miR-103/miR-195/miR-15b Regulate SALL4 and Inhibit Proliferation and Migration in Glioma
Abstract
:1. Introduction
2. Results
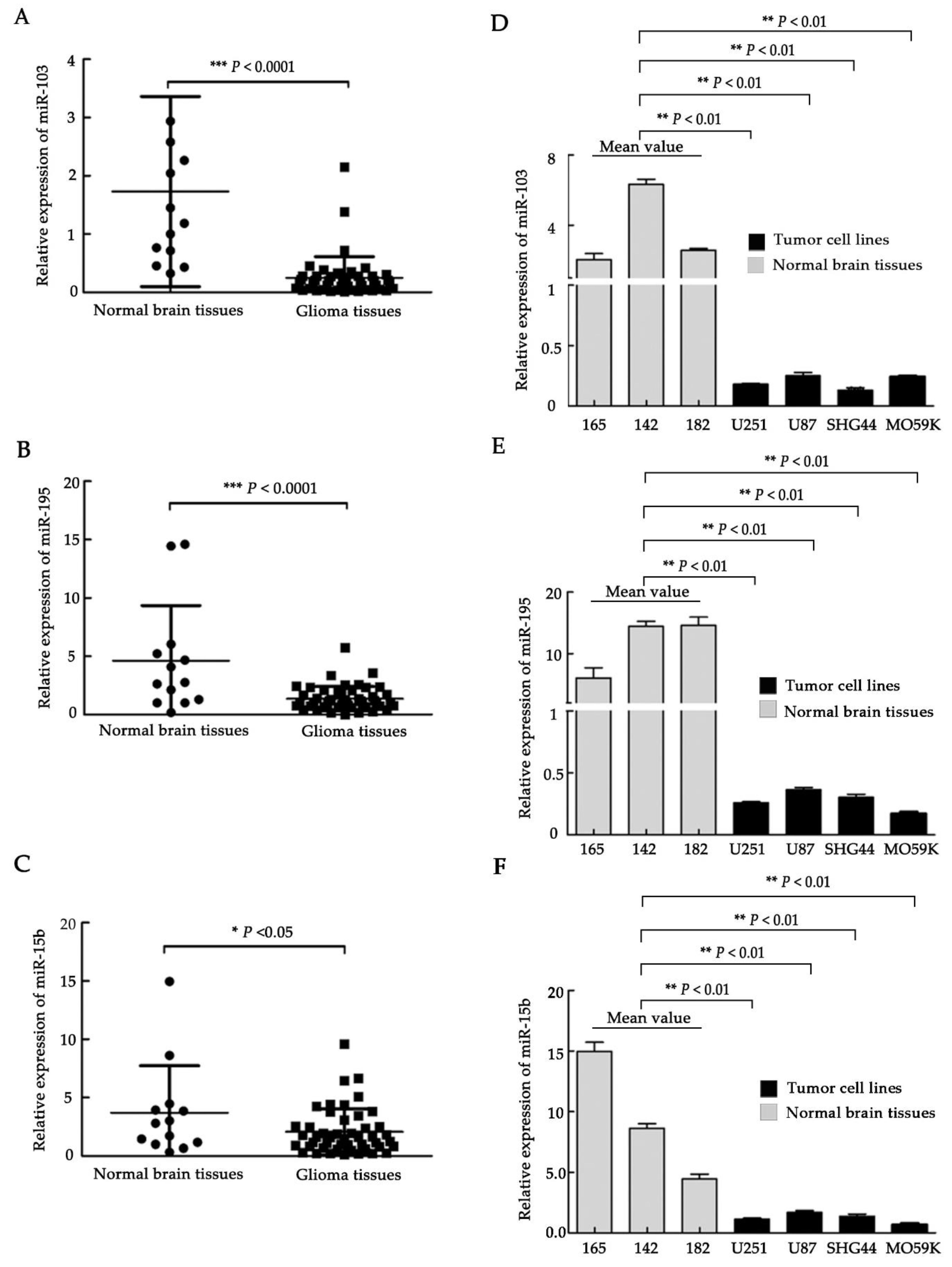
2.1. Downregulation of miR-103, miR-195, and miR-15b in Glioma Clinical Samples and Glioma Cell Lines
2.2. miR-103, miR-195, and miR-15b Impede Cell Proliferation via Apoptosis
2.3. miR-103, miR-195, and miR-15b Suppress Cell Migration and Invasion
2.4. miR-103, miR-195, and miR-15b Directly Target SALL4 3′UTR
2.5. The Correlation between miR-103, miR-195, or miR-15b Expression, and SALL4 Level in Glioma Patients
2.6. SALL4 Overexpression Promotes Cell Proliferation, Migration,and Invasion
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Clinical Tissue Samples
4.3. Cell Culture
4.4. Oligonucleotides and Cell Transfection
4.5. Lentiviral Construct Transduction
4.6. Real-Time Quantitative Reverse-Transcription (RT)-PCR
4.7. Western Blot
4.8. Hematoxylin and Eosin (H&E) Staining
4.9. Cell Proliferation Assays
4.10. Colony Formation Assays
4.11. Transwell Migration and Invasion Assays
4.12. Wound Healing Assay
4.13. Apoptosis Assay
4.14. Luciferase Reporter Assay
4.15. MiRNA Target Site Prediction
4.16. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Adamson, C.; Kanu, O.O.; Mehta, A.I.; Di, C.; Lin, N.; Mattox, A.K.; Bigner, D.D. Glioblastoma multiforme: a review of where we have been and where we are going. Expert Opin. Investig. Drugs 2009, 18, 1061–1083. [Google Scholar] [CrossRef] [PubMed]
- Al-Baradie, R.; Yamada, K.; St Hilaire, C.; Chan, W.M.; Andrews, C.; McIntosh, N.; Nakano, M.; Martonyi, E.J.; Raymond, W.R.; Okumura, S.; et al. Duane radial ray syndrome (Okihiro syndrome) maps to 20q13 and results from mutations in SALL4, a new member of the SAL family. Am. J. Hum. Genet. 2002, 71, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- deCelis, J.F.; Barrio, R. Regulation and function of Spalt proteins during animal development. Int. J. Dev. Biol. 2009, 53, 1385–1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Tam, W.L.; Tong, G.Q.; Wu, Q.; Chan, H.Y.; Soh, B.S.; Lou, Y.; Yang, J.; Ma, Y.; Chai, L.; et al. SALL4 modulates embryonic stem cell pluripotency and early embryonic development by the transcriptional regulation of Pou5f1. Nat. Cell Biol. 2006, 8, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Aguila, J.R.; Liao, W.; Yang, J.; Avila, C.; Hagag, N.; Senzel, L.; Ma, Y. SALL4 is a robust stimulator for the expansion of hematopoietic stem cells. Blood 2011, 118, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Ueno, S.; Lu, J.; He, J.; Li, A.; Zhang, X.X.; Ritz, J.; Silberstein, L.E.; Chai, L. Aberrant expression of SALL4 in acute B cell lymphoblastic leukemia: Mechanism, function, and implication for a potential novel therapeutic target. Exp. Hematol. 2014, 42, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, D.; Kuribayashi, K.; Tanaka, M.; Watanabe, N. Overexpression of SALL4 in lung cancer and its importance in cell proliferation. Oncol. Rep. 2011, 26, 965–970. [Google Scholar] [PubMed]
- Yong, K.J.; Chai, L.; Tenen, D.G. Oncofetal gene SALL4 in aggressive hepatocellular carcinoma. N. Engl. J. Med. 2013, 369, 1170–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Xu, Z.; Xu, X.; Zhang, B.; Wu, H.; Wang, M.; Zhang, X.; Yang, T.; Cai, J.; Yan, Y.; et al. SALL4, a novel marker for human gastric carcinogenesis and metastasis. Oncogene 2014, 33, 5491–9500. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Jiao, Y.; Yong, K.J.; Wang, F.; Gao, C.; Yan, B.; Srivastava, S.; Lim, G.S.; Tang, P.; Yang, H.; et al. SALL4 is a new target in endometrial cancer. Oncogene 2015, 34, 63–72. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, W.; Zhou, Q.; Zhao, T.; Song, Y.; Chai, L.; Li, Y. Low-expression of microRNA-107 inhibits cell apoptosis in glioma by upregulation of SALL4. Int. J. Biochem. Cell B 2013, 45, 1962–1973. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Deng, R.; Zhang, P.; Wu, C.; Wu, K.; Shi, L.; Liu, X.; Bai, J.; Deng, M.; Shuai, X.; et al. miR-219-5p plays a tumor suppressive role in colon cancer by targeting oncogene SALL4. Oncol. Rep. 2015, 34, 1923–1932. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zou, B.; Liu, L.; Cui, K.; Gao, J.; Yuan, S.; Cong, N. MicroRNA-98 acts as a tumor suppressor in hepatocellular carcinoma via targeting SALL4. Oncotarget 2016, 7, 74059–74073. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.G.; Xiao, Y.; Wu, Y.T.; Liu, Y.; Song, Z.Q.; Gao, W.F.; Zhang, J.; Yang, J.L.; Zhang, Y.G.; Guo, T.K.; et al. microRNA-33b suppresses the proliferation and metastasis of hepatocellular carcinoma cells through the inhibition of Sal-like protein 4 expression. Int. J. Mol. Med. 2016, 38, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Li, M.; Ji, F.; Nie, Y. MicroRNA-219 exerts a tumor suppressive role in glioma via targeting Sal-like protein 4. Exp. Ther. Med. 2017, 14, 6213–6221. [Google Scholar] [CrossRef] [PubMed]
- Filipowicz, W.; Bhattacharyya, S.N.; Sonenberg, N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight. Nat. Rev. Genet. 2008, 9, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Esquelakerscher, A.; Slack, F.J. Oncomirs—MicroRNAs with a role in cancer. Nat. Rev. Cancer 2006, 6, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Negrini, M.; Nicoloso, M.S.; Calin, G.A. MicroRNAs and cancer—New paradigms in molecular oncology. Curr. Opin. Cell Biol. 2009, 21, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zhang, W.; Su, Y.; Lu, L.; Wang, D.; Wang, H. MicroRNA-103 suppresses tumor cell proliferation by targeting PDCD10 in prostate cancer. Prostate 2016, 76, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Kfir-Erenfeld, S.; Haggiag, N.; Biton, M.; Stepensky, P.; Assayag-Asherie, N.; Yefenof, E. MiR-103 inhibits proliferation and sensitizes hemopoietic tumor cells for glucocorticoid-induced apoptosis. Oncotarget 2017, 8, 472–489. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.B.; Xiao, K.; Xiao, G.C.; Tong, S.L.; Ding, Y.; Wang, Q.S.; Li, S.B.; Hao, Z.N. MicroRNA-103 promotes tumor growth and metastasis in colorectal cancer by directly targeting. Oncol. Lett. 2016, 12, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.Q.; Zhou, H.J.; Xun, Q.Y.; Xu, X.F.; Ling, J.X.; Hu, Y.L. MicroRNA-103 regulates the growth and invasion of endometrial cancer cells through the downregulation of tissue inhibitor of metalloproteinase 3. Oncol. Lett. 2012, 3, 1221–1226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Q.; Xu, H.; Huang, M.B.; Ma, L.M.; Huang, Q.J.; Yao, Q.; Zhou, H.; Qu, L.H. MicroRNA-195 plays a tumor-suppressor role in human glioblastoma cells by targeting signaling pathways involved in cellular proliferation and invasion. Neuro-Oncol. 2012, 14, 278–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moser, J.J.; Fritzler, M.J. The MicroRNA and Messenger RNA Profile of the RNA-Induced Silencing Complex in Human Primary Astrocyte and Astrocytoma Cells. PLoS ONE 2010, 5, e13445. [Google Scholar] [CrossRef] [PubMed]
- MacLean, J.A.; King, M.L.; Okuda, H.; Hayashi, K. WNT7A Regulation by miR-15b in Ovarian Cancer. PLoS ONE 2016, 11, e156109. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Sheng, L.; Zhang, H.J.; Ji, M.; Qian, W.Q. MiR-15b-5p facilitates the tumorigenicity by targeting RECK and predicts tumour recurrence in prostate cancer. J. Cell. Mol. Med. 2018, 22, 1855–1863. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chai, L.; Gao, C.; Fowles, T.C.; Alipio, Z.; Dang, H.; Xu, D.; Fink, L.M.; Ward, D.C.; Ma, Y. SALL4 is a key regulator of survival and apoptosis in human leukemic cells. Blood 2008, 112, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Ni, L.; Liu, B.; Wei, Y.; Lv, Y.; Qiang, S.; Dong, J.; Liu, X. Upregulation of SALL4 by EGFR activation regulates the stemness of CD44-positive lung cancer. Oncogenesis 2018, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Liang, W.; Gu, J.; Zang, X.; Huang, Z.; Shi, H.; Chen, J.; Fu, M.; Zhang, P.; Xiao, X.; et al. Long noncoding RNA DANCR is activated by SALL4 and promotes the proliferation and invasion of gastric cancer cells. Oncotarget 2018, 9, 1915–1930. [Google Scholar] [CrossRef] [PubMed]
- Böhm, J.; Sustmann, C.; Wilhelm, C.; Kohlhase, J. SALL4 is directly activated by TCF/LEF in the canonical WNT signaling pathway. Biochem. Biophys. Res. Commun. 2006, 348, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Zhou, M.; Chen, X.; Yue, D.; Li, Y.; Qin, G.; Zhen, Z.; Gao, Q.; Dan, W.; Zhang, C. Inhibition of SALL4 reduces tumorigenicity involving epithelial-mesenchymal transition via WNT/β-catenin pathway in esophageal squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2016, 35, 98–110. [Google Scholar]
- Zhou, Y.; Peng, Y.; Liu, M.; Jiang, Y. MicroRNA-181b Inhibits Cellular Proliferation and Invasion of Glioma Cells via Targeting Sal-Like Protein 4. Oncol Res. 2017, 25, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Liu, A.Y.; Fan, C.; Zheng, H.; Li, Y.; Zhang, C.; Wu, S.; Yu, D.; Huang, Z.; Liu, F.; et al. MicroRNA-33b inhibits breast cancer metastasis by targeting HMGA2, SALL4 and Twist1. Sci. Rep. 2015, 5, 9995. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, Y.; Cavazos, D.; Ma, X.; Zhao, Z.; Du, L.; Pertsemlidis, A. MiR-195 targets cyclin D3 and survivin to modulate the tumorigenesis of non-small cell lung cancer. Cell Death Dis. 2018, 9, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.; Kang, X.J.; Sun, Z.Z.; Zeng, M.F.; Yu, S.R.; Ding, Y.; Liang, J.Q.; Li, T.T.; Zhao, J. MiR-497-5p, miR-195-5p and miR-455-3p function as tumor suppressors by targeting hTERT in melanoma A375 cells. Cancer Manag. Res. 2018, 10, 989–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, G.; Shi, L.; Yan, S.; Wan, Z.; Jiang, N.; Fu, L.; Li, M.; Guo, J. MiR-15b targets cyclin D1 to regulate proliferation and apoptosis in glioma cells. Biomed. Res. Int. 2014, 2014, 687826. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, J.; Lee, M.J.; Yu, G.R.; Han, X.; Kim, D.G. OIP5, a target of miR-15b-5p, regulates hepatocellular carcinoma growth and metastasis through the AKT/mTORC1 and β-catenin signaling pathways. Oncotarget 2017, 8, 18129–18144. [Google Scholar] [CrossRef] [PubMed]
- Alberti, C.; Manzenreither, R.A.; Sowemimo, I.; Burkard, T.R.; Wang, J.; Mahofsky, K.; Ameres, S.L.; Cochella, L. Cell-type specific sequencing of microRNAs from complex animal tissues. Nat. Methods 2018, 15, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Liu, Y.; Qiao, Y.; Zhang, L.; Lu, S. MiR-103 Promotes Proliferation and Metastasis by Targeting KLF4 in Gastric Cancer. Int. J. Mol. Sci. 2017, 18, 910. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, H.; Shu, Y.; Li, X. MiR-103 Promotes Neurite Outgrowth and Suppresses Cells Apoptosis by Targeting Prostaglandin-Endoperoxide Synthase 2 in Cellular Models of Alzheimer’s Disease. Front. Cell. Neurosci. 2018, 12, 91. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds miR-103 are available from the authors. |








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.-P.; Zhang, N.-N.; Ren, X.-Q.; He, J.; Li, Y. miR-103/miR-195/miR-15b Regulate SALL4 and Inhibit Proliferation and Migration in Glioma. Molecules 2018, 23, 2938. https://doi.org/10.3390/molecules23112938
Chen L-P, Zhang N-N, Ren X-Q, He J, Li Y. miR-103/miR-195/miR-15b Regulate SALL4 and Inhibit Proliferation and Migration in Glioma. Molecules. 2018; 23(11):2938. https://doi.org/10.3390/molecules23112938
Chicago/Turabian StyleChen, Li-Ping, Na-Na Zhang, Xue-Qing Ren, Jie He, and Yu Li. 2018. "miR-103/miR-195/miR-15b Regulate SALL4 and Inhibit Proliferation and Migration in Glioma" Molecules 23, no. 11: 2938. https://doi.org/10.3390/molecules23112938



