A Brief History of Charcot-Leyden Crystal Protein/Galectin-10 Research
Abstract
:1. Charcot-Leyden Crystals
2. Ambiguous History of CLC Protein/Galecin-10
3. Crystallization Studies of Gal-10
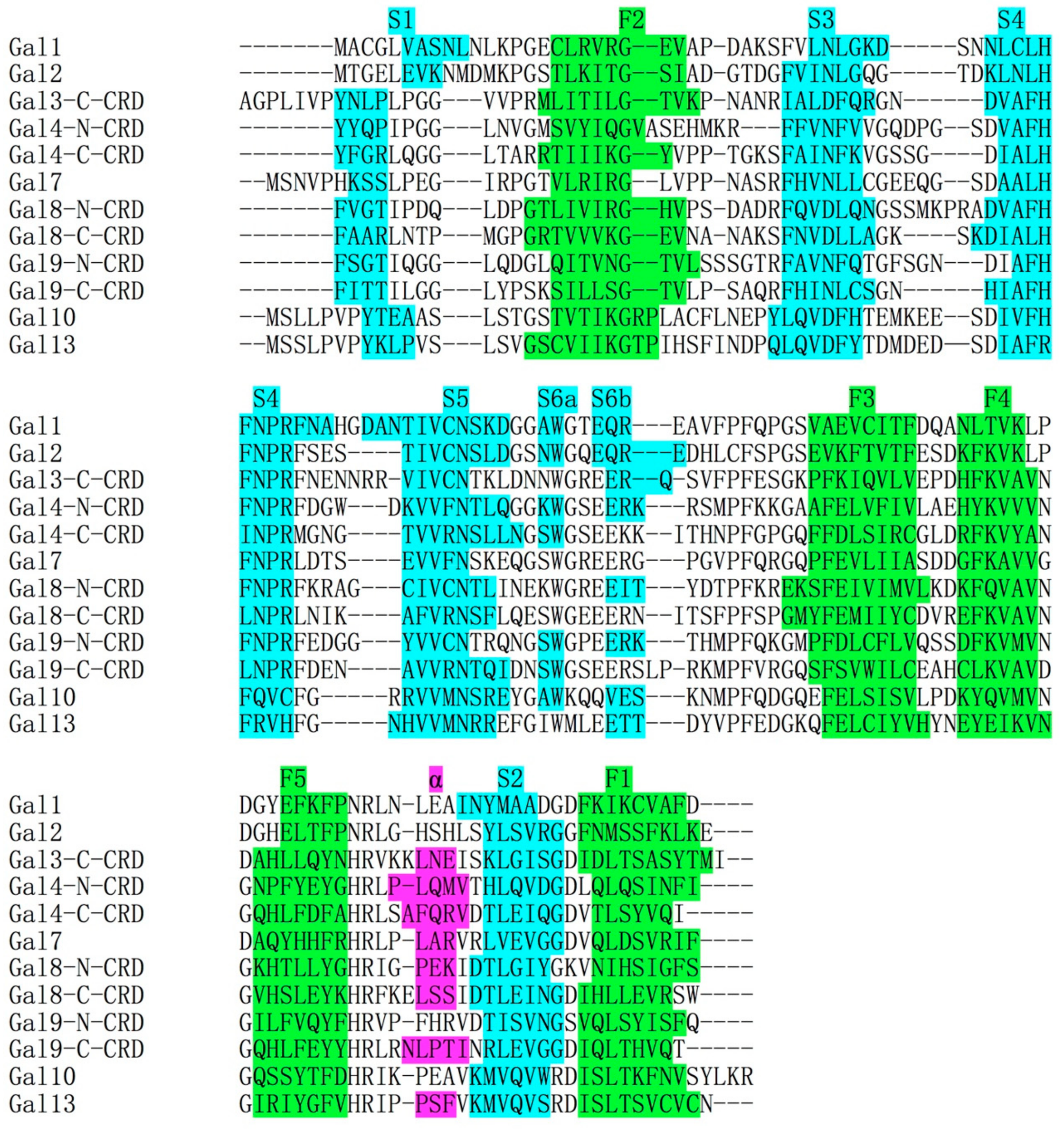
4. Structural Comparisons between Gal-10 and Other Prototype Galectins
5. Ligand Binding Specificity of Gal-10
6. Staining Methods for CLC
7. Promoter Studies of Gal-10 Gene
8. Distribution of Gal-10 in Variable Lymphocytes
9. Transmission Electron Microscopy Studies of CLCs
10. Cellular Distribution of CLCs and Gal-10
11. Relations of CLC/Gal-10 to Variable Diseases
11.1. CLCs in The Lymphoid Organs
11.2. CLCs in Stroma of Solid Tumors
11.3. CLCs and Infections
11.4. CLCs and Celiac Disease
11.5. CLCs in Asthma and Allergy
11.6. CLCs Related to Other Diseases
12. Conclusions and Prospective
Funding
Acknowledgments
Conflicts of Interest
References
- Charcot, J.M.; Robin, C. Observation de leucocythemie. Mem. Soc. Biol. 1853, 5, 44–50. [Google Scholar]
- Leyden, E. Zur Kenntnis des Bronchial-Asthma. Virchows Arch. Path. Anal. 1872, 54, 324–352. [Google Scholar] [CrossRef]
- Liebreich, E. In-vitro Versuch über Eosinophilie. KIin. Wchnschr. 1923, 2, 194–198. [Google Scholar] [CrossRef]
- Neumann, A. Ueber die Natur der Charcot-Leyden-Bottcher-Neumann Krystalle. Ztschr. Phys. Chem. 1927, 173, 69–71. [Google Scholar] [CrossRef]
- Thompson, J.; Paddock, F.K. The significance of Charcot-Leyden crystals. N. Engl. J. Med. 1940, 223, 936–939. [Google Scholar] [CrossRef]
- Schwarz, E. Die Lehre von der allgemeinen und örtlichen “Eosinophilie”. Ergebn. Alig. Path. Path. Anat. 1914, 17, 137–789. [Google Scholar]
- Böttcher, A. Farblose Krystalle eines eiweissartigen Körpers aus dem menschlichen Sperma dargestellt. Virchows Arch. Path. Anat. 1865, 32, 525–535. [Google Scholar] [CrossRef]
- Wrede, Fr.; Boldt, F.; Buch, E. Ueber die Natur der Charcot-Leyden-Böttcher-Neumann Krystalle. Ztschr. Physiol. Chem. 1927, 165, 155. [Google Scholar] [CrossRef]
- Cohn, T. Beitrag zur Kenntnis des Charcot’schen und Böttcher’schen Krystalle. Deutsches Arch. Klin. Med. 1895, 54, 515–524. [Google Scholar]
- Askanazy, M. Handbuch der Speziellen Pathologische Anatomie und Histologie; Springer: Berlin, Germany, 1927; p. 789. [Google Scholar]
- Weleed, E. Charcot-Leyden Crystals. Am. J. Pathol. 1971, 65, 311–324. [Google Scholar]
- Huffnagle, G.B.; Boyd, M.B.; Street, N.E.; Lipscomb, M.F. IL-5 is required for eosinophil recruitment, crystal deposition, and mononuclear cell recruitment during a pulmonary Cryptococcus neoformans infection in genetically susceptible mice (C57BL/6). J. Immunol. 1998, 160, 2393–2400. [Google Scholar] [PubMed]
- Henderson, W.R., Jr.; Tang, L.O.; Chu, S.J.; Tsao, S.M.; Chiang, G.K.; Jones, F.; Jonas, M.; Pae, C.; Wang, H.; Chi, E.Y. A role for cysteinyl leukotrienes in airway remodeling in a mouse asthma model. Am. J. Respir. Crit. Care Med. 2002, 165, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Hoenerhoff, M.J.; Starost, M.F.; Ward, J.M. Eosinophilic crystalline pneumonia as a major cause of death in 129S4/SvJae mice. Vet. Pathol. 2006, 43, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Miller, A.D.; Devenish, E.; Asakawa, M.; McConkey, M.; Peters-Kennedy, J. Charcot-Leyden crystals: Do they exist in veterinary species? A case report and literature review. J. Vet. Diagn. Investig. 2017, 29, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Weller, P.F.; Goetzl, E.J.; Austen, K.F. Identification of human eosinophil lysophospholipase as the constituent of Charcot-Leyden crystals. Proc. Natl. Acad. Sci. USA 1980, 77, 7440–7443. [Google Scholar] [CrossRef] [PubMed]
- Weller, P.F.; Bach, D.S.; Austen, K.F. Biochemical characterization of human eosinophil Charcot-Leyden crystal protein (lysophospholipase). J. Biol. Chem. 1984, 259, 15100–15105. [Google Scholar] [PubMed]
- Holtsberg, F.W.; Ozgur, L.E.; Garsetti, D.E.; Myers, J.; Egan, R.W.; Clark, M.A. Presence in human eosinophils of a lysophospholipase similar to that found in the pancreas. Biochem. J. 1995, 309, 141–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ackerman, S.J.; Liu, L.; Kwatia, M.A.; Savage, M.P.; Leonidas, D.D.; Swaminathan, G.J.; Acharya, K.R. Charcot-Leyden crystal protein (galectin-10) is not a dual function galectin with lysophospholipase activity but binds a lysophospholipase inhibitor in a novel structural fashion. J. Biol. Chem. 2002, 277, 14859–14868. [Google Scholar] [CrossRef] [PubMed]
- Mastrianni, D.M.; Eddy, R.L.; Rosenberg, H.F.; Corrette, S.E.; Shows, T.B.; Tenen, D.G.; Ackerman, S.J. Localization of the human eosinophil Charcot-Leyden crystal protein (lysophospholipase) gene (CLC) to chromosome 19 and the human ribonuclease 2 (eosinophil-derived neurotoxin) and ribonuclease 3 (eosinophil cationic protein) genes (RNS2 and RNS3) to chromosome 14. Genomics 1992, 13, 240–242. [Google Scholar] [PubMed]
- Dyer, K.D.; Handen, J.S.; Rosenberg, H.F. The genomic structure of the human Charcot-Leyden crystal protein gene is analogous to those of the galectin genes. Genomics 1997, 40, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, S.J.; Corrette, S.E.; Rosenberg, H.F.; Bennett, J.C.; Mastrianni, D.M.; Nicholson-Weller, A.; Weller, P.F.; Chin, D.T.; Tenen, D.G. Molecular cloning and characterization of human eosinophil Charcot-Leyden crystal protein (lysophospholipase). Similarities to IgE binding proteins and the S-type animal lectin superfamily. J. Immunol. 1993, 150, 456–468. [Google Scholar] [PubMed]
- Leonidas, D.D.; Elbert, B.L.; Zhou, Z.; Leffler, H.; Ackerman, S.J.; Acharya, K.R. Crystal structure of human Charcot-Leyden crystal protein, an eosinophil lysophospholipase, identifies it as a new member of the carbohydrate-binding family of galectins. Structure 1995, 3, 1379–1393. [Google Scholar] [CrossRef]
- Leffler, H.; Carlsson, S.; Hedlund, M.; Qian, Y.; Poirier, F. Introduction to galectins. Glycoconj. J. 2002, 19, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Gao, J.; Si, Y.; Cui, L.; Song, C.; Wang, Y.; Wu, R.; Tai, G.; Zhou, Y. Galectin-10: A new structural type of prototype galectin dimer and effects on saccharide ligand binding. Glycobiology 2018, 28, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Dyer, K.D.; Rosenberg, H.F. Eosinophil Charcot-Leyden crystal protein binds to beta-galactoside sugars. Life Sci. 1996, 58, 2073–2082. [Google Scholar] [CrossRef]
- Dvorak, A.M.; Letourneau, L.; Weller, P.F.; Ackerman, S.J. Ultrastructural localization of Charcot-Leyden crystal protein (lysophospholipase) to intracytoplasmic crystals in tumor cells of primary solid and papillary epithelial neoplasm of the pancreas. Lab. Investig. 1990, 62, 608–615. [Google Scholar] [PubMed]
- Su, J.; Song, C.; Si, Y.; Cui, L.; Yang, T.; Li, Y.; Wang, H.; Tai, G.; Zhou, Y. Identification of key amino acid residues determining ligand binding specificity, homodimerization and cellular distribution of human Galectin-10. Glycobiology 2018, cwy087. [Google Scholar] [CrossRef] [PubMed]
- Gleich, G.J.; Loegering, D.A.; Mann, K.G.; Maldonado, J.E. Comparative properties of the Charcot-Leyden crystal protein and the major basic protein from human eosinophils. J. Clin. Investig. 1976, 57, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri, B.; Irander, K.; Lindbom, J.; Tagesson, C.; Lindahl, M. Comparative proteomics of nasal fluid in seasonal allergic rhinitis. J. Proteome Res. 2006, 5, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Samter, M. Charcot-leyden crystals- A study of the conditions necessary for their formation. J. Allerg. 1947, 18, 221–230. [Google Scholar] [CrossRef]
- William, A. Production of Charcot-Leyden crystals from eosinophils with aerosol MA. Blood 1949, 4, 595–602. [Google Scholar]
- William, A.; Starkey, N. Studies of Charcot-Leyden crystals. Blood 1950, 5, 254–266. [Google Scholar]
- Swaminathan, G.J.; Leonidas, D.D.; Savage, M.P.; Ackerman, S.J.; Acharya, K.R. Selective recognition of mannose by the human eosinophil Charcot-Leyden crystal protein (galectin-10): A crystallographic study at 1.8 A resolution. J. Biochem. 1999, 38, 13837–13843. [Google Scholar]
- Than, N.G.; Romero, R.; Goodman, M.; Weckle, A.; Xing, J.; Dong, Z.; Xu, Y.; Tarquini, F.; Szilagyi, A.; Gal, P.; et al. A primate subfamily of galectins expressed at the maternal-fetal interface that promote immune cell death. Proc. Natl. Acad. Sci. USA 2009, 106, 9731–9736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.T.; Rabinovich, G.A. Galectins as modulators of tumour progression. Nat. Rev. Cancer 2005, 5, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.Y.; Rabinovich, G.A.; Liu, F.T. Galectins: Structure, function and therapeutic potential. Expert Rev. Mol. Med. 2008, 10, e17. [Google Scholar] [CrossRef] [PubMed]
- Johannes, L.; Jacob, R.; Leffler, H. Galectins at a glance. J. Cell Sci. 2018, 131. [Google Scholar] [CrossRef] [PubMed]
- Banfer, S.; Schneider, D.; Dewes, J.; Strauss, M.T.; Freibert, S.A.; Heimerl, T.; Maier, U.G.; Elsasser, H.P.; Jungmann, R.; Jacob, R. Molecular mechanism to recruit galectin-3 into multivesicular bodies for polarized exosomal secretion. Proc. Natl. Acad. Sci. USA 2018, 115, E4396–E4405. [Google Scholar] [CrossRef] [PubMed]
- Kamitori, S. Three-Dimensional Structures of Galectins. Trends Glycosci. Glycotechnol. 2018, 30, Se41–Se50. [Google Scholar] [CrossRef]
- Cooper, D.N. Galectinomics: Finding themes in complexity. Biochim. Biophys. Acta 2002, 1572, 209–231. [Google Scholar] [CrossRef]
- Kasai, K.; Hirabayashi, J. Galectins: A family of animal lectins that decipher glycocodes. J. Biochem. 1996, 119, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tasumi, S.; Vasta, G.R. A galectin of unique domain organization from hemocytes of the Eastern oyster (Crassostrea virginica) is a receptor for the protistan parasite Perkinsus marinus. J. Immunol. 2007, 179, 3086–3098. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Yamashita, S.; Teraoka, M.; Itoh, A.; Nakakita, S.; Nishi, N.; Kamitori, S. X-ray structure of a protease-resistant mutant form of human galectin-8 with two carbohydrate recognition domains. FEBS J. 2012, 279, 3937–3951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, H.; Nishi, N.; Wada, K.; Nakamura, T.; Hirashima, M.; Kuwabara, N.; Kato, R.; Kamitori, S. X-ray structure of a protease-resistant mutant form of human galectin-9 having two carbohydrate recognition domains with a metal-binding site. Biochem. Biophys. Res. Commun. 2017, 490, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Qiu, D.C.; Chang, W.H.; Yeh, Y.Q.; Jeng, U.S.; Liu, F.T.; Huang, J.R. The intrinsically disordered N-terminal domain of galectin-3 dynamically mediates multisite self-association of the protein through fuzzy interactions. J. Biol. Chem. 2017, 292, 17845–17856. [Google Scholar] [CrossRef] [PubMed]
- Seetharaman, J.; Kanigsberg, A.; Slaaby, R.; Leffler, H.; Barondes, S.H.; Rini, J.M. X-ray crystal structure of the human galectin-3 carbohydrate recognition domain at 2.1-A resolution. J. Biol. Chem. 1998, 273, 13047–13052. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.M.; Hidari, K.I.; Blanchard, H. Slow diffusion of lactose out of galectin-3 crystals monitored by X-ray crystallography: Possible implications for ligand-exchange protocols. Acta Crystallogr. D Biol. Crystallogr. 2007, 63, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Saraboji, K.; Hakansson, M.; Genheden, S.; Diehl, C.; Qvist, J.; Weininger, U.; Nilsson, U.J.; Leffler, H.; Ryde, U.; Akke, M.; et al. The carbohydrate-binding site in galectin-3 is preorganized to recognize a sugarlike framework of oxygens: Ultra-high-resolution structures and water dynamics. Biochemistry 2012, 51, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Zhang, T.; Wang, P.; Liu, F.; Tai, G.; Zhou, Y. The water network in galectin-3 ligand binding site guides inhibitor design. Acta Biochim. Biophys. Sin. (Shanghai) 2015, 47, 192–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ippel, H.; Miller, M.C.; Vertesy, S.; Zheng, Y.; Canada, F.J.; Suylen, D.; Umemoto, K.; Romano, C.; Hackeng, T.; Tai, G.; et al. Intra- and intermolecular interactions of human galectin-3: Assessment by full-assignment-based NMR. Glycobiology 2016, 26, 888–903. [Google Scholar] [CrossRef] [PubMed]
- Flores-Ibarra, A.; Vertesy, S.; Medrano, F.J.; Gabius, H.J.; Romero, A. Crystallization of a human galectin-3 variant with two ordered segments in the shortened N-terminal tail. Sci. Rep. 2018, 8, 9835. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lucendo, M.F.; Solis, D.; Andre, S.; Hirabayashi, J.; Kasai, K.; Kaltner, H.; Gabius, H.J.; Romero, A. Growth-regulatory human galectin-1: Crystallographic characterisation of the structural changes induced by single-site mutations and their impact on the thermodynamics of ligand binding. J. Mol. Biol. 2004, 343, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Feng, S.; Gao, J.; Wang, Y.; Zhang, Z.; Meng, Y.; Zhou, Y.; Tai, G.; Su, J. Human galectin-2 interacts with carbohydrates and peptides non-classically: New insight from X-ray crystallography and hemagglutination. Acta Biochim. Biophys. Sin. (Shanghai) 2016, 48, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Leonidas, D.D.; Vatzaki, E.H.; Vorum, H.; Celis, J.E.; Madsen, P.; Acharya, K.R. Structural basis for the recognition of carbohydrates by human galectin-7. J. Biochem. 1998, 37, 13930–13940. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Wang, Y.; Si, Y.; Gao, J.; Song, C.; Cui, L.; Wu, R.; Tai, G.; Zhou, Y. Galectin-13, a different prototype galectin, does not bind beta-galacto-sides and forms dimers via intermolecular disulfide bridges between Cys-136 and Cys-138. Sci. Rep. 2018, 8, 980. [Google Scholar] [CrossRef] [PubMed]
- Nesmelova, I.V.; Ermakova, E.; Daragan, V.A.; Pang, M.; Menendez, M.; Lagartera, L.; Solis, D.; Baum, L.G.; Mayo, K.H. Lactose binding to galectin-1 modulates structural dynamics, increases conformational entropy, and occurs with apparent negative cooperativity. J. Mol. Biol. 2010, 397, 1209–1230. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Ren, C.H.; Wada, M.; Kawanami, T. Galectin-1 as a potential therapeutic agent for amyotrophic lateral sclerosis. Curr. Drug Targets 2005, 6, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Camby, I.; Le Mercier, M.; Lefranc, F.; Kiss, R. Galectin-1: A small protein with major functions. Glycobiology 2006, 16, 137R–157R. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Cummings, R.D. Galectin-1, a beta-galactoside-binding lectin in Chinese hamster ovary cells. II. Localization and biosynthesis. J. Biol. Chem. 1995, 270, 5207–5212. [Google Scholar] [CrossRef] [PubMed]
- Giudicelli, V.; Lutomski, D.; Levi-Strauss, M.; Bladier, D.; Joubert-Caron, R.; Caron, M. Is human galectin-1 activity modulated by monomer/dimer equilibrium? Glycobiology 1997, 7, viii-x. [Google Scholar] [CrossRef] [PubMed]
- Dias-Baruffi, M.; Zhu, H.; Cho, M.; Karmakar, S.; McEver, R.P.; Cummings, R.D. Dimeric galectin-1 induces surface exposure of phosphatidylserine and phagocytic recognition of leukocytes without inducing apoptosis. J. Biol. Chem. 2003, 278, 41282–41293. [Google Scholar] [CrossRef] [PubMed]
- Horie, H.; Inagaki, Y.; Sohma, Y.; Nozawa, R.; Okawa, K.; Hasegawa, M.; Muramatsu, N.; Kawano, H.; Horie, M.; Koyama, H.; et al. Galectin-1 regulates initial axonal growth in peripheral nerves after axotomy. J. Neurosci. 1999, 19, 9964–9974. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, Y.; Sohma, Y.; Horie, H.; Nozawa, R.; Kadoya, T. Oxidized galectin-1 promotes axonal regeneration in peripheral nerves but does not possess lectin properties. Eur. J. Biochem. 2000, 267, 2955–2964. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Cummings, R.D. Galectin-1, a beta-galactoside-binding lectin in Chinese hamster ovary cells. I. Physical and chemical characterization. J. Biol. Chem. 1995, 270, 5198–5206. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Tenen, D.G.; Dvorak, A.M.; Ackerman, S.J. The gene for human eosinophil Charcot-Leyden crystal protein directs expression of lysophospholipase activity and spontaneous crystallization in transiently transfected COS cells. J. Leukoc. Biol. 1992, 52, 588–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, S.K.; Wong, K.F.; Chan, J.K.; Kwong, Y.L. Refractory cytopenia with t(1;7),+8 abnormality and dysplastic eosinophils showing intranuclear Charcot-Leyden crystals: A fluorescence in situ hybridization study. Br. J. Haematol. 1995, 90, 216–218. [Google Scholar] [CrossRef] [PubMed]
- Del Blanco, J.; Valverde, J.; Mateo, L.; Juanola, X.; Pons, M.; Ferrer, J. Charcot Leyden crystals in synovial fluid. J. Rheumatol. 1991, 18, 1944. [Google Scholar] [PubMed]
- Staribratova, D.; Belovejdov, V.; Staikov, D.; Dikov, D. Demonstration of Charcot-Leyden crystals in eosinophilic cystitis. Arch. Pathol. Lab. Med. 2010, 134, 1420. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, S.J.; Loegering, D.A.; Gleich, G.J. The human eosinophil Charcot-Leyden crystal protein: Biochemical characteristics and measurement by radioimmunoassay. J. Immunol. 1980, 125, 2118–2126. [Google Scholar] [PubMed]
- Ackerman, S.J.; Weil, G.J.; Gleich, G.J. Formation of Charcot-Leyden crystals by human basophils. J. Exp. Med. 1982, 155, 1597–1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dvorak, A.M.; Letourneau, L.; Login, G.R.; Weller, P.F.; Ackerman, S.J. Ultrastructural localization of the Charcot-Leyden crystal protein (lysophospholipase) to a distinct crystalloid-free granule population in mature human eosinophils. Blood 1988, 72, 150–158. [Google Scholar] [PubMed]
- Arora, V.K.; Singh, N.; Bhatia, A. Charcot-Leyden crystals in fine needle aspiration cytology. Acta Cytol. 1997, 41, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Van de Kerkhof, D.; Scharnhorst, V.; Huysentruyt, C.J.; Brands-Nijenhuis, A.V.; Ermens, A.A. Charcot-Leyden crystals in acute myeloid leukemia. Int. J. Lab. Hematol. 2015, 37, e100–e102. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.K.; Ueda, M.; Nishino, T.; Muramatsu, M.; Moritani, S.; Shigematsu, T.; Kohno, Y.; Kaneko, C.; Kushima, R. Eosinophilic ascites. Cytopathology 2003, 14, 84–86. [Google Scholar] [CrossRef] [PubMed]
- Dincsoy, H.P.; Burton, T.J.; van der Bel-Kahn, J.M. Circulating Charcot-Leyden crystals in the hypereosinophilic syndrome. Am. J. Clin. Pathol. 1981, 75, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Lao, L.M.; Kumakiri, M.; Nakagawa, K.; Ishida, H.; Ishiguro, K.; Yanagihara, M.; Ueda, K. The ultrastructural findings of Charcot-Leyden crystals in stroma of mastocytoma. J. Dermatol. Sci. 1998, 17, 198–204. [Google Scholar] [CrossRef]
- Strauchen, J.A.; Gordon, R.E. Crystalline inclusions in granulocytic sarcoma. Arch. Pathol. Lab. Med. 2002, 126, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Lyall, H.; O’Connor, S.; Clark, D. Charcot-Leyden crystals in the trephine biopsy of a patient with a FIP1L1-PDGFRA-positive myeloproliferative disorder. Br. J. Haematol. 2007, 138, 405. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.V.; Mousavi, A.; Karimi, M.; Bedayat, G.R. Fine needle aspiration of Langerhans cell histiocytosis of the lymph nodes. A report of six cases. Acta Cytol. 2002, 46, 753–756. [Google Scholar] [CrossRef] [PubMed]
- Kuk, J.S.; Maceachern, J.A.; Soamboonsrup, P.; McFarlane, A.; Benger, A.; Patterson, W.; Yang, L.; Trus, M.R. Chronic eosinophilic leukemia presenting with autoimmune hemolytic anemia and erythrophagocytosis by eosinophils. Am. J. Hematol. 2006, 81, 458–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomolin, H.I.; Yamaguchi, Y.; Paulpillai, A.V.; Dvorak, L.A.; Ackerman, S.J.; Tenen, D.G. Human eosinophil Charcot-Leyden crystal protein: Cloning and characterization of a lysophospholipase gene promoter. Blood 1993, 82, 1868–1874. [Google Scholar] [PubMed]
- Dyer, K.D.; Rosenberg, H.F. Transcriptional regulation of galectin-10 (eosinophil Charcot-Leyden crystal protein): A GC box (-44 to -50) controls butyric acid induction of gene expression. Life Sci. 2001, 69, 201–212. [Google Scholar] [CrossRef]
- Foell, J.L.; Volkmer, I.; Giersberg, C.; Kornhuber, M.; Horneff, G.; Staege, M.S. Loss of detectability of Charcot-Leyden crystal protein transcripts in blood cells after treatment with dimethyl sulfoxide. J. Immunol. Methods 2008, 339, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Dyer, K.D.; Rosenberg, H.F. Shared features of transcription: Mutational analysis of the eosinophil/basophil Charcot-Leyden crystal protein gene promoter. J. Leukoc. Biol. 2000, 67, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Bryborn, M.; Hallden, C.; Sall, T.; Cardell, L.O. CLC- a novel susceptibility gene for allergic rhinitis? Allergy 2010, 65, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Kubach, J.; Lutter, P.; Bopp, T.; Stoll, S.; Becker, C.; Huter, E.; Richter, C.; Weingarten, P.; Warger, T.; Knop, J.; et al. Human CD4+CD25+ regulatory T cells: Proteome analysis identifies galectin-10 as a novel marker essential for their anergy and suppressive function. Blood 2007, 110, 1550–1558. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.; Jin, S.; Park, C.O.; Lee, Y.S.; Lee, N.; Lee, J.; Shin, J.U.; Kim, S.H.; Yun, K.N.; Kim, J.Y.; et al. Elevated Galectin-10 Expression of IL-22 Producing T Cells in Atopic Dermatitis Patients. J. Investig. Dermatol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Lingblom, C.; Andersson, J.; Andersson, K.; Wenneras, C. Regulatory Eosinophils Suppress T Cells Partly through Galectin-10. J. Immunol. 2017, 198, 4672–4681. [Google Scholar] [CrossRef] [PubMed]
- Calafat, J.; Janssen, H.; Knol, E.F.; Weller, P.F.; Egesten, A. Ultrastructural localization of Charcot-Leyden crystal protein in human eosinophils and basophils. Eur. J. Haematol. 1997, 58, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, A.M.; Furitsu, T.; Letourneau, L.; Ishizaka, T.; Ackerman, S.J. Mature eosinophils stimulated to develop in human cord blood mononuclear cell cultures supplemented with recombinant human interleukin-5. Part I. Piecemeal degranulation of specific granules and distribution of Charcot-Leyden crystal protein. Am. J. Pathol. 1991, 138, 69–82. [Google Scholar] [PubMed]
- Gleich, G.J.; Loegering, D.A.; Adolphson, C.R. Eosinophils and bronchial inflammation. Chest 1985, 87, 10S–13S. [Google Scholar] [CrossRef] [PubMed]
- Khalife, J.; Capron, M.; Cesbron, J.Y.; Tai, P.C.; Taelman, H.; Prin, L.; Capron, A. Role of specific IgE antibodies in peroxidase (EPO) release from human eosinophils. J. Immunol. 1986, 137, 1659–1664. [Google Scholar] [PubMed]
- Tomassini, M.; Tsicopoulos, A.; Tai, P.C.; Gruart, V.; Tonnel, A.B.; Prin, L.; Capron, A.; Capron, M. Release of granule proteins by eosinophils from allergic and nonallergic patients with eosinophilia on immunoglobulin-dependent activation. J. Allergy Clin. Immunol. 1991, 88, 365–375. [Google Scholar] [CrossRef]
- Egesten, A.; Calafat, J.; Weller, P.F.; Knol, E.F.; Janssen, H.; Walz, T.M.; Olsson, I. Localization of granule proteins in human eosinophil bone marrow progenitors. Int. Arch. Allergy Immunol. 1997, 114, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, A.M.; MacGlashan, D.W., Jr.; Warner, J.A.; Letourneau, L.; Morgan, E.S.; Lichtenstein, L.M.; Ackerman, S.J. Vesicular transport of Charcot-Leyden crystal protein in f-Met peptide-stimulated human basophils. Int. Arch. Allergy Immunol. 1997, 113, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, A.M.; MacGlashan, D.W.; Warner, J.A.; Letourneau, L.; Morgan, E.S.; Lichtenstein, L.M.; Ackerman, S.J. Localization of Charcot-Leyden crystal protein in individual morphological phenotypes of human basophils stimulated by f-Met peptide. Clin. Exp. Allergy 1997, 27, 452–474. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, A.M.; Ishizaka, T.; Letourneau, L.; Albee, E.A.; Mitsui, H.; Ackerman, S.J. Charcot-Leyden crystal protein distribution in basophils and its absence in mast cells that differentiate from human umbilical cord blood precursor cells cultured in murine fibroblast culture supernatants or in recombinant human c-kit ligand. J. Histochem. Cytochem. 1994, 42, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, A.M. Immunogold ultrastructural techniques identify subcellular sites of chymase, Charcot-Leyden crystal protein, and histamine in basophils and mast cells. Chem. Immunol. Allergy 2005, 85, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Vermeersch, P.; Zachee, P.; Brusselmans, C. Acute myeloid leukemia with bone marrow necrosis and Charcot Leyden crystals. Am. J. Hematol. 2007, 82, 1029. [Google Scholar] [CrossRef] [PubMed]
- Khrizman, P.; Altman, J.K.; Mohtashamian, A.; Peterson, L.; Chen, Y.H.; Tallman, M.S. Charcot-Leyden crystals associated with acute myeloid leukemia: Case report and literature review. Leuk. Res. 2010, 34, e336–e338. [Google Scholar] [CrossRef] [PubMed]
- Radujkovic, A.; Bellos, F.; Andrulis, M.; Ho, A.D.; Hundemer, M. Charcot-Leyden crystals and bone marrow necrosis in acute myeloid leukemia. Eur. J. Haematol. 2011, 86, 451–452. [Google Scholar] [CrossRef] [PubMed]
- Manny, J.S.; Ellis, L.R. Acute myeloid leukemia with Charcot-Leyden crystals. Blood 2012, 120, 503. [Google Scholar] [CrossRef] [PubMed]
- Lowe, D.; Jorizzo, J.; Hutt, M.S. Tumour-associated eosinophilia: A review. J. Clin. Pathol. 1981, 34, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Charatcharoenwitthaya, P.; Apisarnthanarak, P.; Pongpaibul, A.; Boonyaarunnate, T. Eosinophilic pseudotumour of the liver. Liver Int. 2012, 32, 311. [Google Scholar] [CrossRef] [PubMed]
- Reichman, H.; Karo-Atar, D.; Munitz, A. Emerging Roles for Eosinophils in the Tumor Microenvironment. Trends Cancer 2016, 2, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Ravin, K.A.; Loy, M. The Eosinophil in Infection. Clin. Rev. Allergy Immunol. 2016, 50, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Klion, A.D.; Nutman, T.B. The role of eosinophils in host defense against helminth parasites. J. Allergy Clin. Immunol. 2004, 113, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Thakral, D.; Agarwal, P.; Saran, R.K.; Saluja, S. Significance of Charcot Leyden crystals in liver cytology-A case report. Diagn. Cytopathol. 2015, 43, 392–394. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, R.T.; Neves, J.S. Eosinophils in fungal diseases: An overview. J. Leukoc. Biol. 2018, 104, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Correll, D.P.; Luzi, S.A.; Nelson, B.L. Allergic Fungal Sinusitis. Head Neck Pathol. 2015, 9, 488–491. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Tutumi, T.; Watanabe, K. Middle ear effusion and fungi. Ann. Otol. Rhinol. Laryngol. 2012, 121, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Kay, A.B. The eosinophil in infection diseases. J. Infect. Dis. 1974, 129, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Weller, P.F.; Goetzl, E.J. The human eosinophil: Roles in host defense and tissue injury. Am. J. Pathol. 1980, 100, 791–820. [Google Scholar] [PubMed]
- Fjaerli, H.O.; Bukholm, G.; Krog, A.; Skjaeret, C.; Holden, M.; Nakstad, B. Whole blood gene expression in infants with respiratory syncytial virus bronchiolitis. BMC Infect. Dis. 2006, 6, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, P.; Furuta, G.T. Eosinophils in Gastrointestinal Disorders: Eosinophilic Gastrointestinal Diseases, Celiac Disease, Inflammatory Bowel Diseases, and Parasitic Infections. Immunol. Allergy Clin. N. Am. 2015, 35, 413–437. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.F.; Torpier, G.; Janin, A.; Klein, O.; Cortot, A.; Capron, M. Activated eosinophils in adult coeliac disease: Evidence for a local release of major basic protein. Gut 1992, 33, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- De Re, V.; Simula, M.P.; Caggiari, L.; Orzes, N.; Spina, M.; Da ponte, A.; De Appollonia, L.; Dolcetti, R.; Canzonieri, V.; Cannizzaro, R. Proteins specifically hyperexpressed in a coeliac disease patient with aberrant T cells. Clin. Exp. Immunol. 2007, 148, 402–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Re, V.; Simula, M.P.; Cannizzaro, R.; Pavan, A.; De Zorzi, M.A.; Toffoli, G.; Canzonieri, V. Galectin-10, eosinophils, and celiac disease. Ann. N. Y. Acad. Sci. 2009, 1173, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Busse, W.W.; Sedgwick, J.B. Eosinophils in asthma. Ann. Allergy 1992, 68, 286–290. [Google Scholar] [PubMed]
- Welsh, R.A. The genesis of the Charcot-Leyden crystal in the eosinophilic leukocyte of man. Am. J. Pathol. 1959, 35, 1091–1103. [Google Scholar] [PubMed]
- Dor, P.J.; Ackerman, S.J.; Gleich, G.J. Charcot-Leyden crystal protein and eosinophil granule major basic protein in sputum of patients with respiratory diseases. Am. Rev. Respir Dis. 1984, 130, 1072–1077. [Google Scholar] [CrossRef]
- Fulkerson, P.C.; Rothenberg, M.E. Targeting eosinophils in allergy, inflammation and beyond. Nat. Rev. Drug Discov. 2013, 12, 117–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udell, I.J.; Gleich, G.J.; Allansmith, M.R.; Ackerman, S.J.; Abelson, M.B. Eosinophil granule major basic protein and Charcot-Leyden crystal protein in human tears. Am. J. Ophthalmol 1981, 92, 824–828. [Google Scholar] [CrossRef]
- Hirszel, P.; Cashell, A.W.; Whelan, T.V.; Dolan, R.; Yoshihashi, A. Urinary Charcot-Leyden crystals in the hypereosinophilic syndrome with acute renal failure. Am. J. Kidney Dis. 1988, 12, 319–322. [Google Scholar] [CrossRef]
- Kaur, A.C.; Paksoy, N.; Kara, M. Charcot-Leyden crystals in cervical smear. Diagn. Cytopathol. 1999, 21, 433–434. [Google Scholar] [CrossRef]
- Ayres, W.W.; Silliphant, W.M. Charcot-Leyden crystals in eosinophilic granuloma of bone. Am. J. Clin. Pathol. 1958, 30, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Silver, G.K.; Simon, J.H. Charcot-Leyden crystals within a periapical lesion. J. Endod. 2000, 26, 679–681. [Google Scholar] [CrossRef] [PubMed]
- Kanitakis, J. Charcot-Leyden crystals in pemphigus vegetans. J. Cutan. Pathol. 1987, 14, 127. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, J. A Brief History of Charcot-Leyden Crystal Protein/Galectin-10 Research. Molecules 2018, 23, 2931. https://doi.org/10.3390/molecules23112931
Su J. A Brief History of Charcot-Leyden Crystal Protein/Galectin-10 Research. Molecules. 2018; 23(11):2931. https://doi.org/10.3390/molecules23112931
Chicago/Turabian StyleSu, Jiyong. 2018. "A Brief History of Charcot-Leyden Crystal Protein/Galectin-10 Research" Molecules 23, no. 11: 2931. https://doi.org/10.3390/molecules23112931
APA StyleSu, J. (2018). A Brief History of Charcot-Leyden Crystal Protein/Galectin-10 Research. Molecules, 23(11), 2931. https://doi.org/10.3390/molecules23112931




