Development of New Analytical Microwave-Assisted Extraction Methods for Bioactive Compounds from Myrtle (Myrtus communis L.)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Development of the MAE Methods
2.2. Optimal Conditions
2.3. Kinetics of the Extraction Process
2.4. Precision of MAE Methods
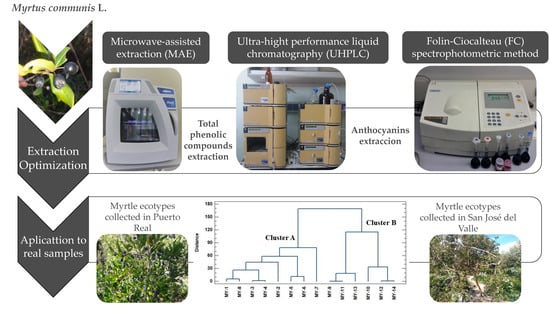
2.5. Application to Real Sample
2.5.1. Study of Myrtle Berries from Different Locations
2.5.2. Multivariate Statistical Analysis
3. Material and Methods
3.1. Myrtle Sample
3.2. Chemicals and Solvents
3.3. Microwave-Assisted Extraction Procedure
3.4. Identification of Anthocyanins
3.5. Determination of Anthocyanins
3.6. Determination of Total Phenolic Compounds
3.7. Optimization Procedure and Data Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bouzabata, A.; Casanova, J.; Bighelli, A.; Cavaleiro, C.; Salgueiro, L.; Tomi, F. The genus Myrtus L. in Algeria: Composition and biological aspects of essential oils from M. communis and M. nivellei: A review. Chem. Biodivers. 2016, 13, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Melito, S.; La Bella, S.; Martinelli, F.; Cammalleri, I.; Tuttolomondo, T.; Leto, C.; Fadda, A.; Molinu, M.G.; Mulas, M. Morphological, chemical, and genetic diversity of wild myrtle (Myrtus communis L.) populations in Sicily. Turk. J. Agric. For. 2016, 40, 249–261. [Google Scholar] [CrossRef]
- Al-Yafeai, A.; Bellstedt, P.; Böhm, V. Bioactive compounds and antioxidant capacity of rosa rugosa depending on degree of ripeness. Antioxidants 2018, 7, 134. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Fu, L.; Du, B.; Chen, B.; Wang, F.; Wang, M. Isolation and characterization of new phenolic compounds with estrogen biosynthesis-inhibiting and antioxidation activities from Broussonetia papyriferia leaves. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Wannes, A.; Mhamdi, B.; Sriti, J.; Ben Jemia, M.; Ouchikh, O.; Hamdaoui, G.; Kchouk, M.E.; Marzouk, B. Antioxidant activities of the essential oils and methanol extracts from myrtle (Myrtus communis var. italica L.) leaf, stem and flower. Food Chem. Toxicol. 2010, 48, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Aleksic, V.; Knezevic, P. Antimicrobial and antioxidative activity of extracts and essential oils of Myrtus communis L. Microbiol. Res. 2014, 169, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Wahid, N.; Chkichekh, A.; Bakry, M. Morphological traits and essential oil yield variation of three Myrtus communis L. populations: Implication for domestication. J. Agric. Food Chem. 2016, 4, 199–207. [Google Scholar]
- Bouzabata, A.; Castola, V.; Bighelli, A.; Abed, L.; Casanova, J.; Tomi, F. Chemical variability of algerian Myrtus communis L. Chem. Biodivers. 2013, 10, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Fadda, A.; Mulas, M. Chemical changes during myrtle (Myrtus communis L.) fruit development and ripening. Sci. Hortic. 2010, 125, 477–485. [Google Scholar] [CrossRef]
- Serreli, G.; Jerković, I.; Gil, K.A.; Marijanović, Z.; Pacini, V.; Tuberoso, C.I.G. Phenolic compounds, volatiles and antioxidant capacity of white myrtle berry liqueurs. Plant Foods Hum. Nutr. 2017, 72, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Messaoud, C.; Boussaid, M. Myrtus communis berry color morphs: A comparative analysis of essential oils, fatty acids, phenolic compounds, and antioxidant activities. Chem. Biodivers. 2011, 8, 300–310. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, C.B.; Comunello, L.N.; Lunardelli, A.; Amaral, R.H.; Pires, M.G.S.; Da Silva, G.L.; Manfredini, V.; Vargas, C.R.; Gnoatto, S.C.B.; de Oliveira, J.R.; et al. Phenolic enriched extract of baccharis trimera presents anti-inflammatory and antioxidant activities. Molecules 2017, 17, 1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarais, G.; D’Urso, G.; Lai, C.; Pirisi, FM.; Pizza, C.; Montoro, P. Targeted and untargeted mass spectrometric approaches in discrimination between Myrtus communis cultivars from Sardinia region. J. Mass. Spectrom. 2016, 51, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Tuberoso, C.I.G.; Rosa, A.; Bifulco, E.; Melis, M.P.; Atzeri, A.; Pirisi, F.M.; Dessì, M.A. Chemical composition and antioxidant activities of Myrtus communis L. berries extracts. Food Chem. 2010, 123, 1242–1251. [Google Scholar] [CrossRef]
- Montoro, P.; Tuberoso, C.I.G.; Perrone, A.; Piacente, S.; Cabras, P.; Pizza, C. Characterisation by liquid chromatography-electrospray tandem mass spectrometry of anthocyanins in extracts of Myrtus communis L. berries used for the preparation of myrtle liqueur. J. Chromatogr. A 2006, 1112, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Tuberoso, C.I.G.; Melis, M.; Angioni, A.; Pala, M.; Cabras, P. Myrtle hydroalcoholic extracts obtained from different selections of Myrtus communis L. Food Chem. 2007, 101, 806–811. [Google Scholar] [CrossRef]
- Spigno, G.; De Faveri, D.M. Microwave-assisted extraction of tea phenols: A phenomenological study. J. Food Eng. 2009, 93, 210–217. [Google Scholar] [CrossRef]
- Zhao, C.-N.; Zhang, J.; Li, Y.; Meng, X.; Li, H. Microwave-assisted extraction of phenolic compounds from Melastoma sanguineum fruit: Optimization and identification. Molecules 2018, 23, 2498. [Google Scholar] [CrossRef] [PubMed]
- Bouras, M.; Chadni, M.; Barba, F.J.; Grimi, N.; Bals, O.; Vorobiev, E. Optimization of microwave-assisted extraction of polyphenols from Quercus bark. Ind. Crops. Prod. 2015, 77, 590–601. [Google Scholar] [CrossRef]
- Haddadi-Guemghar, H.; Janel, N.; Dairou, J.; Remini, H.; Madani, K. Optimisation of microwave-assisted extraction of prune (Prunus domestica) antioxidants by response surface methodology. Int. J. Food Sci. Technol. 2014, 49, 2158–2166. [Google Scholar] [CrossRef]
- Liazid, A.; Guerrero, R.F.; Cantos, E.; Palma, M.; Barroso, C.G. Microwave assisted extraction of anthocyanins from grape skins. Food Chem. 2011, 124, 1238–1243. [Google Scholar] [CrossRef]
- Li, H.; Deng, Z.; Wu, T.; Liu, R.; Loewen, S.; Tsao, R. Microwave-assisted extraction of phenolics with maximal antioxidant activities in tomatoes. Food Chem. 2012, 130, 928–936. [Google Scholar] [CrossRef]
- Wen, Y.; Chen, H.; Zhou, X.; Deng, Q.; Zhao, Y.; Zhao, C.; Gong, X. Optimization of the microwave-assisted extraction and antioxidant activities of anthocyanins from blackberry using a response surface methodology. RSC Adv. 2015, 5, 19686–19695. [Google Scholar] [CrossRef]
- Dahmoune, F.; Nayak, B.; Moussi, K.; Remini, H.; Madani, K. Optimization of microwave-assisted extraction of polyphenols from Myrtus communis L. leaves. Food Chem. 2015, 166, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Routray, W.; Orsat, V. Microwave-assisted extraction of flavonoids: A review. Food Bioprocess Technol. 2012, 5, 409–424. [Google Scholar] [CrossRef]
- Dong, C.H.; Xie, X.Q.; Wang, X.L.; Zhan, Y.; Yao, Y.J. Application of Box–Behnken design in optimisation for polysaccharides extraction from cultured mycelium of Cordyceps sinensis. Food Bioprod. Process 2009, 87, 139–144. [Google Scholar] [CrossRef]
- Hou, W.; Zhang, W.; Chen, G.; Luo, Y. Optimization of extraction conditions for maximal phenolic, flavonoid and antioxidant activity from melaleuca bracteata leaves using the response surface methodology. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Razali, M.A.A.; Sanusi, N.; Ismail, H.; Othman, N.; Ariffin, A. Application of response surface methodology (RSM) for optimization of cassava starch grafted polyDADMAC synthesis for cationic properties. Starch/Staerke 2012, 64, 935–943. [Google Scholar] [CrossRef]
- Castañeda-ovando, A.; Pacheco-hernández, M.D.L.; Páez-hernández, M.E.; Rodríguez, J.A.; Galán-vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; De Faveri, D.M. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Pereira, D.T.V.; Tarone, A.G.; Cazarin, C.B.B.; Barbero, G.F.; Martínez, J. Pressurized liquid extraction of bioactive compounds from grape marc. J. Food Eng. 2019, 240, 105–113. [Google Scholar] [CrossRef]
- Espada-Bellido, E.; Ferreiro-González, M.; Barbero, G.F.; Carrera, C.; Palma, M.; Barroso, C.G. Alternative extraction method of bioactive compounds from Mulberry (Morus nigra L.) pulp using pressurized-liquid extraction. Food Anal. Methods 2018, 11, 2384–2395. [Google Scholar] [CrossRef]
- D’Alessandro, L.G.; Kriaa, K.; Nikov, L.; Dimitrov, K. Ultrasound assited extraction of polyphenols from black chokeberry. Sep. Purif. Technol. 2012, 93, 42–47. [Google Scholar] [CrossRef]
- Pereira, P.; Cebola, M.-J.; Oliveira, M.C.; Bernardo Gil, M.G. Antioxidant capacity and identification of bioactive compounds of Myrtus communis L. extract obtained by ultrasound-assisted extraction. J. Food Sci. Technol. 2017, 54, 4362–4369. [Google Scholar] [CrossRef] [PubMed]
- Aidi Wannes, W.; Marzouk, B. Differences between myrtle fruit parts (Myrtus communis var. italica) in phenolics and antioxidant contents. J. Food Biochem. 2013, 37, 585–594. [Google Scholar] [CrossRef]
- Scorrano, S.; Lazzoi, M.R.; Mergola, L.; Di Bello, M.P.; Del Sole, R.; Vasapollo, G. Anthocyanins profile by Q-TOF LC/MS in Myrtus communis berries from salento area. Food Anal. Methods 2017, 10, 2404–2411. [Google Scholar] [CrossRef]
- Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Barroso, C.G.; Barbero, G.F. Optimization of the ultrasound-assisted extraction of anthocyanins and total phenolic compounds in mulberry (Morus nigra) pulp. Food Chem. 2017, 219, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Stipcovich, T.; Barbero, G.F.; Ferreiro-González, M.; Palma, M.; Barroso, C.G. Fast analysis of capsaicinoids in Naga Jolokia extracts (Capsicum chinense) by high-performance liquid chromatography using fused core columns. Food Chem. 2018, 239, 217–224. [Google Scholar] [CrossRef] [PubMed]
- AOAC Peer Verified Methods Advisory Committee. AOAC Peer Verified Methods Program; AOAC International: Gaithersburg, MD, USA, 1998; pp. 1–35. [Google Scholar]
- Vázquez-Espinosa, M.; Espada-Bellido, E.; González de Peredo, A.V.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Barroso, C.G.; Barbero, G.F. Optimization of microwave-assisted extraction for the recovery of bioactive compounds from the Chilean superfruit (Aristotelia chilensis (mol.) stuntz). Agronomy 2018, 8, 240. [Google Scholar] [CrossRef]
- Garzón, G.A.; Narváez-Cuenca, C.E.; Vincken, J.P.; Gruppen, H. Polyphenolic composition and antioxidant activity of açai (Euterpe oleracea Mart.) from Colombia. Food Chem. 2017, 217, 364–372. [Google Scholar] [CrossRef] [PubMed]
- González-Varo, J.P.; Albaladejo, R.G.; Aparicio, A. Mating patterns and spatial distribution of conspecific neighbours in the Mediterranean shrub Myrtus communis (Myrtaceae). Plant Ecol. 2009, 203, 207–215. [Google Scholar] [CrossRef]
- Mulas, M.; Cani, M.R. Germplasm Evaluation of spontaneous Myrtle (Myrtus communis L.) for cultivar selection and crop development. J. Herbs Spices Med. Plants 1999, 6, 31–49. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Santos, U.P.; Campos, J.F.; Heron, F.V.; Paredes-Gamero, E.J.; Carollo, C.A.; Estevinho, L.M.; de Picol Souza, K.; Dos Santos, E.L. Antioxidant, antimicrobial and cytotoxic properties as well as the phenolic content of the extract from Hancornia speciosa Gomes. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; da Silva, E.G.P.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; dos Santos, W.N.L. Box–Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples with the compounds delphinidin 3,5-O-diglucoside, delphinidin 3-O-glucoside, cyanidin 3-O-galactoside, cyanidin 3-O-glucoside, cyanidin 3-O-arabinoside, petunidin 3-O-glucoside, delphinidin 3-O-arabinoside, peonidin 3-O-glucoside, malvidin 3-O-glucoside, petunidin 3-O-arabinoside and malvidin 3-O-arabinoside are available from the authors. |





| Variable | Source | Coefficient | Sum of Squares | Degrees of Freedom | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|---|---|
| Model | 1016.28 | 14 | 72.59 | 2.92 | 0.0352 | ||
| Methanol | X1 | −4.39257 | 231.54 | 1 | 231.54 | 9.31 | 0.0101 |
| pH | X2 | −3.62017 | 157.27 | 1 | 157.27 | 6.32 | 0.0272 |
| Temperature | X3 | 0.915742 | 10.06 | 1 | 10.06 | 0.4047 | 0.5366 |
| Ratio | X4 | 1.11842 | 15.01 | 1 | 15.01 | 0.6036 | 0.4522 |
| Methanol-pH | X1X2 | −5.57764 | 3.80 | 1 | 3.80 | 0.1528 | 0.7027 |
| Methanol-Temperature | X1X3 | 0.974675 | 47.75 | 1 | 47.75 | 1.92 | 0.1911 |
| Methanol-Ratio | X1X4 | −3.45505 | 3.43 | 1 | 3.43 | 0.1380 | 0.7167 |
| pH-Temperature | X2X3 | 0.926275 | 51.01 | 1 | 51.01 | 2.05 | 0.1776 |
| pH-Ratio | X2X4 | 4.93415 | 25.11 | 1 | 25.11 | 1.01 | 0.3348 |
| Temperature-Ratio | X3X4 | −3.571 | 8.33 | 1 | 8.33 | 0.3350 | 0.5734 |
| Methanol-Methanol | X12 | −2.50565 | 165.92 | 1 | 165.92 | 6.67 | 0.0240 |
| pH-pH | X22 | 1.45055 | 129.84 | 1 | 129.84 | 5.22 | 0.0413 |
| Temperature-Temperature | X32 | −1.44313 | 11.22 | 1 | 11.22 | 0.4513 | 0.5145 |
| Ratio-Ratio | X42 | 0.889012 | 4.22 | 1 | 4.22 | 0.1695 | 0.6878 |
| Residual | 42.6799 | 298.41 | 12 | 24.87 | |||
| Lack of Fit | 266.57 | 10 | 26.66 | 1.67 | 0.4311 | ||
| Pure Error | 31.83 | 2 | 15.92 | ||||
| Total | 1314.68 | 26 |
| Variable | Source | Coefficient | Sum of Squares | Degrees of Freedom | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|---|---|
| Model | 445.11 | 14 | 31.79 | 4.05 | 0.0100 | ||
| Methanol | X1 | 0.249033 | 0.7442 | 1 | 0.7442 | 0.0949 | 0.7633 |
| pH | X2 | −0.207067 | 0.5145 | 1 | 0.5145 | 0.0656 | 0.8022 |
| Temperature | X3 | −2.56032 | 78.66 | 1 | 78.66 | 10.03 | 0.0081 |
| Ratio | X4 | −0.92215 | 10.20 | 1 | 10.20 | 1.30 | 0.2762 |
| Methanol-pH | X1X2 | 5.98483 | 15.51 | 1 | 15.51 | 1.98 | 0.1850 |
| Methanol-Temperature | X1X3 | −1.9692 | 21.20 | 1 | 21.20 | 2.70 | 0.1260 |
| Methanol-Ratio | X1X4 | 2.3022 | 0.6398 | 1 | 0.6398 | 0.0816 | 0.7800 |
| pH-Temperature | X2X3 | 0.39995 | 41.29 | 1 | 41.29 | 5.27 | 0.0406 |
| pH-Ratio | X2X4 | −0.758496 | 0.5627 | 1 | 0.5627 | 0.0718 | 0.7933 |
| Temperature-Ratio | X3X4 | 3.21277 | 9.76 | 1 | 9.76 | 1.24 | 0.2864 |
| Methanol-Methanol | X12 | 0.375075 | 191.03 | 1 | 191.03 | 24.36 | 0.0003 |
| pH-pH | X22 | −0.830096 | 3.07 | 1 | 3.07 | 0.3913 | 0.5433 |
| Temperature-Temperature | X32 | −1.56213 | 3.67 | 1 | 3.67 | 0.4687 | 0.5066 |
| Ratio-Ratio | X42 | 1.0868 | 6.30 | 1 | 6.30 | 0.8034 | 0.3877 |
| Residual | 18.6496 | 94.10 | 12 | 7.84 | |||
| Lack of Fit | 87.43 | 10 | 8.74 | 2.62 | 0.3076 | ||
| Pure Error | 6.67 | 2 | 3.33 | ||||
| Total | 539.21 | 26 |
| Compounds | Myrtle Ecotypes of Puerto Real | Myrtle Ecotypes of San José del Valle | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MY-1 | MY-2 | MY-3 | MY-4 | MY-5 | MY-6 | MY-7 | MY-8 | MY-9 | MY-10 | MY-11 | MY-12 | MY-13 | MY-14 | |
| Del-3,5-diGl | 0.434 ± 0.012 | 0.440 ± 0.015 | 0.367 ± 0.013 | 0.352 ± 0.035 | 0.514 ± 0.0132 | 0.397 ± 0.018 | 0.210 ± 0.001 | 0.498± 0.023 | 0.156 ± 0.004 | 0.073 ± 0.003 | 0.1456 ± 0.004 | 0.181 ± 0.005 | 0.157 ± 0.004 | 0.180 ± 0.007 |
| Del-3-Glu | 9.405 ± 0.256 | 13.232 ± 0.369 | 9.555 ± 0.051 | 9.704 ± 0.159 | 15.110 ± 0.160 | 10.935 ± 0.001 | 10.164 ± 0.171 | 9.664 ± 0.497 | 8.049 ± 0.283 | 1.798 ± 0.072 | 7.897 ± 0.301 | 6.102 ± 0.234 | 5.432 ± 0.234 | 6.192 ± 0.236 |
| Cy-3-Ga | 0.159 ± 0.0006 | 0.288 ± 0.014 | 0.211 ± 0.010 | 0.309 ± 0.010 | 0.372 ± 0.010 | 0.326 ± 0.003 | 0.184 ± 0.004 | 0.426 ± 0.023 | 0.504 ± 0.023 | 0.047 ± 0.001 | 0.490 ± 0.023 | 0.133 ± 0.003 | 0.341 ± 0.013 | 0.154 ± 0.005 |
| Cy-3-Gl | 1.702 ± 0.063 | 2.326 ± 0.075 | 1.013 ± 0.019 | 1.067 ± 0.022 | 1.854 ± 0.048 | 1.142 ± 0.015 | 2.276 ± 0.016 | 1.626 ± 0.079 | 1.011 ± 0.039 | 0.321 ± 0.013 | 1.002 ± 0.035 | 1.191 ± 0.043 | 0.988 ± 0.038 | 1.235 ± 0.031 |
| Cy-3-Ar | 0.090 ± 0.002 | 0.098 ± 0.002 | 0.075 ± 0.002 | 0.145 ± 0.009 | 0.136 ± 0.0014 | 0.611 ± 0.656 | 2.124 ± 0.015 | 0.134 ± 0.005 | 0.943 ± 0.037 | 0.299 ± 0.012 | 0.898 ± 0.032 | 0.084 ± 0.002 | 0.765 ± 0.029 | 0.085 ± 0.038 |
| Pet-3-Gl | 4.738 ± 0.065 | 8.680 ± 0.239 | 6.512 ± 0.027 | 7.480 ± 0.159 | 9.958 ± 0.426 | 4.614 ± 0.196 | 2.036 ± 0.023 | 6.058 ± 0.330 | 0.094 ± 0.010 | 0.036 ± 0.001 | 0.091 ± 0.003 | 2.346 ± 0.087 | 1.247 ± 0.051 | 2.988 ± 0.002 |
| Del-3-Ara | 1.979 ± 0.073 | 1.838 ± 0.072 | 1.527 ± 0.069 | 1.601 ± 0.032 | 2.320 ± 0.062 | 4.974 ± 0.234 | 4.022 ± 0.052 | 1.627 ± 0.079 | 6.256 ± 0.222 | 1.634 ± 0.072 | 5.990 ± 0.189 | 0.900 ± 0.023 | 3.257 ± 0.138 | 0.912 ± 0.119 |
| Peo-3-Gl | 0.614 ± 0.021 | 0.856 ± 0.027 | 0.573 ± 0.023 | 0.608 ± 0.022 | 0.705 ± 0.019 | 0.7206 ± 0.056 | 0.869 ± 0.052 | 0.406 ± 0.022 | 0.627 ± 0.022 | 0.330 ± 0.011 | 0.599 ± 0.019 | 0.488 ± 0.023 | 0.178 ± 0.07 | 0.375 ± 0.029 |
| Mal-3-Gl | 6.893 ± 0.245 | 13.124 ± 0.417 | 14.346 ± 0.024 | 15.495 ± 0.331 | 0.750 ± 0.020 | 0.767 ± 0.060 | 0.925 ± 0.0004 | 6.227 ± 0.339 | 0.667 ± 0.024 | 0.352 ± 0.013 | 0.601 ± 0.023 | 9.832 ± 0.342 | 5.563 ± 0.190 | 6.877 ± 0.013 |
| Pet-3-Ar | 0.295 ± 0.0003 | 0.430 ± 0.010 | 0.411 ± 0.0174 | 0.504 ± 0.020 | 0.590 ± 0.013 | 0.656 ± 0.021 | 0.3891 ± 0.0004 | 0.378 ± 0.018 | 0.765 ± 0.023 | 0.203 ± 0.001 | 0.679 ± 0.021 | 0.184 ± 0.007 | 0.634 ± 0.022 | 0.199 ± 0.268 |
| Mal-3-Ar | 0.2451 ± 0.002 | 0.231 ± 0.007 | 0.356 ± 0.002 | 0.372 ± 0.002 | 0.328 ± 0.006 | 0.5402 ± 0.001 | 0.289 ± 0.002 | 0.154 ± 0.010 | 0.520 ± 0.024 | 0.263 ± 0.01 | 0.492 ± 0.012 | 0.299 ± 0.013 | 0.5757 ± 0.020 | 0.213 ± 0.007 |
| Total anthocyanins | 26.555 ± 0.395 | 41.544 ± 1.390 | 34.947 ± 0.183 | 37.638 ± 0.730 | 32.637 ± 0.854 | 25.682 ± 0.846 | 23.487 ± 0.234 | 35.846 ± 0.896 | 19.595 ± 0.577 | 5.355 ± 0.242 | 18.884 ± 0.762 | 21.740 ± 0.865 | 19.138 ± 0.645 | 19.409 ± 0.567 |
| Total phenolic compounds | 88.598 ± 2.983 | 124.684 ± 0.934 | 82.603 ± 0.343 | 63.457 ± 2.541 | 86.251 ± 2.934 | 81.225 ± 2.199 | 88.340 ± 3.899 | 90.682 ± 4.706 | 55.934 ± 2.743 | 69.550 ± 3.123 | 56.790 ± 2.065 | 60.654 ± 2.127 | 61.193 ± 2.356 | 59.898 ± 2.967 |
| Run | Factors | Responses | ||||||
|---|---|---|---|---|---|---|---|---|
| Solvent | pH | Temp. | Ratio | YTP (mg g−1) | YTA (mg g−1) | |||
| Measured | Predicted | Measured | Predicted | |||||
| 1 | −1 | −1 | 0 | 0 | 50.66 | 51.02 | 26.04 | 21.86 |
| 2 | 1 | −1 | 0 | 0 | 34.64 | 40.29 | 24.74 | 26.30 |
| 3 | −1 | 1 | 0 | 0 | 43.86 | 41.83 | 26.73 | 25.39 |
| 4 | 1 | 1 | 0 | 0 | 31.74 | 35.00 | 17.54 | 21.95 |
| 5 | 0 | 0 | −1 | −1 | 44.30 | 41.54 | 20.05 | 20.83 |
| 6 | 0 | 0 | 1 | −1 | 42.60 | 46.26 | 20.50 | 18.83 |
| 7 | 0 | 0 | −1 | 1 | 46.71 | 46.67 | 20.21 | 22.11 |
| 8 | 0 | 0 | 1 | 1 | 39.23 | 45.61 | 14.41 | 13.86 |
| 9 | 0 | 0 | 0 | 0 | 38.17 | 42.68 | 16.56 | 18.65 |
| 10 | −1 | 0 | 0 | −1 | 38.51 | 42.19 | 25.34 | 26.79 |
| 11 | 1 | 0 | 0 | −1 | 31.50 | 31.55 | 26.42 | 26.49 |
| 12 | −1 | 0 | 0 | 1 | 40.14 | 42.58 | 25.04 | 24.15 |
| 13 | 1 | 0 | 0 | 1 | 36.83 | 35.64 | 27.71 | 25.45 |
| 14 | 0 | −1 | −1 | 0 | 44.07 | 48.20 | 22.79 | 23.04 |
| 15 | 0 | 1 | −1 | 0 | 46.49 | 48.10 | 18.40 | 16.20 |
| 16 | 0 | −1 | 1 | 0 | 56.28 | 57.17 | 10.11 | 11.50 |
| 17 | 0 | 1 | 1 | 0 | 44.42 | 42.79 | 18.57 | 17.51 |
| 18 | 0 | 0 | 0 | 0 | 44.14 | 42.68 | 19.45 | 18.65 |
| 19 | 0 | −1 | 0 | −1 | 53.28 | 48.50 | 20.59 | 20.48 |
| 20 | 0 | 1 | 0 | −1 | 46.14 | 46.27 | 19.82 | 19.32 |
| 21 | 0 | −1 | 0 | 1 | 62.00 | 55.75 | 16.79 | 17.89 |
| 22 | 0 | 1 | 0 | 1 | 44.84 | 43.50 | 17.52 | 18.22 |
| 23 | −1 | 0 | −1 | 0 | 39.22 | 38.57 | 26.60 | 28.42 |
| 24 | 1 | 0 | −1 | 0 | 39.00 | 36.70 | 26.86 | 24.31 |
| 25 | −1 | 0 | 1 | 0 | 51.14 | 47.32 | 15.60 | 18.69 |
| 26 | 1 | 0 | 1 | 0 | 37.10 | 31.62 | 25.03 | 23.80 |
| 27 | 0 | 0 | 0 | 0 | 45.74 | 42.68 | 19.94 | 18.65 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
V. González de Peredo, A.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Jiménez-Cantizano, A.; Ferreiro-González, M.; Amores-Arrocha, A.; Palma, M.; G. Barroso, C.; F. Barbero, G. Development of New Analytical Microwave-Assisted Extraction Methods for Bioactive Compounds from Myrtle (Myrtus communis L.). Molecules 2018, 23, 2992. https://doi.org/10.3390/molecules23112992
V. González de Peredo A, Vázquez-Espinosa M, Espada-Bellido E, Jiménez-Cantizano A, Ferreiro-González M, Amores-Arrocha A, Palma M, G. Barroso C, F. Barbero G. Development of New Analytical Microwave-Assisted Extraction Methods for Bioactive Compounds from Myrtle (Myrtus communis L.). Molecules. 2018; 23(11):2992. https://doi.org/10.3390/molecules23112992
Chicago/Turabian StyleV. González de Peredo, Ana, Mercedes Vázquez-Espinosa, Estrella Espada-Bellido, Ana Jiménez-Cantizano, Marta Ferreiro-González, Antonio Amores-Arrocha, Miguel Palma, Carmelo G. Barroso, and Gerardo F. Barbero. 2018. "Development of New Analytical Microwave-Assisted Extraction Methods for Bioactive Compounds from Myrtle (Myrtus communis L.)" Molecules 23, no. 11: 2992. https://doi.org/10.3390/molecules23112992
APA StyleV. González de Peredo, A., Vázquez-Espinosa, M., Espada-Bellido, E., Jiménez-Cantizano, A., Ferreiro-González, M., Amores-Arrocha, A., Palma, M., G. Barroso, C., & F. Barbero, G. (2018). Development of New Analytical Microwave-Assisted Extraction Methods for Bioactive Compounds from Myrtle (Myrtus communis L.). Molecules, 23(11), 2992. https://doi.org/10.3390/molecules23112992