Imidazo[1,2-b]pyrazole-7-carboxamides Induce Apoptosis in Human Leukemia Cells at Nanomolar Concentrations
Abstract
:1. Introduction
2. Results
2.1. Synthesis of Imidazo[1,2-b]pyrazole-7-carboxamides
2.2. Imidazo[1,2-b]pyrazole-7-carboxamides Hampered the Viability of Leukemia Cells with Different Potential
2.3. Leukemia Cells Died by Apoptosis Upon Treatment by Imidazo[1,2-b]pyrazole-carboxamides
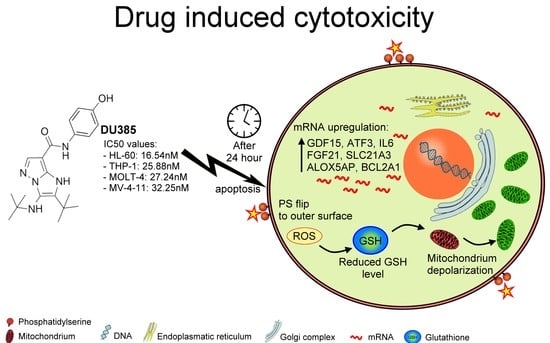
2.4. Toxicogenomic Data upon Treatment by Imidazo[1,2-b]pyrazole-7-carboxamide DU385
2.5. Imidazo[1,2-b]pyrazole-7-carboxamide DU385 Exerted Oxidative Stress of MV-4-11 Cells
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
4.2. Skin Biopsies and Cell Culture of Human Primary Fibroblasts
4.3. Cell Culturing, 3D Spheroid Formation and Treatments
4.4. Resazurin Viability Assay
4.5. Detection of Phosphatidylserine Exposure
4.6. Quantitative-Real Time PCR
4.7. Detection of the Oxidative Stress
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
 GJSz was supported BY the UNKP-18-4 New National Excellence Program of the Ministry of Human Capacities (UNKP-18-4-SZTE-73).
GJSz was supported BY the UNKP-18-4 New National Excellence Program of the Ministry of Human Capacities (UNKP-18-4-SZTE-73).Conflicts of Interest
References
- McGuire, S. World cancer report 2014. Geneva, switzerland: World health organization, international agency for research on cancer, who press, 2015. Adv. Nutr. 2016, 7, 418–419. [Google Scholar] [CrossRef] [PubMed]
- Greaves, M. Leukaemia ‘firsts’ in cancer research and treatment. Nat. Rev. Cancer 2016, 16, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Vardiman, J.W.; Thiele, J.; Arber, D.A.; Brunning, R.D.; Borowitz, M.J.; Porwit, A.; Harris, N.L.; Le Beau, M.M.; Hellstrom-Lindberg, E.; Tefferi, A.; et al. The 2008 revision of the world health organization (who) classification of myeloid neoplasms and acute leukemia: Rationale and important changes. Blood 2009, 114, 937–951. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.M.; Catovsky, D.; Daniel, M.T.; Flandrin, G.; Galton, D.A.; Gralnick, H.R.; Sultan, C. Proposals for the classification of the acute leukaemias. French-American-British (fab) co-operative group. Br. J. Haematol. 1976, 33, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.M.; Catovsky, D.; Daniel, M.T.; Flandrin, G.; Galton, D.A.; Gralnick, H.R.; Sultan, C. Criteria for the diagnosis of acute leukemia of megakaryocyte lineage (M7). A report of the French-American-British cooperative group. Ann. Intern. Med. 1985, 103, 460–462. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.J. The HL-60 promyelocytic leukemia cell line: Proliferation, differentiation, and cellular oncogene expression. Blood 1987, 70, 1233–1244. [Google Scholar] [PubMed]
- Tasseff, R.; Jensen, H.A.; Congleton, J.; Dai, D.; Rogers, K.V.; Sagar, A.; Bunaciu, R.P.; Yen, A.; Varner, J.D. An effective model of the retinoic acid induced HL-60 differentiation program. Sci. Rep. 2017, 7, 14327. [Google Scholar] [CrossRef] [PubMed]
- Mannarino, L.; Paracchini, L.; Craparotta, I.; Romano, M.; Marchini, S.; Gatta, R.; Erba, E.; Clivio, L.; Romualdi, C.; D’Incalci, M.; et al. A systems biology approach to investigate the mechanism of action of trabectedin in a model of myelomonocytic leukemia. Pharmacogenom. J. 2018, 18, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Mieczkowski, A.; Psurski, M.; Baginski, M.; Bieszczad, B.; Mroczkowska, M.; Wilczek, M.; Czajkowska, J.; Trzybinski, D.; Wozniak, K.; Wietrzyk, J. Novel (S)-1,3,4,12a-tetrahydropyrazino[2,1-c][1,4]benzodiazepine-6,12(2H,11H)-dione derivatives: Selective inhibition of MV-4-11 biphenotypic b myelomonocytic leukemia cells’ growth is accompanied by reactive oxygen species overproduction and apoptosis. Bioorg. Med. Chem. Lett. 2018, 28, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Battin, C.; Hennig, A.; Mayrhofer, P.; Kunert, R.; Zlabinger, G.J.; Steinberger, P.; Paster, W. A human monocytic NF-κB fluorescent reporter cell line for detection of microbial contaminants in biological samples. PLoS ONE 2017, 12, e0178220. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, S.; Yamabe, M.; Yamaguchi, Y.; Kobayashi, Y.; Konno, T.; Tada, K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 1980, 26, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, F.; Chang, C.K.; He, Q.; Wu, L.Y.; Zhang, Z.; Li, X. Mycn contributes to the malignant characteristics of erythroleukemia through EZH2-mediated epigenetic repression of p21. Cell Death Dis. 2017, 8, e3126. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wang, L.; Luo, X.; Chen, L.; Yang, Z.; Liu, L. SPAG6 silencing inhibits the growth of the malignant myeloid cell lines SKM-1 and K562 via activating p53 and caspase activation-dependent apoptosis. Int. J. Oncol. 2015, 46, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Karrouchi, K.; Radi, S.; Ramli, Y.; Taoufik, J.; Mabkhot, Y.N.; Al-Aizari, F.A.; Ansar, M. Synthesis and pharmacological activities of pyrazole derivatives: A review. Molecules 2018, 23, 134. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, A.; Eskandari-Vashareh, M.; Alizadeh-Kouzehrash, M. Synthesis of 3-(benzylideneamino)-2-phenyl-5H-imidazo[1,2-b]pyrazole-7-carbonitriles via a four-component condensation reaction. Tetrahedron 2013, 69, 4199–4204. [Google Scholar] [CrossRef]
- Baviskar, A.T.; Madaan, C.; Preet, R.; Mohapatra, P.; Jain, V.; Agarwal, A.; Guchhait, S.K.; Kundu, C.N.; Banerjee, U.C.; Bharatam, P.V. N-fused imidazoles as novel anticancer agents that inhibit catalytic activity of topoisomerase IIα and induce apoptosis in G1/S phase. J. Med. Chem. 2011, 54, 5013–5030. [Google Scholar] [CrossRef] [PubMed]
- Demjen, A.; Alfoldi, R.; Angyal, A.; Gyuris, M.; Hackler, L., Jr.; Szebeni, G.J.; Wolfling, J.; Puskas, L.G.; Kanizsai, I. Synthesis, cytotoxic characterization, and sar study of imidazo[1,2-b]pyrazole-7-carboxamides. Arch. Pharm. 2018, 351, e1800062. [Google Scholar] [CrossRef] [PubMed]
- Puskas, L.G.; Feher, L.Z.; Vizler, C.; Ayaydin, F.; Raso, E.; Molnar, E.; Magyary, I.; Kanizsai, I.; Gyuris, M.; Madacsi, R.; et al. Polyunsaturated fatty acids synergize with lipid droplet binding thalidomide analogs to induce oxidative stress in cancer cells. Lipids Health Dis. 2010, 9, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.D.; Berntenis, N.; Roth, A.; Ebeling, M. Data mining reveals a network of early-response genes as a consensus signature of drug-induced in vitro and in vivo toxicity. Pharmacogenom. J. 2014, 14, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fong, C.C.; Tzang, C.H.; Xiao, P.; Han, R.; Yang, M. Gene expression analysis of human promyelocytic leukemia HL-60 cell differentiation and cytotoxicity induced by natural and synthetic retinoids. Life Sci. 2009, 84, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Fabian, G.; Farago, N.; Feher, L.Z.; Nagy, L.I.; Kulin, S.; Kitajka, K.; Bito, T.; Tubak, V.; Katona, R.L.; Tiszlavicz, L.; et al. High-density real-time pcr-based in vivo toxicogenomic screen to predict organ-specific toxicity. Int. J. Mol. Sci. 2011, 12, 6116–6134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietrich, P.A.; Yang, C.; Leung, H.H.; Lynch, J.R.; Gonzales, E.; Liu, B.; Haber, M.; Norris, M.D.; Wang, J.; Wang, J.Y. GPR84 sustains aberrant β-catenin signaling in leukemic stem cells for maintenance of mll leukemogenesis. Blood 2014, 124, 3284–3294. [Google Scholar] [CrossRef] [PubMed]
- Stevens, A.M.; Miller, J.M.; Munoz, J.O.; Gaikwad, A.S.; Redell, M.S. Interleukin-6 levels predict event-free survival in pediatric aml and suggest a mechanism of chemotherapy resistance. Blood Adv. 2017, 1, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Sedger, L.M.; McDermott, M.F. Tnf and tnf-receptors: From mediators of cell death and inflammation to therapeutic giants—Past, present and future. Cytokine Growth Factor Rev. 2014, 25, 453–472. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Glutathione and its role in cellular functions. Free Radic. Biol. Med. 1999, 27, 916–921. [Google Scholar] [CrossRef]
- Zanoni, M.; Piccinini, F.; Arienti, C.; Zamagni, A.; Santi, S.; Polico, R.; Bevilacqua, A.; Tesei, A. 3D tumor spheroid models for in vitro therapeutic screening: A systematic approach to enhance the biological relevance of data obtained. Sci. Rep. 2016, 6, 19103. [Google Scholar] [CrossRef] [PubMed]
- Verjans, E.T.; Doijen, J.; Luyten, W.; Landuyt, B.; Schoofs, L. Three-dimensional cell culture models for anticancer drug screening: Worth the effort? J. Cell Physiol. 2018, 233, 2993–3003. [Google Scholar] [CrossRef] [PubMed]
- Broemer, M.; Meier, P. Ubiquitin-mediated regulation of apoptosis. Trends Cell Biol. 2009, 19, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Ottina, E.; Tischner, D.; Herold, M.J.; Villunger, A. A1/Bfl-1 in leukocyte development and cell death. Exp. Cell Res. 2012, 318, 1291–1303. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.; Crinelli, R.; Arbore, V.; Magnani, M. Induction of ubiquitin C (UBC) gene transcription is mediated by HSF1: Role of proteotoxic and oxidative stress. FEBS Open Bio 2018, 8, 1471–1485. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.Y.; Maehr, R.; Gilchrist, C.A.; Long, M.A.; Bouley, D.M.; Mueller, B.; Ploegh, H.L.; Kopito, R.R. The mouse polyubiquitin gene UBC is essential for fetal liver development, cell-cycle progression and stress tolerance. EMBO J. 2007, 26, 2693–2706. [Google Scholar] [CrossRef] [PubMed]
- Jenal, M.; Batliner, J.; Reddy, V.A.; Haferlach, T.; Tobler, A.; Fey, M.F.; Torbett, B.E.; Tschan, M.P. The anti-apoptotic gene BCL2A1 is a novel transcriptional target of PU.1. Leukemia 2010, 24, 1073–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kucharczak, J.F.; Simmons, M.J.; Duckett, C.S.; Gelinas, C. Constitutive proteasome-mediated turnover of Bfl-1/A1 and its processing in response to TNF receptor activation in FL5.12 pro-B cells convert it into a prodeath factor. Cell Death Differ. 2005, 12, 1225–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Circu, M.L.; Aw, T.Y. Glutathione and modulation of cell apoptosis. Biochim. Biophys. Acta 2012, 1823, 1767–1777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, R.W.; Rotstein, O.D.; Parodo, J.; Bitar, R.; Hackam, D.; Marshall, J.C. Granulocytic differentiation of HL-60 cells results in spontaneous apoptosis mediated by increased caspase expression. FEBS Lett. 1997, 412, 603–609. [Google Scholar] [CrossRef] [Green Version]
- Doyle, B.T.; O’Neill, A.J.; Fitzpatrick, J.M.; Watson, R.W. Differentiation-induced HL-60 cell apoptosis: A mechanism independent of mitochondrial disruption? Apoptosis 2004, 9, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Doyle, B.T.; O’Neill, A.J.; Newsholme, P.; Fitzpatrick, J.M.; Watson, R.W. The loss of iap expression during HL-60 cell differentiation is caspase-independent. J. Leukoc. Biol. 2002, 71, 247–254. [Google Scholar] [PubMed]
- Brown, G.; Hughes, P. Retinoid differentiation therapy for common types of acute myeloid leukemia. Leukemia Res. Treat. 2012, 2012, 939021. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, B.; Hering, T.M.; Caplan, A.I.; Goldberg, V.M.; Yoo, J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp. Cell Res. 1998, 238, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Szebeni, G.J.; Balazs, A.; Madarasz, I.; Pocz, G.; Ayaydin, F.; Kanizsai, I.; Fajka-Boja, R.; Alfoldi, R.; Hackler, L., Jr.; Puskas, L.G. Achiral mannich-base curcumin analogs induce unfolded protein response and mitochondrial membrane depolarization in panc-1 cells. Int. J. Mol. Sci. 2017, 18, 2105. [Google Scholar] [CrossRef] [PubMed]
- Molnar, J.; Szebeni, G.J.; Csupor-Loffler, B.; Hajdu, Z.; Szekeres, T.; Saiko, P.; Ocsovszki, I.; Puskas, L.G.; Hohmann, J.; Zupko, I. Investigation of the antiproliferative properties of natural sesquiterpenes from artemisia asiatica and onopordum acanthium on HL-60 cells in vitro. Int. J. Mol. Sci. 2016, 17, 83. [Google Scholar] [CrossRef] [PubMed]
- Szebeni, G.J.; Tancos, Z.; Feher, L.Z.; Alfoldi, R.; Kobolak, J.; Dinnyes, A.; Puskas, L.G. Real architecture for 3D tissue (raft) culture system improves viability and maintains insulin and glucagon production of mouse pancreatic islet cells. Cytotechnology 2017, 69, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Nagy, L.I.; Molnar, E.; Kanizsai, I.; Madacsi, R.; Ozsvari, B.; Feher, L.Z.; Fabian, G.; Marton, A.; Vizler, C.; Ayaydin, F.; et al. Lipid droplet binding thalidomide analogs activate endoplasmic reticulum stress and suppress hepatocellular carcinoma in a chemically induced transgenic mouse model. Lipids Health. Dis. 2013, 12, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanizsai, I.; Madacsi, R.; Hackler, L., Jr.; Gyuris, M.; Szebeni, G.J.; Huzian, O.; Puskas, L.G. Synthesis and cytoprotective characterization of 8-hydroxyquinoline betti products. Molecules 2018, 23, 1934. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |




| HL-60 | MOLT-4 | MV-4-11 | THP-1 | K-562 | Human Fibroblasts | |
|---|---|---|---|---|---|---|
| DU283 | 266.9 | 146.2 | 209.4 | 352.5 | 493.7 | n.d. |
| DU325 # | 66.31 | 39.35 | 50.21 | 73.78 | 194.9 | n.d. |
| DU385 # | 16.54 | 27.24 | 32.25 | 25.88 | 54.31 | n.d. |
| DU441 # | 62.04 | 60.48 | 87.56 | 94.9 | 190.9 | n.d. |
| DU442 # | 58.71 | 51.03 | 68.08 | 95.22 | 163.6 | n.d. |
| DU443 | 130.7 | 104.2 | 173.7 | 108.9 | 303.1 | n.d. |
| DU455 | 2169 | 1211 | 2892 | 2354 | inactive | n.d. |
| DU456 | 446.5 | 324.8 | 524.5 | 761.4 | 901.8 | n.d. |
| 4T1 2D | 4T1 3D | MCF7 2D | MCF7 3D | |
|---|---|---|---|---|
| DU325 | 3.938 | 10.800 | 3.445 | 8.216 |
| DU385 | 4.558 | 9.520 | 3.531 | 8.853 |
| DU441 | 3.363 | 4.914 | 3.032 | 3.537 |
| DU442 | 2.255 | 5.656 | 1.559 | 5.514 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szebeni, G.J.; Balog, J.A.; Demjén, A.; Alföldi, R.; Végi, V.L.; Fehér, L.Z.; Mán, I.; Kotogány, E.; Gubán, B.; Batár, P.; et al. Imidazo[1,2-b]pyrazole-7-carboxamides Induce Apoptosis in Human Leukemia Cells at Nanomolar Concentrations. Molecules 2018, 23, 2845. https://doi.org/10.3390/molecules23112845
Szebeni GJ, Balog JA, Demjén A, Alföldi R, Végi VL, Fehér LZ, Mán I, Kotogány E, Gubán B, Batár P, et al. Imidazo[1,2-b]pyrazole-7-carboxamides Induce Apoptosis in Human Leukemia Cells at Nanomolar Concentrations. Molecules. 2018; 23(11):2845. https://doi.org/10.3390/molecules23112845
Chicago/Turabian StyleSzebeni, Gábor J., József A. Balog, András Demjén, Róbert Alföldi, Vanessza L. Végi, Liliána Z. Fehér, Imola Mán, Edit Kotogány, Barbara Gubán, Péter Batár, and et al. 2018. "Imidazo[1,2-b]pyrazole-7-carboxamides Induce Apoptosis in Human Leukemia Cells at Nanomolar Concentrations" Molecules 23, no. 11: 2845. https://doi.org/10.3390/molecules23112845
APA StyleSzebeni, G. J., Balog, J. A., Demjén, A., Alföldi, R., Végi, V. L., Fehér, L. Z., Mán, I., Kotogány, E., Gubán, B., Batár, P., Hackler, L., Kanizsai, I., & Puskás, L. G. (2018). Imidazo[1,2-b]pyrazole-7-carboxamides Induce Apoptosis in Human Leukemia Cells at Nanomolar Concentrations. Molecules, 23(11), 2845. https://doi.org/10.3390/molecules23112845