Volatile Terpenes and Terpenoids from Workers and Queens of Monomorium chinense (Hymenoptera: Formicidae)
Abstract
:1. Introduction
2. Results
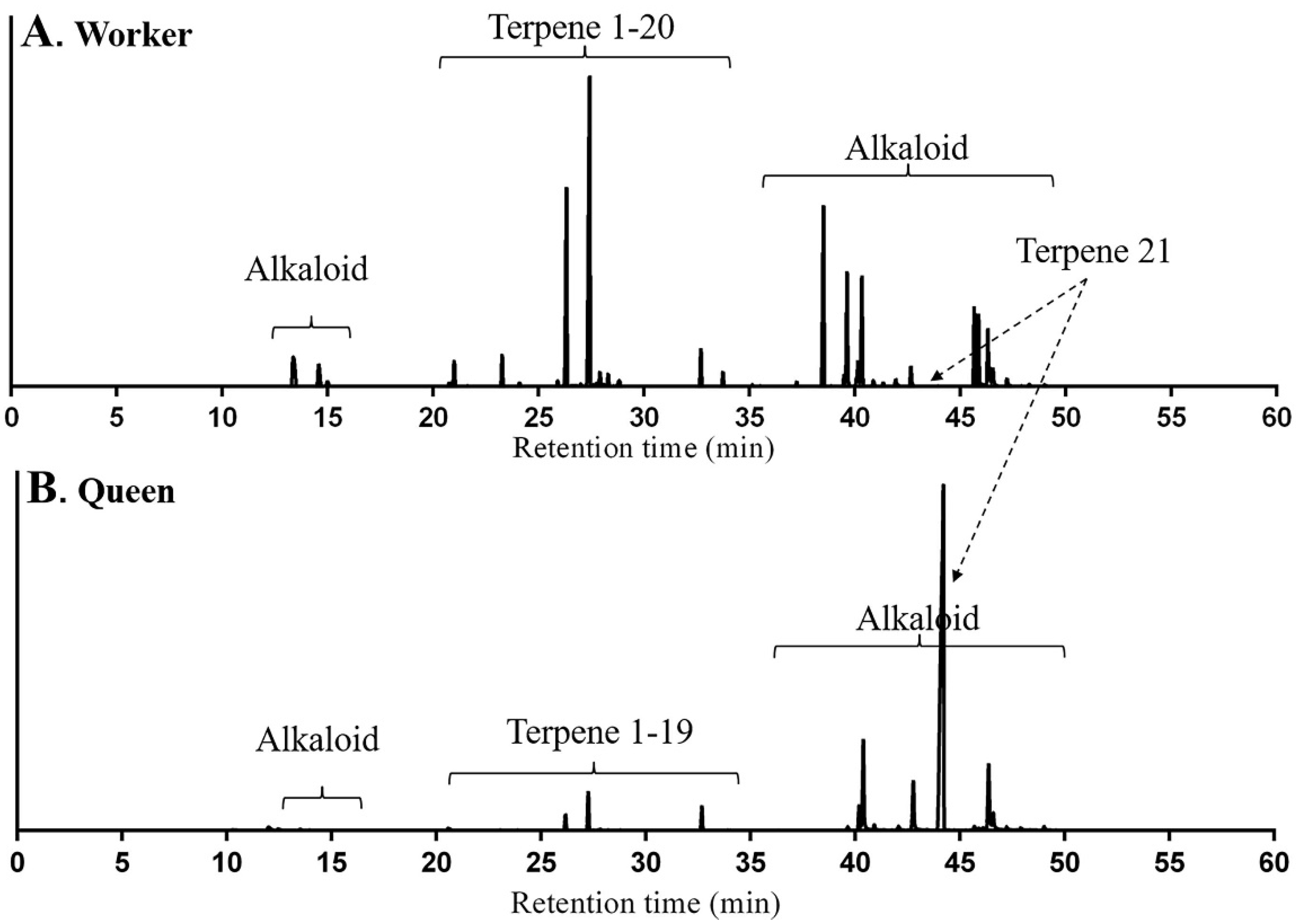
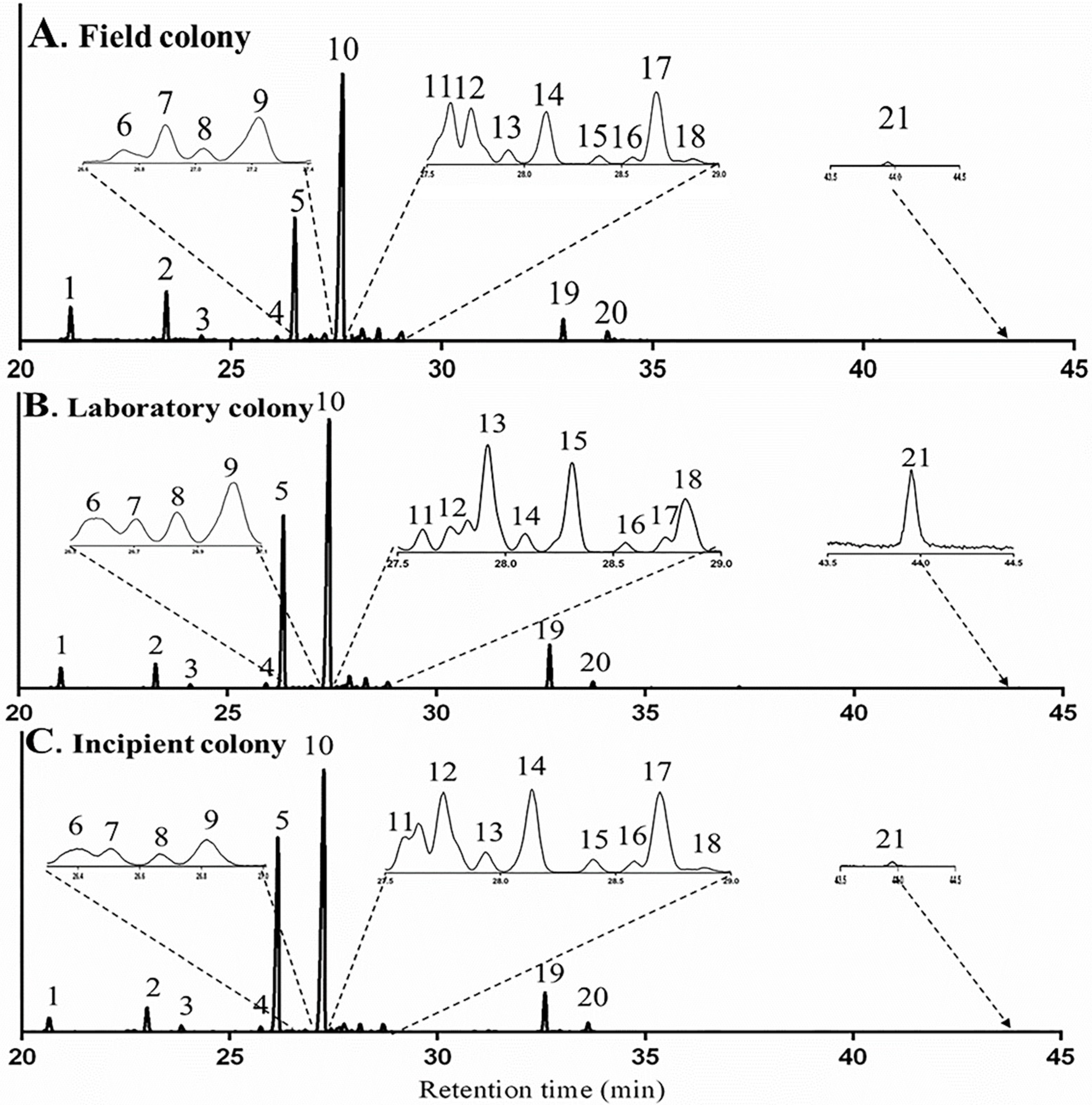
2.1. Identification of Terpenes and Terpenoids from Whole Bodies of Ants
2.2. Origin of Terpenes and Terpenoids
2.2.1. Body Parts
2.2.2. Exocrine Glands
2.2.3. Influence of Diet on Terpene and Terpenoid Profile
3. Discussion
4. Materials and Methods
4.1. Ants
4.1.1. Maintenance of Field-Collected Ant Colonies
4.1.2. Establishment of Incipient Colonies
4.2. Chemical Analysis of Ant Volatile Terpenes and Terpenoids
4.2.1. Ant Sample Preparation and Extraction by HS-SPME
4.2.2. Determination of Glandular Sources of Terpenes and Terpenoids
4.2.3. Gas Chromatography and Mass Spectrometry (GC-MS)
4.3. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Connolly, J.D.; Hill, R.A. Dictionary of Terpenoids, 1st ed.; Chapman & Hall: London, UK, 1991; ISBN 0-412-25770-X. [Google Scholar]
- Chen, X.; Köllner, T.G.; Jia, Q.; Norris, A.; Santhanam, B.; Rabe, P.; Dickschat, J.S.; Shaulsky, G.; Gershenzon, J.; Chen, F. Terpene synthase genes in eukaryotes beyond plants and fungi: Occurrence in social amoebae. Proc. Natl. Acad. Sci. USA 2016, 113, 12132–12137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, Y.; Kuzuyama, T.; Komatsu, M.; Shinya, K.; Omura, S.; Cane, D.E.; Ikeda, H. Terpene synthases are widely distributed in bacteria. Proc. Natl. Acad. Sci. USA 2015, 112, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Breitmaier, E. Terpenes: Flavors, Fragrances, Pharmaca, Pheromones, 1st ed.; Wiley-VCH: Wallingford, UK, 2006; p. 214. ISBN 3-527-31786-4. [Google Scholar]
- Gershenzon, J.; Dudareva, N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 2007, 3, 408. [Google Scholar] [CrossRef] [PubMed]
- Gnankiné, O.; Bassolé, I. Essential oils as an alternative to pyrethroids’ resistance against Anopheles species complex giles (Diptera: Culicidae). Molecules 2017, 22, 1321. [Google Scholar] [CrossRef] [PubMed]
- Leavell, M.D.; Mcphee, D.J.; Paddon, C.J. Developing fermentative terpenoid production for commercial usage. Curr. Opin. Biotechnol. 2016, 37, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.O.; Regnier, F.E. The evolution of the alarm-defense system in the Formicine ants. Am. Nat. 1971, 105, 279–289. [Google Scholar] [CrossRef]
- Meer, R.K.V.; Alvarez, F.; Lofgren, C.S. Isolation of the trail recruitment pheromone of Solenopsis invicta. J. Chem. Ecol. 1988, 14, 825–838. [Google Scholar] [CrossRef] [PubMed]
- Ritter, F.J.; Brüggemann-Rotgans, I.E.M.; Verwiel, P.E.J.; Persoons, C.J.; Talman, E. Trail pheromone of the Pharaoh’s ant, Monomorium pharaonis: Isolation and identification of faranal, a terpenoid related to juvenile hormone II. Tetrahedron Lett. 1977, 18, 2617–2618. [Google Scholar] [CrossRef]
- Meinwald, J.; Wiemer, D.F.; Hölldobler, B. Pygidial gland secretions of the ponerine ant Rhytidoponera metallica. Naturwissenschaften 1983, 70, 46–47. [Google Scholar] [CrossRef]
- Cavill, G.W.K.; Robertson, P.L.; Brophy, J.J.; Clark, D.V.; Duke, R.; Orton, C.J.; Plant, W.D. Defensive and other secretions of the Australian cocktail ant, Iridomyrmex nitidiceps. Tetrahedron 1982, 38, 1931–1938. [Google Scholar] [CrossRef]
- Welzel, K.F.; Lee, S.H.; Dossey, A.T.; Chauhan, K.R.; Choe, D.H. Verification of Argentine ant defensive compounds and their behavioral effects on heterospecific competitors and conspecific nestmates. Sci. Rep. 2018, 8, 1477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oldham, N.J.; Morgan, E.D.; Gobin, B.; Schoeters, E.; Billen, J. Volatile secretions of old world army ant Aenictus rotundatus and chemotaxonomic implications of army ant Dufour’s gland chemistry. J. Chem. Ecol. 1994, 20, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Keegans, S.J.; Billen, J.; Morgan, E.D.; Gökcen, O.A. Volatile glandular secretions of three species of new world army ants, Eciton burchelli, Labidus coecus, and Labidus praedator. J. Chem. Ecol. 1993, 19, 2705–2719. [Google Scholar] [CrossRef] [PubMed]
- Hick, A.J.; Luszniak, M.C.; Pickett, J.A. Volatile isoprenoids that control insect behaviour and development. Nat. Prod. Rep. 1999, 16, 39–54. [Google Scholar] [CrossRef]
- Kunert, M.; Søe, A.; Bartram, S.; Discher, S.; Tolzin-Banasch, K.; Nie, L.; David, A.; Pasteels, J.; Boland, W. De novo biosynthesis versus sequestration: A network of transport systems supports in iridoid producing leaf beetle larvae both modes of defense. Insect Biochem. Mol. 2008, 38, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Bowers, M.D.; Puttick, G.M. Fate of ingested iridoid glycosides in lepidopteran herbivores. J. Chem. Ecol. 1986, 12, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Taft, S.; Najar, A.; Erbilgin, N. Pheromone production by an invasive bark beetle varies with monoterpene composition of its naïve host. J. Chem. Ecol. 2015, 41, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Burse, A.; Boland, W. Deciphering the route to cyclic monoterpenes in Chrysomelina leaf beetles: Source of new biocatalysts for industrial application? Z. Naturforsch. C 2017, 72, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Gilg, A.B.; Tittiger, C.; Blomquist, G.J. Unique animal prenyltransferase with monoterpene synthase activity. Naturwissenschaften 2009, 96, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Bolton, B. A review of the Solenopsis genus-group and revision of Afrotropical Monomorium Mayr (Hymenoptera: Formicidae). Bull. Br. Mus. Entomol. 1987, 39, 263–452. [Google Scholar] [CrossRef]
- Chen, Y.C.; Kafle, L.; Shih, C.J. Interspecific competition between Solenopsis invicta and two native ant species, Pheidole fervens and Monomorium chinense. J. Econ. Entomol. 2011, 104, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.P.; Chambers, J. Identification and source of a queen-specific chemical in the pharaoh’s ant, Monomorium pharaonis (L.). J. Chem. Ecol. 1984, 10, 1731–1747. [Google Scholar] [CrossRef] [PubMed]
- Everaerts, C.; Roisin, Y.; Quéré, J.L.L.; Bonnard, O.; Pasteels, J.M. Sesquiterpenes in the frontal gland secretions of nasute soldier termites from New Guinea. J. Chem. Ecol. 1993, 19, 2865–2879. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.F.; Jackson, B.D.; Morgan, E.D. Contents of the poison apparatus of some species of Pheidole ants. Biochem. Syst. Ecol. 2007, 35, 641–651. [Google Scholar] [CrossRef]
- Cruz-López, L.; Rojas, J.C.; De, L.C.R.; Morgan, E.D. Behavioral and chemical analysis of venom gland secretion of queens of the ant Solenopsis geminata. J. Chem. Ecol. 2001, 27, 2437–2445. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. Freeze-thaw sample preparation method improves detection of volatile compounds in insects using headspace solid-phase microextraction. Anal. Chem. 2017, 89, 8366–8371. [Google Scholar] [CrossRef] [PubMed]
- Borges, M.; Millar, J.G.; Laumann, R.A.; Moraes, M.C. A male-produced sex pheromone from the neotropical redbanded stink bug, Piezodorus guildinii (W.). J. Chem. Ecol. 2007, 33, 1235–1248. [Google Scholar] [CrossRef] [PubMed]
- Frankfater, C.; Tellez, M.R.; Slattery, M. The scent of alarm: Ontogenetic and genetic variation in the osmeterial gland chemistry of Papilio glaucus (Papilionidae) caterpillars. Chemoecology 2009, 19, 81–96. [Google Scholar] [CrossRef]
- Guevara, R.; Hutcheson, K.A.; Mee, A.C.; Rayner, A.D.M.; Reynolds, S.E. Resource partitioning of the host fungus Coriolus versicolor by two ciid beetles: The role of odour compounds and host ageing. Oikos 2000, 91, 184–194. [Google Scholar] [CrossRef]
- Mcbrien, H.L.; Millar, J.G.; Rice, R.E.; Mcelfresh, J.S.; Cullen, E.; Zalom, F.G. Sex attractant pheromone of the red-shouldered stink bug Thyanta pallidovirens: A pheromone blend with multiple redundant components. J. Chem. Ecol. 2002, 28, 1797–1818. [Google Scholar] [CrossRef] [PubMed]
- Mitaka, Y.; Mori, N.; Matsuura, K. Multi-functional roles of a soldier-specific volatile as a worker arrestant, primer pheromone and an antimicrobial agent in a termite. Proc. Biol. Sci. 2017, 284, 20171134. [Google Scholar] [CrossRef] [PubMed]
- Hisashi, Ô.; Noguchi, T.; Nehira, T. New oxygenated himachalenes in male-specific odor of the Chinese windmill butterfly, Byasa alcinousalcinous. Nat. Prod. Res. 2016, 30, 406–411. [Google Scholar] [CrossRef]
- Degenhardt, J.; Köllner, T.G.; Gershenzon, J. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 2009, 70, 1621–1637. [Google Scholar] [CrossRef] [PubMed]
- Lücker, J.; Bowen, P.; Bohlmann, J. Vitis vinifera terpenoid cyclases: Functional identification of two sesquiterpene synthase cDNAs encoding (+)-valencene synthase and (−)-germacrene D synthase and expression of mono- and sesquiterpene synthases in grapevine flowers and berries. Phytochemistry 2004, 65, 2649–2659. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Hayashi, N. Chemical nature of larval osmeterial secretions of papilionid butterflies in the genera Parnassius, Sericinus and Pachliopta. J. Chem. Ecol. 1995, 21, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Krasulová, J.; Hanus, R.; Kutalová, K.; Jan, Š.; Sillam-Dussès, D.; Tichý, M.; Valterová, I. Chemistry and anatomy of the frontal gland in soldiers of the sand termite Psammotermes hybostoma. J. Chem. Ecol. 2012, 38, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Ohmura, W.; Hishiyama, S.; Nakashima, T.; Kato, A.; Makihara, H.; Ohira, T.; Irei, H. Chemical composition of the defensive secretion of the longhorned beetle, Chloridolum loochooanum. J. Chem. Ecol. 2009, 35, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Boonen, S.; Billen, J. Caste regulation in the ant Monomorium pharaonis (L.) with emphasis on the role of queens. Insects Soc. 2017, 64, 113–121. [Google Scholar] [CrossRef]
- Chen, J.; Cantrell, C.L.; Oi, D.; Grodowitz, M.J. Update on the defensive chemicals of the little black ant, Monomorium minimum (Hymenoptera: Formicidae). Toxicon 2016, 122, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Lu, L.; Shi, Q.; Chen, J.; He, Y. Contents of Dufour’s Gland of the Queens of Monomorium Species (Hymenoptera: Formicidae). 2018, unpublished work. [Google Scholar]
- Brand, J.M.; Blum, M.S.; Lloyd, H.A.; Fletcher, D.J.C. Monoterpene hydrocarbons in the poison gland secretion of the ant Myrmicaria natalensis (Hymenoptera: Formicidae). Ann. Entomol. Soc. Am. 1974, 67, 525–526. [Google Scholar] [CrossRef]
- Jones, T.H.; Blum, M.S.; Howard, R.W.; Mcdaniel, C.A.; Fales, H.M.; Dubois, M.B.; Torres, J. Venom chemistry of ants in the genus Monomorium. J. Chem. Ecol. 1982, 8, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.N.; Blum, M.S.; Jones, T.H. Venom alkaloids in Monomorium “rothsteini” Forel repel other ants: Is this the secret to success by Monomorium in Australian ant communities? Oecologia 1991, 88, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Kirby, J.; Keasling, J.D. Biosynthesis of plant isoprenoids: Perspectives for microbial engineering. Annu. Rev. Plant Biol. 2009, 60, 335–355. [Google Scholar] [CrossRef] [PubMed]
- Pichersky, E.; Gang, D.R. Genetics and biochemistry of secondary metabolites in plants: An evolutionary perspective. Trends Plant Sci. 2000, 5, 439–445. [Google Scholar] [CrossRef]
- García-Martínez, M.A.; Martínez-Tlapa, D.L.; Pérez-Toledo, G.R.; Quiroz-Robledo, L.N.; Castaño-Meneses, G.; Laborde, J.; Valenzuela-González, J.E. Taxonomic, species and functional group diversity of ants in a tropical anthropogenic landscape. Trop. Conserv. Sci. 2015, 8, 1017–1032. [Google Scholar] [CrossRef]
- Ivarsson, P.; Schlyter, F.; Birgersson, G. Demonstration of de novo pheromone biosynthesis in Ips duplicatus (Coleoptera: Scolytidae): Inhibition of ipsdienol and E-myrcenol production by compactin. Insect Biochem. Mol. 1993, 23, 655–662. [Google Scholar] [CrossRef]
- Seybold, S.J.; Quilici, D.R.; Tillman, J.A.; Vanderwel, D.; Wood, D.L.; Blomquist, G.J. De novo biosynthesis of the aggregation pheromone components ipsenol and ipsdienol by the pine bark beetles Ips paraconfusus Lanier and Ips pini (Say) (Coleoptera: Scolytidae). Proc. Natl. Acad. Sci. USA 1995, 92, 8393–8397. [Google Scholar] [CrossRef] [PubMed]
- Beran, F.; Rahfeld, P.; Luck, K.; Nagel, R.; Vogel, H.; Wielsch, N.; Irmisch, S.; Ramasamy, S.; Gershenzon, J.; Heckel, D.G. Novel family of terpene synthases evolved from trans-isoprenyl diphosphate synthases in a flea beetle. Proc. Natl. Acad. Sci. USA 2016, 113, 2922–2927. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Business Media: Carol Stream, IL, USA, 2009; pp. 53–788. ISBN 0-931710-85-5. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |



| Peak No. | Compound | RT (min) | AI | KI | Identification Proposal * | Glandular Source ** | Relative Content (Mean ± SE) (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| Worker | Queen | Worker | Queen | ||||||
| 1 | δ-elemene | 21.024 | 1338 | 1341 | B | PG | Abd | 2.7 ± 0.44 | 0.29 ± 0.14 |
| 2 | β-elemene | 23.292 | 1392 | 1393 | A | PG | Abd | 3.11 ± 0.77 | 0.3 ± 0.12 |
| 3 | β-cedrene | 24.436 | 1420 | 1421 | A | MG | Head | 0.71 ± 0.19 | 0.07 ± 0.05 |
| 4 | (E)-β-farnesene | 25.917 | 1457 | 1459 | A | PG | Abd | 0.76 ± 0.28 | 0.03 ± 0.01 |
| 5 | β-acoradiene | 26.323 | 1467 | 1469 | B | MG, PG | PG, Head | 24.13 ± 2.11 | 2.01 ± 0.18 |
| 6 | α-neocallitropsene | 26.569 | 1473 | 1475 | B | Abd | Abd | 0.24 ± 0.09 | 0.02 ± 0.00 |
| 7 | β-chamigrene | 26.708 | 1477 | 1478 | B | Abd | Abd | 0.7 ± 0.28 | 0.04 ± 0.01 |
| 8 | γ-curcumene | 26.836 | 1480 | 1481 | B | Abd | Abd | 0.22 ± 0.14 | 0.01 ± 0.01 |
| 9 | aristolochene | 27.012 | 1484 | 1485 | B | Abd | Abd | 0.62 ± 0.49 | 0.31 ± 0.05 |
| 10 | terpene 1 | 27.4 | 1494 | 1494 | C | PG | PG | 48.15 ± 2.97 | 4.75 ± 0.48 |
| 11 | β-himachalene | 27.617 | 1499 | 1499 | B | MG | Head | 0.84 ± 0.72 | 0.14 ± 0.11 |
| 12 | (Z)-α-bisabolene | 27.747 | 1503 | 1503 | B | Abd | Abd | 0.63 ± 0.47 | 0.23 ± 0.12 |
| 13 | terpene 2 | 27.974 | 1509 | 1509 | C | PG | Abd | 1.99 ± 0.85 | 0.15 ± 0.11 |
| 14 | β-curcumene | 28.093 | 1512 | 1512 | B | Abd | Abd | 0.33 ± 0.14 | 0.07 ± 0.01 |
| 15 | 7-epi-α-Selinene | 28.311 | 1517 | 1518 | B | PG | Abd | 1.51 ± 0.2 | 0.07 ± 0.05 |
| 16 | β-sesquiphellandrene | 28.577 | 1524 | 1525 | B | Abd | Abd | 0.59 ± 0.44 | 0.05 ± 0.04 |
| 17 | terpene 3 | 28.743 | 1529 | 1530 | C | Abd | Abd | 1.19 ± 0.81 | 0.01 ± 0.00 |
| 18 | γ-cuprenene | 28.853 | 1531 | 1533 | B | MG, PG | Abd, Head | 1.68 ± 0.72 | 0.09 ± 0.04 |
| 19 | 8-cedren-13-ol | 32.721 | 1633 | 1634 | B | MG | MG | 7.04 ± 5.08 | 2.34 ± 0.62 |
| 20 | terpenoid 1 | 33.751 | 1661 | 1662 | C | DG | - | 2.51 ± 0.67 | 0 |
| 21 | neocembrene | 43.951 | 1959 | 1959 | B | DG | DG | 0.33 ± 0.27 | 89.00 ± 1.46 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, R.; Lu, L.; Shi, Q.; Chen, J.; He, Y. Volatile Terpenes and Terpenoids from Workers and Queens of Monomorium chinense (Hymenoptera: Formicidae). Molecules 2018, 23, 2838. https://doi.org/10.3390/molecules23112838
Zhao R, Lu L, Shi Q, Chen J, He Y. Volatile Terpenes and Terpenoids from Workers and Queens of Monomorium chinense (Hymenoptera: Formicidae). Molecules. 2018; 23(11):2838. https://doi.org/10.3390/molecules23112838
Chicago/Turabian StyleZhao, Rui, Lihua Lu, Qingxing Shi, Jian Chen, and Yurong He. 2018. "Volatile Terpenes and Terpenoids from Workers and Queens of Monomorium chinense (Hymenoptera: Formicidae)" Molecules 23, no. 11: 2838. https://doi.org/10.3390/molecules23112838
APA StyleZhao, R., Lu, L., Shi, Q., Chen, J., & He, Y. (2018). Volatile Terpenes and Terpenoids from Workers and Queens of Monomorium chinense (Hymenoptera: Formicidae). Molecules, 23(11), 2838. https://doi.org/10.3390/molecules23112838






