic toxin (Hannalgesin) from the venom of King cobra (Ophiophagus hannah). Toxicon 1995, 33, 1425–1431. [Google Scholar] [CrossRef]
- Liu, C.-C.; Yang, H.; Zhang, L.-L.; Zhang, Q.; Chen, B.; Wang, Y. Biotoxins for cancer therapy. Asian Pac. J. Cancer Prev. 2014, 15, 4753–4758. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ke, M.; Tian, Y.; Wang, J.; Li, B.; Wang, Y.; Dou, J.; Zhou, C. BF-30 selectively inhibits melanoma cell proliferation via cytoplasmic membrane permeabilization and DNA-binding in vitro and in B16F10-bearing mice. Eur. J. Pharmacol. 2013, 707, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadoon, M.K.; Rabah, D.M.; Badr, G. Enhanced anticancer efficacy of snake venom combined with silica nanoparticles in a murine model of human multiple myeloma: Molecular targets for cell cycle arrest and apoptosis induction. Cell. Immunol. 2013, 284, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.H. A badykinin-potrntia factor (bpf) present in the venom of bothrops jararca. Br. J. Pharmacol. Chemother. 1965, 24, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.S.; Cheung, R.C.F.; Xia, L.; Wong, J.H.; Ng, T.B.; Chan, W.Y. Snake venom toxins: Toxicity and medicinal applications. Appl. Microbiol. Biotechnol. 2016, 100, 6165–6181. [Google Scholar] [CrossRef] [PubMed]
- Hashemzadeh, M.; Furukawa, M.; Goldsberry, S.; Movahed, M.R. Chemical structures and mode of action of intravenous glycoprotein IIb/IIIa receptor blockers: A review. Exp. Clin. Cardiol. 2008, 13, 192–197. [Google Scholar] [PubMed]
- Ding, G.; Li, S.; Pan, Z.; Gao, C.; Ma, H. Effects of batroxobin on perioperative blood loss and coagulation in patients with low molecular weight heparin when undergoing the total hip replacement. Zhonghua Liu Xing Bing Xue Za Zhi 2014, 35, 737–740. [Google Scholar] [PubMed]
- Lodha, A.; Kamaluddeen, M.; Akierman, A.; Amin, H. Role of hemocoagulase in pulmonary hemorrhage in preterm Infants: A systematic review. Indian J. Pediatr. 2011, 78, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, K.V.; Baliga, M.; Mahajan, S.; Ramesh, K.V. The effects of topical hemocoagulase solution on the healing process of post-extraction wounds: A split mouth design. J. Maxillofac. Oral Surg. 2015, 14, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, J.S.A.M.; Evangelista, J.J.F.; Evangelista, I.L.; Nojosa, D.M.B.; Nascimento, N.R.F.; Souza, M.H.L.P.; Alves, R.S.; Martins, A.M.C.; Moraes, M.E.A.; Monteiro, H.S.A. Hypotensive effects of the crotalus durissus cascavella venom: Involvement of NO. Nat. Prod. Commun. 2011, 6, 871–874. [Google Scholar] [PubMed]
- Ferquel, E.; de Haro, L.; Jan, V.; Guillemin, I.; Jourdain, S.; Teynié, A.; D’Alayer, J.; Choumet, V. Reappraisal of Vipera aspis venom neurotoxicity. PLoS ONE 2007, 2, e1194. [Google Scholar] [CrossRef] [PubMed]
- Hraoui-Bloquet, S.; Sadek, R.; Accary, C.; Hleihel, W.; Fajloun, Z. An ecological study of the Lebanon mountain viper Montivipera bornmuelleri (Werner, 1898) with a preliminary biochemical characterization of its venom. Leban. Sci. J. 2012, 13, 89–101. [Google Scholar]
- Mousa Disi, A.M.; Hraoui-Bloquet, S.; Sadek, R.; Werner, Y.V. Montivipera bornmuelleri (Lebanon Viper). IUCN Red List Threat. Species. 2015. Available online: www.iucnredlist.org/details/61445/0.55 (accessed on 30 September 2018).
- Accary, C.; Hraoui-Bloquet, S.; Hamze, M.; Mallem, Y.; El Omar, F.; Sabatier, J.-M.; Desfontis, J.-C.; Fajloun, Z. Protein content analysis and antimicrobial activity of the crude venom of montivipera bornmuelleri; a viper from Lebanon. Infect. Disord. Drug Targets 2014, 14, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Rima, M.; Accary, C.; Haddad, K.; Sadek, R.; Hraoui-Bloquet, S.; Desfontis, J.C.; Fajloun, Z. Identification of L-amino acid oxidase (Mb-LAAO) with antibacterial activity in the venom of montivipera bornmuelleri, a viper from Lebanon. Infect. Disord. Drug Targets 2013, 13, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Accary, C.; Rima, M.; Kouzahya, A.; Hleihel, W.; Sadek, R.; Desfontis, J.C.; Fajloun, Z.; Hraoui-Bloquet, S. Effect of the montivipera bornmuelleri snake venom on human blood: Coagulation disorders and hemolytic activities. Open J. Hematol. 2014, 8. [Google Scholar] [CrossRef]
- Suntravat, M.; Nuchprayoon, I.; Pérez, J.C. Comparative study of anticoagulant and procoagulant properties of 28 snake venoms from families elapidae, viperidae, and purified Russell’s viper venom-Factor X activator (RVV-X). Toxicon 2010, 56, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Accary, C.; Mantash, A.; Mallem, Y.; Fajloun, Z.; Elkak, A. Separation and biological activities of phospholipase A2 (Mb-PLA2) from the venom of Montivipera bornmuelleri, a Lebanese Viper. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 833–839. [Google Scholar] [CrossRef]
- Accary, C.; Hraoui-Bloquet, S.; Sadek, R.; Alameddine, A.; Fajloun, Z.; Desfontis, J.-C.; Mallem, Y. The relaxant effect of the montivipera bornmuelleri snake venom on vascular contractility. J. Venom Res. 2016, 7, 10–15. [Google Scholar] [PubMed]
- Sawan, S.; Yaacoub, T.; Hraoui-Bloquet, S.; Sadek, R.; Hleihel, W.; Fajloun, Z.; Karam, M. Montivipera bornmuelleri venom selectively exhibits high cytotoxic effects on keratinocytes cancer cell lines. Exp. Toxicol. Pathol. 2017, 69, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Abi-Rizk, A.; Rima, M.; Bloquet, S.H.; Sadek, R.; Sleiman, Y.; Fajloun, Z.; Hleihel, W. Lethal, hemorrhagic, and necrotic effects of Montivipera bornmuelleri venom. Curr. Herpetol. 2017, 36, 58–62. [Google Scholar] [CrossRef]
- Yacoub, T.; Rima, M.; Sadek, R.; Hleihel, W.; Fajloun, Z.; Karam, M. Montivipera bornmuelleri venom has immunomodulatory effects mainly up-regulating pro-inflammatory cytokines in the spleens of mice. Toxicol. Rep. 2018, 5, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Sanz, L.; Ayvazyan, N.; Calvete, J.J. Snake venomics of the Armenian Mountain vipers Macrovipera lebetina obtusa and Vipera raddei. J. Proteomics 2008, 71, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Mallow, D.; Ludwig, D.; Nilson, G. True Vipers: Natural History and Toxinology of Old World Vipers; Krieger Publishing Company: Malabar, FL, USA, 2003. [Google Scholar]
- Morad, R.; Zohreh, A.; Mozhgan, N. Study of alkaline phosphatase activity in isolation fractions from Iranian snake Vipera lebetina venom. Clin. Biochem. 2011, 44, S88–S89. [Google Scholar] [CrossRef]
- Igci, N.; Demiralp, D.O. A preliminary investigation into the venom proteome of Macrovipera Lebetina Obtusa (Dwigubsky, 1832) from southeastern Anatolia by MALDI-TOF mass spectrometry and comparison of venom protein profiles with macrovipera Lebetina lebetina (Linnaeus, 1758) Fro. Arch. Toxicol. 2012, 86, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Hamza, L.; Girardi, T.; Castelli, S.; Gargioli, C.; Cannata, S.; Patamia, M.; Luly, P.; Laraba-Djebari, F.; Petruzzelli, R.; Rufini, S. Isolation and characterization of a myotoxin from the venom of Macrovipera lebetina transmediterranea. Toxicon 2010, 56, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Ozen, M.O.; İğci, N.; Yalçin, H.T.; Goçmen, B.; Nalbantsoy, A. Screening of cytotoxic and antimicrobial activity potential of anatolian macrovipera Lebetina obtusa (Ophidia: Viperidae) crude venom. Front. Life Sci. 2015, 8, 363–370. [Google Scholar] [CrossRef]
- Marrakchi, N.; Sarray, S.; Marvaldi, J.; El Ayeb, M.; Luis, J. Effect of Macrovipera lebetina and Cerastes cerastes venoms on adherence to integrins of cancerous cells (IGR39, HT29-D4 and IGROV1)]. Arch. Inst. Pasteur Tunis 2002, 79, 3–9. [Google Scholar] [PubMed]
- Bazaa, A.; Luis, J.; Srairi-Abid, N.; Kallech-Ziri, O.; Kessentini-Zouari, R.; Defilles, C.; Lissitzky, J.-C.; El Ayeb, M.; Marrakchi, N. MVL-PLA2, a phospholipase A2 from Macrovipera lebetina Transmediterranea venom, inhibits tumor cells adhesion and migration. Matrix Biol. 2009, 28, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Bazaa, A.; Pasquier, E.; Defilles, C.; Limam, I.; Kessentini-Zouari, R.; Kallech-Ziri, O.; El Battari, A.; Braguer, D.; El Ayeb, M.; Marrakchi, N.; et al. MVL-PLA2, a snake venom phospholipase A2, inhibits angiogenesis through an increase in microtubule dynamics and disorganization of focal adhesions. PLoS ONE 2010, 5, e10124. [Google Scholar] [CrossRef] [PubMed]
- Zakraoui, O.; Marcinkiewicz, C.; Aloui, Z.; Othman, H.; Grépin, R.; Haoues, M.; Essafi, M.; Srairi-Abid, N.; Gasmi, A.; Karoui, H.; et al. Lebein, a snake venom disintegrin, suppresses human colon cancer cells proliferation and tumor-induced angiogenesis through cell cycle arrest, apoptosis induction and inhibition of VEGF expression. Mol. Carcinog. 2017, 56, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Hammouda, M.B.; Montenegro, M.F.; Sánchez-Del-Campo, L.; Zakraoui, O.; Aloui, Z.; Riahi-Chebbi, I.; Karoui, H.; Rodríguez-López, J.N.; Essafi-Benkhadir, K. Lebein, a snake venom disintegrin, induces apoptosis in human melanoma cells. Toxins 2016, 8, 206. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.C.; Staniszewska, I.; Del Valle, L.; Tuszynski, G.P.; Marcinkiewicz, C. Angiostatic activity of obtustatin as α1β1 integrin inhibitor in experimental melanoma growth. Int. J. Cancer 2008, 123, 2195–2203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghazaryan, N.A.; Ghulikyan, L.A.; Kishmiryan, A.V.; Kirakosyan, G.R.; Nazaryan, O.H.; Ghevondyan, T.H.; Zakaryan, N.A.; Ayvazyan, N.M. Anti-tumor effect investigation of obtustatin and crude macrovipera Lebetina Obtusa venom in S-180 sarcoma bearing mice. Eur. J. Pharmacol. 2015, 764, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Limam, I.; Bazaa, A.; Srairi-Abid, N.; Taboubi, S.; Jebali, J.; Zouari-Kessentini, R.; Kallech-Ziri, O.; Mejdoub, H.; Hammami, A.; El Ayeb, M.; et al. Leberagin-C, a disintegrin-like/cysteine-rich protein from Macrovipera lebetina transmediterranea venom, inhibits αvβ3 integrin-mediated cell adhesion. Matrix Biol. 2010, 29, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Sarray, S.; Delamarre, E.; Marvaldi, J.; El Ayeb, M.; Marrakchi, N.; Luis, J. Lebectin and Lebecetin, two C-type lectins from snake venom, inhibit α5β1 and αV-containing integrins. Matrix Biol. 2007, 26, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Sarray, S.; Berthet, V.; Calvete, J.J.; Secchi, J.; Marvaldi, J.; El-Ayeb, M.; Marrakchi, N.; Luis, J. Lebectin, a novel C-type lectin from Macrovipera lebetina venom, inhibits integrin-mediated adhesion, migration and invasion of human tumour cells. Lab. Investig. 2004, 84, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Sarray, S.; Srairi, N.; Hatmi, M.; Luis, J.; Louzir, H.; Regaya, I.; Slema, H.; Marvaldi, J.; El Ayeb, M.; Marrakchi, N. Lebecetin, a potent antiplatelet C-type lectin from Macrovipera Lebetina venom. Biochim. Biophys. Acta 2003, 1651, 30–40. [Google Scholar] [CrossRef]
- Sarray, S.; Srairi, N.; Luis, J.; Marvaldi, J.; El Ayeb, M.; Marrakchi, N. Lebecetin, a C-lectin protein from the venom of Macrovipera Lebetina that inhibits platelet aggregation and adhesion of cancerous cells. Haemostasis 2002, 31, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Jebali, J.; Fakhfekh, E.; Morgen, M.; Srairi-Abid, N.; Majdoub, H.; Gargouri, A.; El Ayeb, M.; Luis, J.; Marrakchi, N.; Sarray, S. Lebecin, a new C-type lectin like protein from Macrovipera lebetina venom with anti-tumor activity against the breast cancer cell line MDA-MB231. Toxicon 2014, 86, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Pilorget, A.; Conesa, M.; Sarray, S.; Michaud-Levesque, J.; Daoud, S.; Kim, K.S.; Demeule, M.; Marvaldi, J.; El Ayeb, M.; Marrakchi, N.; et al. Lebectin, a Macrovipera Lebetina Venom-Derived C-Type Lectin, Inhibits Angiogenesis Both in Vitro and in Vivo. J. Cell. Physiol. 2007, 211, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Montassar, F.; Darche, M.; Blaizot, A.; Augustin, S.; Conart, J.-B.; Millet, A.; Elayeb, M.; Sahel, J.-A.; Réaux-Le Goazigo, A.; Sennlaub, F.; et al. Lebecetin, a C-type lectin, inhibits choroidal and retinal neovascularization. FASEB J. 2017, 31, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Sarray, S.; Siret, C.; Lehmann, M.; Marrakchi, N.; Luis, J.; El Ayeb, M.; André, F. Lebectin increases N-cadherin-mediated adhesion through PI3K/AKT pathway. Cancer Lett. 2009, 285, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Hammouda, M.B.; Riahi-Chebbi, I.; Souid, S.; Othman, H.; Aloui, Z.; Srairi-Abid, N.; Karoui, H.; Gasmi, A.; Magnenat, E.M.; Wells, T.N.C.; et al. Macrovipecetin, a C-type lectin from Macrovipera lebetina venom, inhibits proliferation migration and invasion of SK-MEL-28 human melanoma cells and enhances their sensitivity to cisplatin. Biochim. Biophys. Acta 2018, 1862, 600–614. [Google Scholar] [CrossRef] [PubMed]
- Tourki, B.; Matéo, P.; Morand, J.; Elayeb, M.; Godin-Ribuot, D.; Marrakchi, N.; Belaidi, E.; Messadi, E. Lebetin 2, a snake venom-derived natriuretic peptide, attenuates acute myocardial ischemic injury through the modulation of mitochondrial permeability transition pore at the time of reperfusion. PLoS ONE 2016, 11, e0162632. [Google Scholar] [CrossRef] [PubMed]
- Morjen, M.; Kallech-Ziri, O.; Bazaa, A.; Othman, H.; Mabrouk, K.; Zouari-Kessentini, R.; Sanz, L.; Calvete, J.J.; Srairi-Abid, N.; El Ayeb, M.; et al. PIVL, a new serine protease inhibitor from Macrovipera lebetina Transmediterranea venom, impairs motility of human glioblastoma cells. Matrix Biol. 2013, 32, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Morjen, M.; Honoré, S.; Bazaa, A.; Abdelkafi-Koubaa, Z.; Ellafi, A.; Mabrouk, K.; Kovacic, H.; El Ayeb, M.; Marrakchi, N.; Luis, J. PIVL, a snake venom Kunitz-type serine protease inhibitor, inhibits in vitro and in vivo angiogenesis. Microvasc. Res. 2014, 95, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, A.; Bourcier, C.; Aloui, Z.; Srairi, N.; Marchetti, S.; Gimond, C.; Wedge, S.R.; Hennequin, L.; Pouysségur, J. Complete structure of an increasing capillary permeability protein (ICPP) purified from vipera Lebetina venom. ICPP is angiogenic via vascular endothelial growth factor receptor signalling. J. Biol. Chem. 2002, 277, 29992–29998. [Google Scholar] [CrossRef] [PubMed]
- Aloui, Z.; Hoos, S.; Geretti, E.; Kharmachi, H.; Haumont, P.Y.; Mejdoub, H.; Klagsbrun, M.; England, P.; Gasmi, A. Novel svVEGF isoforms from Macrovipera lebetina venom interact with neuropilins. Biochem. Biophys. Res. Commun. 2009, 389, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Ovadia, M.; Kochva, E.; Moav, B. Purification and partial characterization of lethal synergistic components from the venom of Vipera palaestinae. Toxicon 1977, 15, 549–560. [Google Scholar] [CrossRef]
- Staniszewska, I.; Walsh, E.M.; Rothman, V.L.; Gaathon, A.; Tuszynski, G.P.; Calvete, J.J.; Lazarovici, P.; Marcinkiewicz, C. Effect of VP12 and viperistatin on inhibition of collagen-receptor-dependent melanoma metastasis. Cancer Biol. Ther. 2009, 8, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Arlinghaus, F.T.; Momic, T.; Ammar, N.A.; Shai, E.; Spectre, G.; Varon, D.; Marcinkiewicz, C.; Heide, H.; Lazarovici, P.; Eble, J.A. Identification of α2β1 integrin inhibitor VP-I with anti-platelet properties in the venom of Vipera palaestinae. Toxicon 2013, 64, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Shaham, N.; Bdolah, A. l-Amino acid oxidase from Vipera palaestinae venom: Purification and assay. Comp. Biochem. Physiol. B. 1973, 46, 691–698. [Google Scholar] [CrossRef]
- Nakar, O.; Ovadia, M.; Kochva, E. Isolation and characterization of a proteolytic factor from the venom of Vipera palaestinae. Toxicon 1986, 24, 293–304. [Google Scholar] [CrossRef]
- Krizaj, I.; Bdolah, A.; Gubensek, F.; Bencina, P.; Pungercar, J. Protein and cDNA structures of an acidic phospholipase A2, the enzymatic part of an unusual, two-component toxin from Vipera palaestinae. Biochem. Biophys. Res. Commun. 1996, 227, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.; Bdolah, A.; Kochva, E. The two-component toxin of Vipera Palaestinae: Contribution of phospholipase A to its activity. Toxicon 1980, 18, 249–259. [Google Scholar] [CrossRef]
- Barzilay, M.; Kaminsky, E.; Condrea, E. Exposure of human red blood cell membrane phospholipids to snake venom phospholipases. A-II. hydrolysis of substrates in intact and resealed cells by phospholipase from ringhals (Hemachatus haemachates) venom: Effect of calcium ions. Toxicon 1978, 16, 153–161. [Google Scholar] [CrossRef]

| Snake | Venom/Molecule | Biological Activities | References |
|---|---|---|---|
| Montivipera bornmuelleri | Crude Venom | Pro- and anti-coagulant activities Indirect hemolysis of human RBCs Reduction of blood pressure Selective cytotoxicity on benign and malignant cells, but not on non-tumorigenic cells Up-regulates pro-inflammatory cytokines and downregulates anti-inflammatory cytokines Antibacterial and anti-fungal activities | [57] [57] [60] [61] [63] [55] |
| PLA2 | Antibacterial, hemolytic, anticoagulant, and pro-inflammatory activities | [59] | |
| l-AAO | Antibacterial activity | [56] | |
| Macrovipera lebetina | Crude venom | Cytotoxicity against normal and cancer cell lines Antibacterial activity and antifungal activities Inhibits adhesion of melanoma and colon adenocarcinoma cells to ECM Anti-tumor activity | [69] [69] [70] [76] |
| Metalloproteinase | Myotoxicity | [68] | |
| PLA2 | Inhibits tumor cell adhesion and migration in vitro Inhibits angiogenesis in vitro and in vivo | [71] [72] | |
| Lebein | Reduces proliferation and induces apoptosis of melanoma cells Inhibits human colon cancer cells proliferation, migration and angiogenesis | [74] [73] | |
| Obtustatin | Fights melanoma by restricting vascularization Decreases malignant sarcoma size in mice | [75] [76] | |
| Leberagin-C | Inhibits cell adhesion and shows anti-platelet aggregation potential | [77] | |
| Lebecetin | Decreases platelet aggregation and inhibits adhesion of cancer cells | [80,81] | |
| Lebectin | Anti-angiogenic activities in vitro and in vivo Inhibits adhesion, migration and invasion of human tumour cells | [83] [79] | |
| Lebecin | Anti-tumor activity against breast cancer cells | [82] | |
| Macrovipecetin | Anti-neoplastic properties | [86] | |
| Lebetin 2 | Displays cardioprotective properties | [87] | |
| Serine proteinase inhibitors | Anti-neoplastic and anti-angiogenic properties | [88,89] | |
| Vipera palaestinae | Crude venom | Hemorrhagic activity and neurotoxicity | [92] |
| Integrin antagonists | Anti-neoplastic properties Inhibits cell migration and cell adhesion to type I collagen | [93] [94] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rima, M.; Alavi Naini, S.M.; Karam, M.; Sadek, R.; Sabatier, J.-M.; Fajloun, Z. Vipers of the Middle East: A Rich Source of Bioactive Molecules. Molecules 2018, 23, 2721. https://doi.org/10.3390/molecules23102721
Rima M, Alavi Naini SM, Karam M, Sadek R, Sabatier J-M, Fajloun Z. Vipers of the Middle East: A Rich Source of Bioactive Molecules. Molecules. 2018; 23(10):2721. https://doi.org/10.3390/molecules23102721
Chicago/Turabian StyleRima, Mohamad, Seyedeh Maryam Alavi Naini, Marc Karam, Riyad Sadek, Jean-Marc Sabatier, and Ziad Fajloun. 2018. "Vipers of the Middle East: A Rich Source of Bioactive Molecules" Molecules 23, no. 10: 2721. https://doi.org/10.3390/molecules23102721
APA StyleRima, M., Alavi Naini, S. M., Karam, M., Sadek, R., Sabatier, J. -M., & Fajloun, Z. (2018). Vipers of the Middle East: A Rich Source of Bioactive Molecules. Molecules, 23(10), 2721. https://doi.org/10.3390/molecules23102721