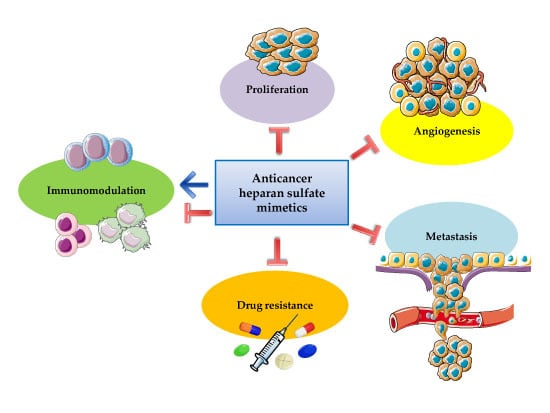
Heparan Sulfate Mimetics in Cancer Therapy: The Challenge to Define Structural Determinants and the Relevance of Targets for Optimal Activity
Abstract
Share and Cite
Lanzi, C.; Cassinelli, G. Heparan Sulfate Mimetics in Cancer Therapy: The Challenge to Define Structural Determinants and the Relevance of Targets for Optimal Activity. Molecules 2018, 23, 2915. https://doi.org/10.3390/molecules23112915
Lanzi C, Cassinelli G. Heparan Sulfate Mimetics in Cancer Therapy: The Challenge to Define Structural Determinants and the Relevance of Targets for Optimal Activity. Molecules. 2018; 23(11):2915. https://doi.org/10.3390/molecules23112915
Chicago/Turabian StyleLanzi, Cinzia, and Giuliana Cassinelli. 2018. "Heparan Sulfate Mimetics in Cancer Therapy: The Challenge to Define Structural Determinants and the Relevance of Targets for Optimal Activity" Molecules 23, no. 11: 2915. https://doi.org/10.3390/molecules23112915
APA StyleLanzi, C., & Cassinelli, G. (2018). Heparan Sulfate Mimetics in Cancer Therapy: The Challenge to Define Structural Determinants and the Relevance of Targets for Optimal Activity. Molecules, 23(11), 2915. https://doi.org/10.3390/molecules23112915