Antitumor Effect of the Essential Oil from the Leaves of Croton matourensis Aubl. (Euphorbiaceae)
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition of the Essential Oil
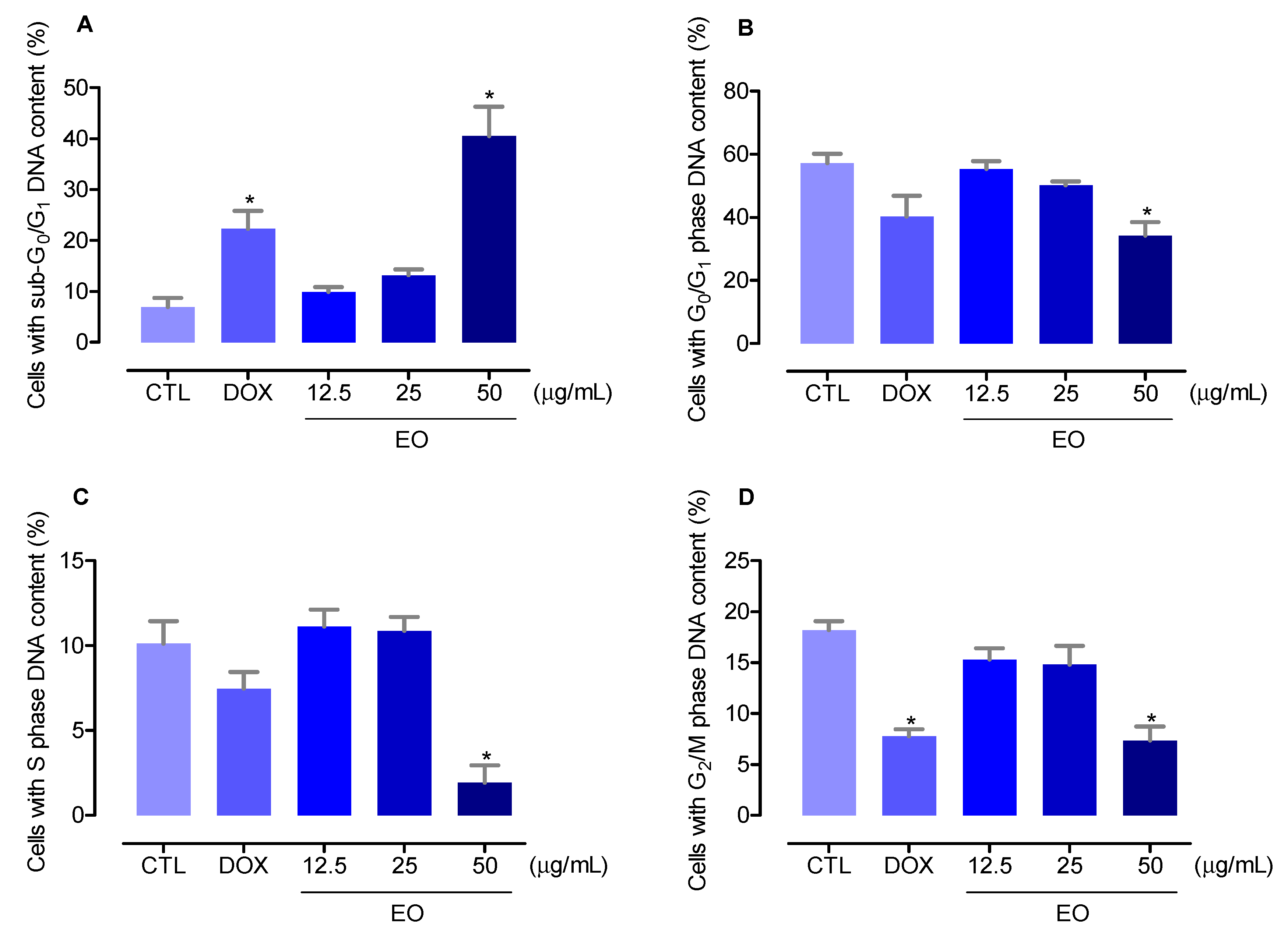
2.2. In Vitro Cytotoxicity
2.3. In Vivo Antitumor Activity
3. Discussion
4. Material and Methods
4.1. Botanical Material
4.2. Essential Oil Extraction
4.3. Chemical Analysis
4.4. In Vitro Assays
4.4.1. Cells
4.4.2. Cell Viability Assay
4.4.3. Annexin-V/PI Staining Assay
4.4.4. Internucleosomal DNA Fragmentation and Cell Cycle Distribution
4.5. In Vivo Assays
4.5.1. Animals
4.5.2. Human Hepatocellular Carcinoma Xenograft Model
4.5.3. Toxicological Evaluation
4.6. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Xu, W.H.; Liu, W.Y.; Liang, Q. Chemical constituents from Croton species and their biological activities. Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Itokawa, H.; Ichihara, Y.; Mochizuki, M.; Enomori, T.; Morita, H.; Shirota, O.; Inamatsu, M.; Takeya, K. A cytotoxic substance from Sangre de Grado. Chem. Pharm. Bull. 1991, 39, 1041–1042. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, M.; Okuhama, N.N.; Clark, M.; Angeles, F.M.; Lao, J.; Bustamante, S.; Miller, M.J. Sangre de grado Croton palanostigma induces apoptosis in human gastrointestinal cancer cells. J. Ethnopharmacol. 2002, 80, 121–129. [Google Scholar] [CrossRef]
- Chen, Z.P.; Cai, Y.; Phillipson, J.D. Studies on the anti-tumour, anti-bacterial, and wound-healing properties of dragon’s blood. Planta Med. 1994, 60, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Kupchan, S.M.; Uchida, I.; Branfman, A.R.; Dailey, R.G.; Fei, B.Y. Antileukemic principles isolated from euphorbiaceae plants. Science 1976, 191, 571–572. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, D.P.; Marinho-Filho, J.D.; Alves, A.P.; Pessoa, C.; Moraes, M.O.; Pessoa, O.D.; Torres, M.C.; Silveira, E.R.; Viana, F.A.; Costa-Lotufo, L.V. Antitumor activity of the essential oil from the leaves of Croton regelianus and its component ascaridole. Chem. Biodivers. 2009, 6, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Castro, A.J.; Ortiz-Sánchez, E.; Domínguez, F.; López-Toledo, G.; Chávez, M.; Ortiz-Tello, A.J.; García-Carrancá, A. Antitumor effect of Croton lechleri Mull. Arg. (Euphorbiaceae). J. Ethnopharmacol. 2012, 140, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Cândido-Bacani, P.M.; Figueiredo, P.O.; Matos, M.F.; Garcez, F.R.; Garcez, W.S. Cytotoxic orbitide from the latex of Croton urucurana. J. Nat. Prod. 2015, 78, 2754–2760. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.L.; Oliveira, M.N.; Silva, A.B.; Pitanga, B.P.; Silva, V.D.; Faria, G.P.; Sampaio, G.P.; Costa, M.F.; Braga-de-Souza, S.; Costa, S.L. The flavonoid apigenin from Croton betulaster Mull inhibits proliferation, induces differentiation and regulates the inflammatory profile of glioma cells. Anticancer Drugs 2016, 27, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Zhao, Y.; Liu, H.; Tang, C.; Zhang, M.; Ke, C.; Ye, Y. Isolation and structure characterization of cytotoxic phorbol esters from the seeds of Croton tiglium. Planta Med. 2017, 83, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.Q.; Tang, S.; Song, W.B.; Wang, W.Q.; Huang, M.; Xuan, L.J. Crassins A-H, diterpenoids from the roots of Croton crassifolius. J. Nat. Prod. 2017, 80, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.R.; Mitja, D. Use of vegetal resources by family farmers in Manacapuru, Amazonas state. Acta Amaz. 2010, 40, 49–58. [Google Scholar] [CrossRef]
- Rodrigues, L. Inventory, Valorization of Forest Resources and Dynamics of Fragmentation in the Region of Influence of Juruena National Park, Mato Grosso. Ph.D. Thesis, Postgraduate Program in Ecology and Conservation, Universidade do Estado de Mato Grosso, Nova Xavantina, Brazil, 2013. (In Portuguese). [Google Scholar]
- Trindade, M.J.S.; Lameira, O.A. Species from the Euphorbiaceae family used for medicinal purposes in Brazil. Rev. Cubana Plan. Medicinales 2014, 19, 292–309. [Google Scholar]
- Cruz-Silva, S.C.B. Biodiversity History and Use in the Rural Black Community Quilombola Buriti Farm, Campo Grande, Mato Grosso Do Sul, Brazil. Ph.D. Thesis, Postgraduate Program in Environment and Regional Development, Universidade Anhanguera, Campo Grande, MS, Brazil, 2016. (In Portuguese). [Google Scholar]
- Gottlieb, O.R.; Koketsu, M.; Magalhães, M.T.; Maia, J.G.S.; Mendes, P.H.; Rocha, A.I.; Silva, M.L.; Wiiberg, V.C. Óleos essenciais da Amazônia VII. Acta Amaz. 1981, 11, 143–148. [Google Scholar] [CrossRef]
- Leão, I.M.S.; Andrade, C.H.S.; Pinheiro, M.L.B.; Rocha, A.F.I.; Machado, M.I.L.; Craveiro, A.A.; Alencar, J.W.; Matos, F.J.A. Essential oil of Croton lanjouwensis Jablonski from brazilian amazonian region. J. Essent. Oil Res. 1998, 10, 643–644. [Google Scholar] [CrossRef]
- Schneider, C.; Breitmaier, E.; Bayma, J.; Franca, L.F.; Kneifel, H.; Krebs, H.C. Maravuic acid, a new seco-labdane diterpene from Croton matourensis. Liebigs Ann. 1995, 709–710. [Google Scholar] [CrossRef]
- Compagnone, R.S.; Chavez, K.; Mateu, E.; Orsini, G.; Arvelo, F.; Suárez, A.I. Composition and cytotoxic activity of essential oils from Croton matourensis and Croton micans from Venezuela. Rec. Nat. Prod. 2010, 4, 101–108. [Google Scholar]
- Silva, T.B.; Costa, C.O.; Galvão, A.F.; Bomfim, L.M.; Rodrigues, A.C.; Mota, M.C.; Dantas, A.A.; Santos, T.R.; Soares, M.B.; Bezerra, D.P. Cytotoxic potential of selected medicinal plants in northeast Brazil. BMC Complement. Altern. Med. 2016, 16, 199. [Google Scholar] [CrossRef] [PubMed]
- Bomfim, L.M.; Menezes, L.R.; Rodrigues, A.C.; Dias, R.B.; Rocha, C.A.; Soares, M.B.; Neto, A.F.; Nascimento, M.P.; Campos, A.F.; Silva, L.C.; et al. Antitumour activity of the microencapsulation of Annona vepretorum essential oil. Basic Clin. Pharmacol. Toxicol. 2016, 118, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.V.; Menezes, L.R.; Rocha, S.L.; Baliza, I.R.; Dias, R.B.; Rocha, C.A.; Soares, M.B.; Bezerra, D.P. Antitumor properties of the leaf essential oil of Zornia brasiliensis. Planta Med. 2015, 81, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Sylvestre, M.; Pichette, A.; Longtin, A.; Nagau, F.; Legault, J. Essential oil analysis and anticancer activity of leaf essential oil of Croton flavens L. from Guadeloupe. J. Ethnopharmacol. 2006, 103, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, P.A.; Zelioli, I.A.M.; Sousa, I.M.O.; Ruiz, A.L.T.G.; Vendramini-Costa, D.B.; Foglio, M.A.; Carvalho, J.E. Chemical composition and antiproliferative activity of Croton campestris A.St.-Hil. essential oil. Nat. Prod. Res. 2017, 9, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, R.P.; Bomfim, D.S.; Carvalho, N.C.; Soares, M.B.; Silva, T.B.; Machado, W.J.; Prata, A.P.; Costa, E.V.; Moraes, V.R.; Nogueira, P.C.; et al. Cytotoxic effect of leaf essential oil of Lippia gracilis Schauer (Verbenaceae). Phytomedicine 2013, 20, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Al-Footy, K.O.; Alarif, W.M.; Zubair, M.S.; Ghandourah, M.A.; Aly, M.M. Antibacterial and cytotoxic properties of isoprenoids from the red sea soft coral, Lobophytum sp. Trop. J. Pharm. Res. 2016, 15, 1431–1438. [Google Scholar] [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-caryophyllene and β-caryophyllene oxide-natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Jacob, J.A.; Loganathachetti, D.S.; Nainangu, P.; Chen, B. β-Elemene: Mechanistic studies on cancer cell interaction and its chemosensitization effect. Front. Pharmacol. 2017, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, R.P.C.; Bomfim, D.S.; Carvalho, N.C.; Soares, M.B.P.; Pinheiro, M.L.B.; Costa, E.V.; Bezerra, D.P. Cytotoxic properties of the leaf essential oils of Guatteria blepharophylla and Guatteria hispida (Annonaceae). Flavour Fragr J. 2014, 29, 228–232. [Google Scholar] [CrossRef]
- Russo, A.; Cardile, V.; Graziano, A.C.E.; Avola, R.; Bruno, M.; Rigano, D. Involvement of Bax and Bcl-2 in induction of apoptosis by essential oils of three Lebanese Salvia species in human prostate cancer cells. Int. J. Mol. Sci. 2018, 19, E292. [Google Scholar] [CrossRef] [PubMed]
- Sajid, A.; Manzoor, Q.; Iqbal, M.; Tyagi, A.K.; Sarfraz, R.A.; Sajid, A. Pinus Roxburghii essential oil anticancer activity and chemical composition evaluation. EXCLI J. 2018, 17, 233–245. [Google Scholar] [PubMed]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Gogal, R.M., Jr.; Walsh, J.E. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: An alternative to [3H]-thymidine incorporation assay. J. Immunol. Methods 1994, 170, 211–224. [Google Scholar] [CrossRef]
- Santos, L.S.; Silva, V.R.; Menezes, L.R.A.; Soares, M.B.P.; Costa, E.V.; Bezerra, D.P. Xylopine induces oxidative stress and causes G2/M phase arrest, triggering caspase-mediated apoptosis by p53-independent pathway in HCT116 cells. Oxid. Med. Cell. Longev. 2017, 2017, 7126872. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.R.; Corrêa, R.S.; Santos, L.S.; Soares, M.B.P.; Batista, A.A.; Bezerra, D.P. A ruthenium-based 5-fluorouracil complex with enhanced cytotoxicity and apoptosis induction action in HCT116 cells. Sci. Rep. 2018, 8, 288. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, I.; Migliorati, G.; Pagliacci, M.C.; Grignani, F.; Riccardi, C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 1991, 139, 271–279. [Google Scholar] [CrossRef]
- Rodrigues, A.C.; Bomfim, L.M.; Neves, S.P.; Menezes, L.R.; Dias, R.B.; Soares, M.B.; Prata, A.P.; Rocha, C.A.; Costa, E.V.; Bezerra, D.P. Antitumor properties of the essential oil from the leaves of Duguetia gardneriana. Planta Med. 2015, 81, 798–803. [Google Scholar]
Sample Availability: Sample of the EO is not available from the authors. |


| Number | Compound | Retention Time (min) | RI Theo. a | RI Exp. b | Proportion Area (%) c |
|---|---|---|---|---|---|
| 1 | α-thujene | 5.28 | 924 | 924 | 1.99 ± 0.21 |
| 2 | α-pinene | 5.48 | 932 | 931 | 3.41 ± 0.34 |
| 3 | sabinene | 6.60 | 969 | 969 | 0.11 ± 0.03 |
| 4 | β-pinene | 6.72 | 974 | 974 | 0.25 ± 0.04 |
| 5 | β-myrcene | 7.11 | 988 | 987 | 0.26 ± 0.04 |
| 6 | α-phellandrene | 7.59 | 1002 | 1002 | 3.90 ± 0.28 |
| 7 | α-terpinene | 8.02 | 1014 | 1014 | 0.54 ± 0.30 |
| 8 | p-cymene | 8.31 | 1020 | 1020 | 5.05 ± 0.49 |
| 9 | limonene | 8.46 | 1024 | 1023 | 1.85 ± 0.18 |
| 10 | eucalyptol | 8.57 | 1026 | 1026 | 0.15 ± 0.02 |
| 11 | β-ocimene | 9.18 | 1032 | 1032 | 0.13 ± 0.02 |
| 12 | γ-terpinene | 9.62 | 1054 | 1054 | 2.02 ± 0.23 |
| 13 | terpinolene | 10.85 | 1086 | 1086 | 2.15 ± 0.18 |
| 14 | linalool | 11.30 | 1095 | 1095 | 3.85 ± 0.34 |
| 15 | 4-terpineol | 14.87 | 1130 | 1130 | 0.25 ± 0.05 |
| 16 | α-terpineol | 15.52 | 1131 | 1131 | 0.21 ± 0.04 |
| 17 | α-copaene | 24.62 | 1374 | 1373 | 1.92 ± 0.15 |
| 18 | α-bourbonene | 25.06 | 1387 | 1387 | 0.36 ± 0.05 |
| 19 | β-elemene | 25.48 | 1389 | 1388 | 4.94 ± 0.35 |
| 20 | β-caryophyllene | 26.74 | 1417 | 1417 | 12.41 ± 1.02 |
| 21 | α-humulene | 28.36 | 1436 | 1436 | 2.52 ± 0.20 |
| 22 | β-farnesene | 28.62 | 1440 | 1440 | 2.33 ± 0.14 |
| 23 | aromadendrane | 28.72 | 1460 | 1559 | 0.64 ± 0.11 |
| 24 | germacrene B | 29.52 | 1480 | 1480 | 1.07 ± 0.29 |
| 25 | α-amorphene | 29.71 | 1483 | 1483 | 0.90 ± 0.13 |
| 26 | α-selinene | 30.37 | 1498 | 1498 | 0.95 ± 0.08 |
| 27 | β-bisabolene | 31.06 | 1505 | 1505 | 0.59 ± 0.05 |
| 28 | γ-cadinene | 31.74 | 1513 | 1513 | 0.99 ± 0.11 |
| 29 | α-elemol | 32.91 | 1548 | 1548 | 0.96 ± 0.09 |
| 30 | spathulenol | 34.18 | 1577 | 1577 | 1.29 ± 0.15 |
| 31 | caryophyllene oxide | 34.41 | 1582 | 1582 | 3.19 ± 0.31 |
| 32 | globulol | 34.81 | 1590 | 1590 | 0.65 ± 0.12 |
| 33 | viridiflorol | 35.33 | 1592 | 1592 | 0.24 ± 0.07 |
| 34 | salvial-4(14)-en-1-one | 35.60 | 1593 | 1593 | 0.34 ± 0.09 |
| 35 | α-cadinol | 37.30 | 1638 | 1638 | 0.38 ± 0.08 |
| 36 | τ-muurolol | 37.41 | 1643 | 1643 | 0.48 ± 0.10 |
| 37 | β-eudesmol | 37.55 | 1649 | 1649 | 0.52 ± 0.13 |
| 38 | α-eudesmol | 37.61 | 1652 | 1652 | 0.48 ± 0.11 |
| 39 | bulnesol | 48.93 | 1670 | 1670 | 4.37 ± 0.48 |
| 40 | cembrene | 53.40 | 1937 | 1938 | 7.12 ± 0.55 |
| 41 | thunbergol | 56.69 | 2061 | 2063 | 11.74 ± 1.11 |
| 42 | geranyllinalool | 56.90 | 2125 | 2125 | 2.30 ± 0.34 |
| Σtotal identified | 90.00% |
| Cell Lines | Origin | Histological Type | IC50 in µg/mL | |
|---|---|---|---|---|
| EO | DOX | |||
| Cancer cells | ||||
| MCF-7 | Human | Breast adenocarcinoma | 23.3 18.2–29.7 | 0.3 0.2–0.4 |
| HCT116 | Human | Colon carcinoma | 28.9 22.1–37.8 | 0.1 0.1–0.2 |
| HepG2 | Human | Hepatocellular carcinoma | 28.5 17.1–37.3 | 0.03 0.01–0.2 |
| HL-60 | Human | Promyelocytic leukemia | 17.8 14.6–21.7 | 0.04 0.02–0.08 |
| Non-cancer cell | ||||
| MRC-5 | Human | Lung fibroblast | 25.8 22.0–30.4 | 0.2 0.1–0.5 |
| Parameters | CTL | 5-FU | EO | |
|---|---|---|---|---|
| Dose (mg/kg/day) | - | 10 | 40 | 80 |
| Initial body weight (g) | 21.4 ± 0.5 | 19.6 ± 0.6 | 22.0 ± 0.5 | 19.9 ± 0.6 |
| Final body weight (g) | 22.1 ± 0.5 | 20.5 ± 0.5 | 21.0 ± 0.3 | 20.9 ± 0.4 |
| Liver (g/100 g body weight) | 4.8 ± 0.2 | 4.8 ± 0.2 | 5.0 ± 0.3 | 5.2 ± 0.3 |
| Kidney (g/100 g body weight) | 1.5 ± 0.1 | 1.5 ± 0.1 | 1.4 ± 0.1 | 1.5 ± 0.1 |
| Heart (g/100 g body weight) | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.5 ± 0.1 |
| Lung (g/100 g body weight) | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.7 ± 0.1 |
| Parameters | CTL | 5-FU | EO | |
|---|---|---|---|---|
| Dose (mg/kg/day) | - | 10 | 40 | 80 |
| Erythrocytes (106/mm3) | 5.2 ± 1.1 | 7.6 ± 0.8 | 4.9 ± 1.2 | 7.6 ± 1.1 |
| Hemoglobin (g/dL) | 21.2 ± 4.8 | 17.7 ± 3.1 | 26.9 ± 0.7 | 17.7 ± 2.2 |
| Hematocrit (%) | 22.0 ± 4.7 | 38.9 ± 0.3 | 9.5 ± 0.3 | 40.1 ± 1.4 |
| MCV (fL) | 43.8 ± 0.4 | 45.0 ± 3.0 | 42.0 ± 0.0 | 45.3 ± 0.5 |
| Platelets (103/mm3) | 247.2 ± 38.5 | 222.1 ± 41.6 | 519.3 ± 136.6 | 279.7 ± 37.2 |
| Leukocytes (103/mm3) | 5.2 ± 0.8 | 2.5 ± 0.6 | 7.1 ± 0.6 | 3.5 ± 0.5 |
| Differential leukocytes (%) | ||||
| Granulocytes | 24.1 | 28.4 | 26.8 | 26.7 |
| Lymphocytes | 41.5 | 46.1 | 45.4 | 51.7 |
| Monocytes | 33.6 | 25.5 | 27.8 | 21.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, E.J.S.P.d.; Alves, R.G.; D´Elia, G.M.A.; Anunciação, T.A.d.; Silva, V.R.; Santos, L.d.S.; Soares, M.B.P.; Cardozo, N.M.D.; Costa, E.V.; Silva, F.M.A.d.; et al. Antitumor Effect of the Essential Oil from the Leaves of Croton matourensis Aubl. (Euphorbiaceae). Molecules 2018, 23, 2974. https://doi.org/10.3390/molecules23112974
Lima EJSPd, Alves RG, D´Elia GMA, Anunciação TAd, Silva VR, Santos LdS, Soares MBP, Cardozo NMD, Costa EV, Silva FMAd, et al. Antitumor Effect of the Essential Oil from the Leaves of Croton matourensis Aubl. (Euphorbiaceae). Molecules. 2018; 23(11):2974. https://doi.org/10.3390/molecules23112974
Chicago/Turabian StyleLima, Emilly J. S. P. de, Rafaela G. Alves, Gigliola M. A. D´Elia, Talita A. da Anunciação, Valdenizia R. Silva, Luciano de S. Santos, Milena B. P. Soares, Nállarett M. D. Cardozo, Emmanoel V. Costa, Felipe M. A. da Silva, and et al. 2018. "Antitumor Effect of the Essential Oil from the Leaves of Croton matourensis Aubl. (Euphorbiaceae)" Molecules 23, no. 11: 2974. https://doi.org/10.3390/molecules23112974
APA StyleLima, E. J. S. P. d., Alves, R. G., D´Elia, G. M. A., Anunciação, T. A. d., Silva, V. R., Santos, L. d. S., Soares, M. B. P., Cardozo, N. M. D., Costa, E. V., Silva, F. M. A. d., Koolen, H. H. F., & Bezerra, D. P. (2018). Antitumor Effect of the Essential Oil from the Leaves of Croton matourensis Aubl. (Euphorbiaceae). Molecules, 23(11), 2974. https://doi.org/10.3390/molecules23112974







