Chemometric Assessment and Best-Fit Function Modelling of the Toxic Potential of Selected Food Packaging Extracts
Abstract
1. Introduction
2. Results
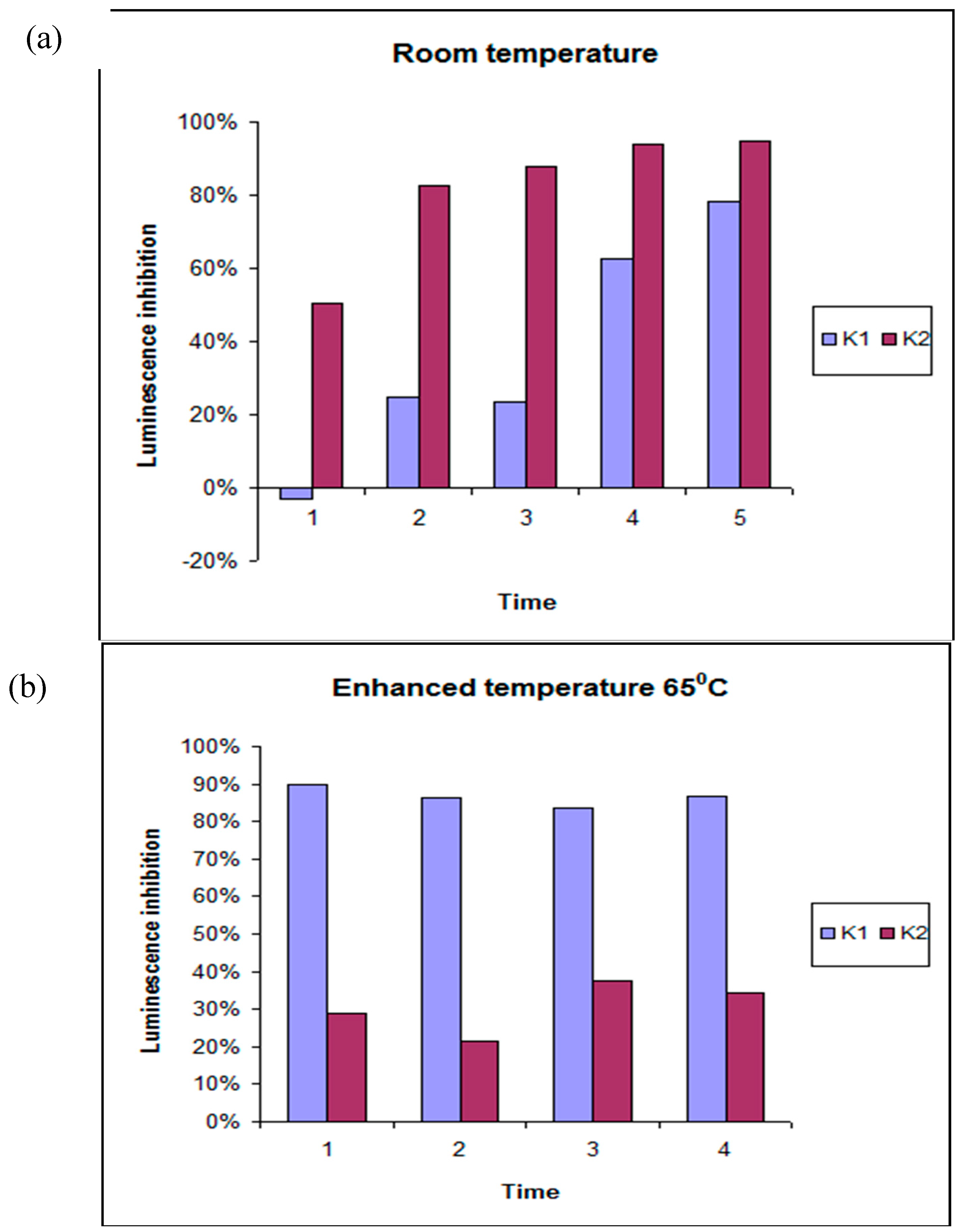
2.1. Exploratory Data Analysis
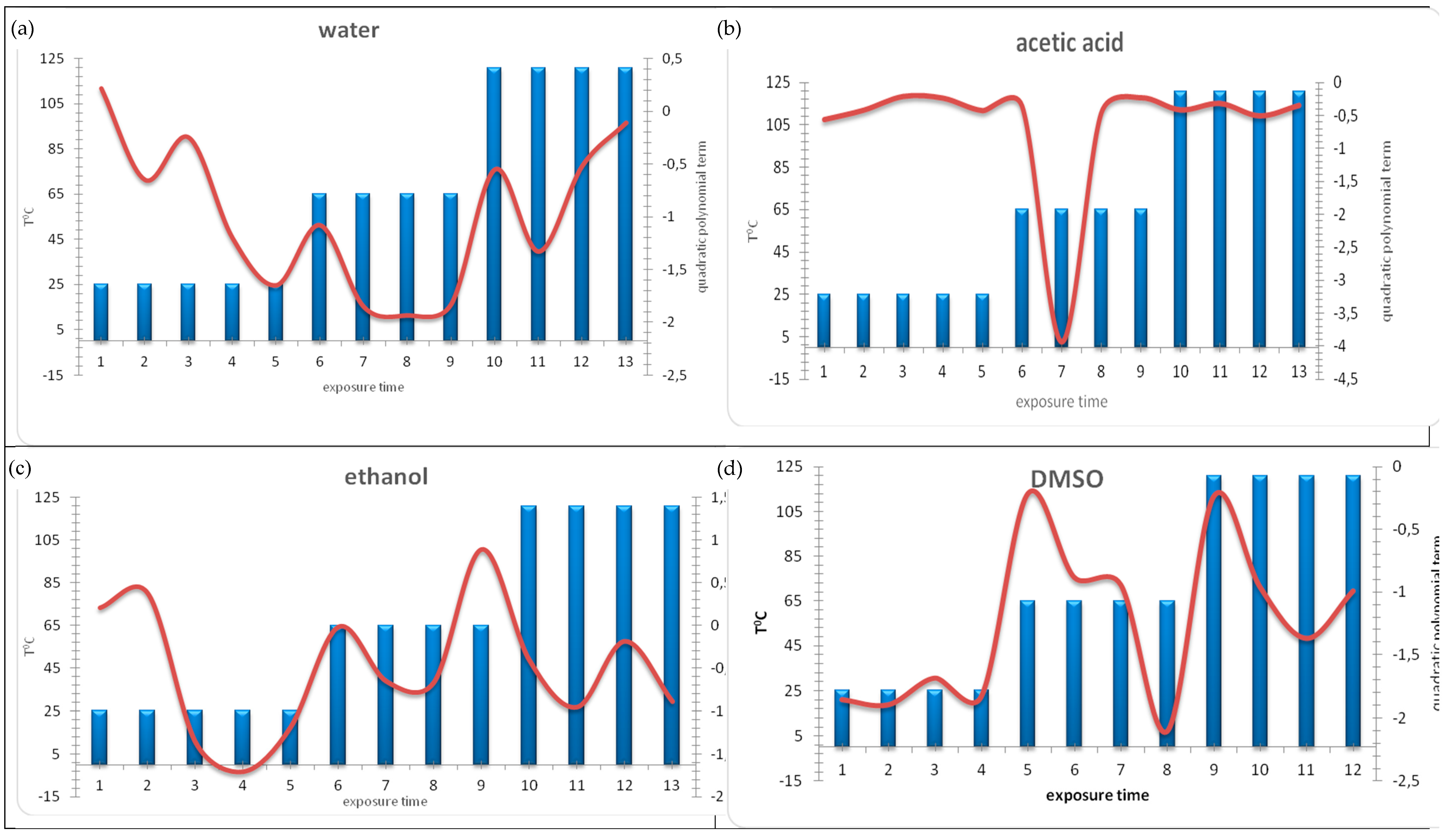
2.2. Best-Fit Function Modelling
3. Discussion
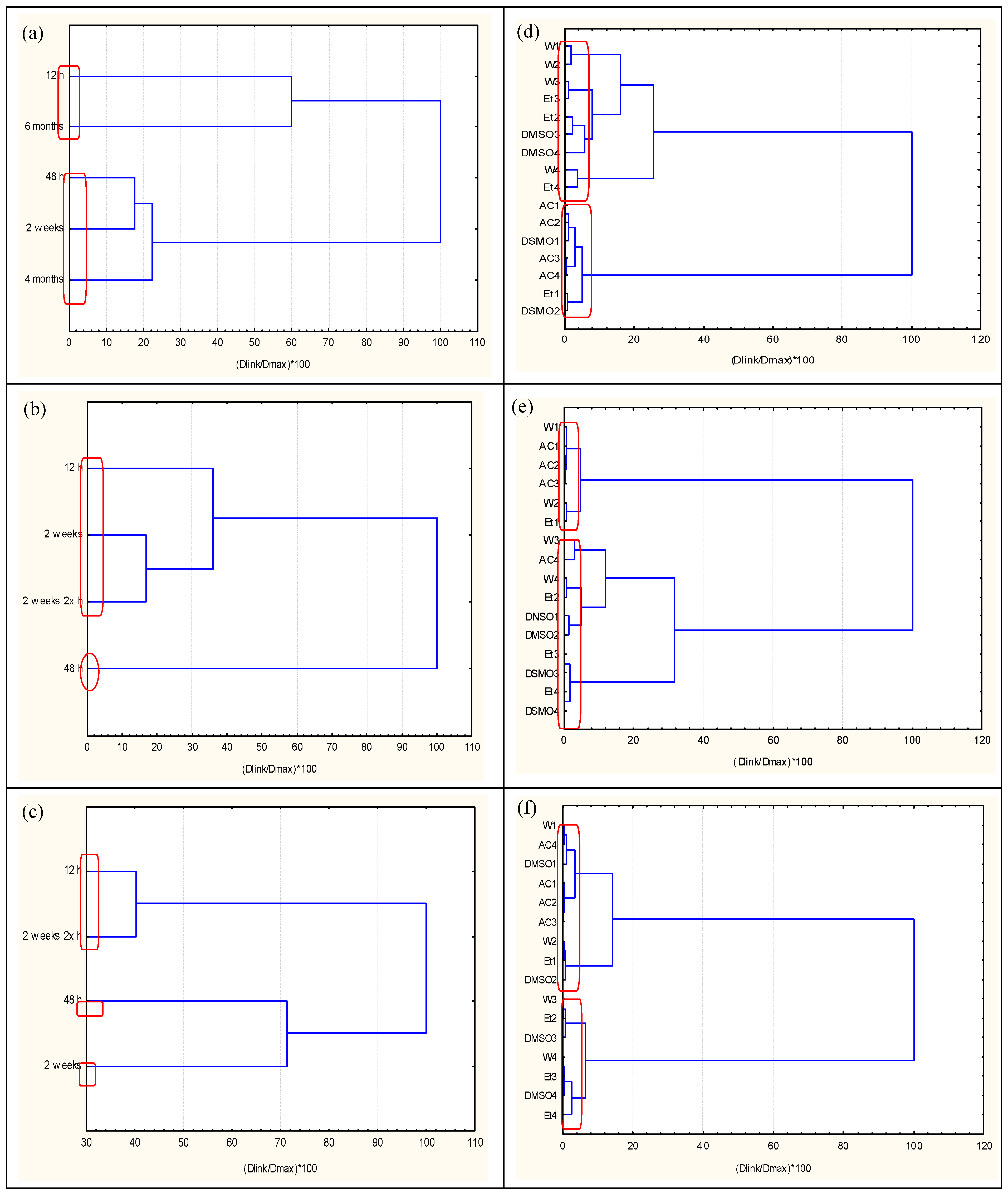
3.1. Discussion of Clustering Analyses
- K1 (W1, W2, W3, W4, Et2, Et3, Et4, DMSO3, DMSO4)—low toxicity
- K2 (AC1, AC2, AC3, AC4, Et1, DMSO1, DMSO2)—high toxicity
- K2 (W3, W4, AC4, Et2, Et3, Et4, DMSO1, DMSO2, DMSO3, DMSO4)—low toxicity
- K1 (W1, W2, AC1, AC2, AC3, Et1)—high toxicity
- K1 (W3, W4, Et2, Et3, Et3, DMSO3, DMSO4)—low toxicity
- K2 (W1, W2, AC1, AC2, AC3, AC4, Et1, DMSO1, DMSO2)—high toxicity
3.2. Discussion on Results of Best-Fit Function Modelling
- positive coefficients—indicate minimal ecotoxicity and negative values of bioluminescence inhibition;
- low negative coefficient—indicates higher or intermediate ecotoxicity and low positive values of bioluminescence inhibition;
- very negative coefficient—indicates maximal ecotoxicity and high positive values of bioluminescence inhibition.
4. Materials and Methods
4.1. Instruments, Chemicals, and Reagents
4.2. Sample Preparation
4.3. Toxicity Studies Using Microtox®
4.4. Chemometric Data Treatment
5. Conclusions
- hierarchical cluster analysis of input data,
- best-fit function modelling using the value of the regression coefficients from the derived polynomial models.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fasano, E.; Bono-Blay, F.; Cirillo, T.; Montuori, P.; Lacorte, S. Migration of phthalates, alkylphenols, bisphenol A and di(2-ethylhexyl)adipate from food packaging. Food Control 2012, 27, 132–138. [Google Scholar] [CrossRef]
- Parkar, J.; Rakesh, M. Leaching of elements from packaging material into canned foods marketed in India. Food Control 2014, 40, 177–184. [Google Scholar] [CrossRef]
- Marsh, K.; Bugusu, B. Food Packaging—Roles, Materials, and Environmental Issues. J. Food Sci. 2007, 72, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Muncke, J. Endocrine disrupting chemicals and other substances of concern in foohd contact materials: An updated review of exposure, effect and risk assessment. J. Steroid Biochem. Mol. Biol. 2011, 127, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, S.; Zurfluh, M.; Grob, K.; Vedani, K.; Brüschweiler, B.J. Migration of cyclo-diBA from coatings into canned food: Method of analysis, concentration determined in a survey and in silico hazard profiling. Food Chem. Toxicol. 2013, 58, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Buculei, A.; Amariei, S.; Oroian, M.; Gutt, G.; Gaceu, L.; Birca, A. Metals migration between product and metallic package in canned Meat. LWT-Food Sci. Technicol. 2014, 58, 364–374. [Google Scholar] [CrossRef]
- Grob, K.; Camus, P.; Gontard, N.; Hoellinger, H.; Joly, C.; Macherey, A.C.; Masset, D.; Nesslany, F.; Régnier, J.F.; Riquet, A.M.; et al. Need for a better safety evaluation of food contact materials produced from resins. Food Control 2010, 21, 763–769. [Google Scholar] [CrossRef]
- Gallart-Ayala, H.; Moyano, E.; Galceran, M.T. Fast liquid chromatography–tandem mass spectrometry for the analysis of bisphenol A-diglycidyl ether, bisphenol F-diglycidyl ether and their derivatives in canned food and beverages. J. Chromatogr. A 2011, 1218, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Fattore, M.; Russo, G.; Barbato, F.; Grumetto, L.; Albrizio, S. Monitoring of bisphenols in canned tuna from Italian markets. Food Chem. Toxicol. 2015, 83, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Gallo, P.; Pisciottano, I.M.; Esposito, F.; Fasano, E.; Scognamiglio, G.; Mita, G.D.; Cirillo, T. Determination of BPA, BPB, BPF, BADGE and BFDGE in canned energy drinks by molecularly imprinted polymer cleaning up and UPLC with fluorescence detection. Food Chem. 2016, 220, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Changa, Y.; Nguyena, C.; Paranjpea, W.R.; Gillilanda, F.; Zhang, J. Analysis of bisphenol A diglycidyl ether (BADGE) and its hydrolytic metabolites in biological specimens by high-performance liquid chromatography and tandem mass spectrometry. J. Chromatogr. B 2014, 965, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Szczepańska, N.; Kudłak, B.; Namieśnik, J. Assessing ecotoxicity and the endocrine potential of selected phthalates, BADGE and BFDGE derivatives in relation to environmentally detectable levels. Sci. Total Environ. 2018, 610–611, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.; Rajapakse, N.; Kortenkamp, A. Something from “Nothing”—Eight Weak Estrogenic Chemicals Combined at Concentrations below NOECs Produce Significant Mixture Effects. Environ. Sci. Technol. 2002, 36, 1751–1756. [Google Scholar] [CrossRef] [PubMed]
- Szczepańska, N.; Kudłak, B.; Namieśnik, J. Recent advances in assessing xenobiotics migrating from packaging material—A review. Anal. Chim. Acta 2018, 1023, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fernández, F.J.; Bayo, J.; Pérez de los Ríos, A.; Vicente, M.A.; Bernal, F.J.; Quesada-Medina, J. Discovering less toxic ionic liquids by using the Microtox® toxicity test. Ecol. Environ. Safe 2015, 116, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Vandeginste, B.G.M.; Massart, D.L.; Buydens, L.M.C.; De Jong, S.; Lewi, P.J.; Smeyers-Verbeke, J. Handbook of Chemometrics and Qualimetrics; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Massart, D.L.; Kaufman, L. The Interpretation of Analytical Chemical Data by the Use of Cluster Analysis; Elsevier: Amsterdam, The Netherlands, 1983. [Google Scholar]
- Szczepańska, N.; Kudłak, B.; Tsakovski, S.; Yotova, G.; Nedyalkova, M.; Simeonov, V.; Dołęga, A.; Namieśnik, J. Modeling and MANOVA studies on toxicity and endocrine potential of packaging materials exposed to different extraction schemes. Environ. Res. 2018, 165, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Kudłak, B.; Owczarek, K.; Namieśnik, J. Selected issues related to the toxicity of ionic liquids and deep eutectic solvents—A review. Environ. Sci. Pollut. Res. 2015, 22, 11975–11992. [Google Scholar] [CrossRef] [PubMed]
- Box, G.E.P.; Draper, N.R. Response Surfaces, Mixtures, and Ridge Analyses, 2nd ed.; Wiley: New York, NY, USA, 2007. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |

| WATER | r2 | 12 h | 48 h | 2 Weeks | 4 Months | 6 Months | 12 h | 48 h | 2 Weeks | 2 Weeks 2x h | 12 h | 48 h | 2 Weeks | 2 Weeks 2x h |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| linear | 0.857 | 0.987 | 0.994 | 0.598 | 0.605 | 0.745 | 0.842 | 0.605 | 0.783 | 0.982 | 0.838 | 0.984 | 0.857 | |
| log | 0.991 | 0.970 | 0.878 | 0.855 | 0.855 | 0.939 | 0.960 | 0.853 | 0.964 | 0.952 | 0.986 | 0.956 | 0.990 | |
| Polynom. | 0.983 | 0.995 | 0.995 | 0.956 | 0.928 | 0.940 | 0.992 | 0.918 | 0.995 | 0.996 | 0.992 | 0.998 | 0.997 | |
| ACETIC ACID | r2 | |||||||||||||
| linear | 0.890 | 0.641 | 0.492 | 0.709 | 0.651 | 0.644 | 0.377 | 0.783 | 0.783 | 0.936 | 0.871 | 0.618 | 0.652 | |
| log | 0.960 | 0.880 | 0.766 | 0.927 | 0.873 | 0.880 | 0.643 | 0.963 | 0.964 | 0.998 | 0.975 | 0.849 | 0.891 | |
| Polynom. | 0.985 | 0.934 | 0.897 | 0.981 | 0.888 | 0.991 | 0.775 | 0.966 | 0.976 | 0.990 | 0.980 | 0.870 | 0.950 | |
| ETHANOL | r2 | |||||||||||||
| linear | 0.998 | 0.989 | 0.948 | 0.648 | 0.582 | 1.000 | 0.983 | 0.970 | 0.955 | 0.992 | 0.948 | 0.948 | 0.983 | |
| log | 0.890 | 0.847 | 0.973 | 0.871 | 0.835 | 0.916 | 0.970 | 0.985 | 0.993 | 0.956 | 0.994 | 0.994 | 0.945 | |
| Polynom. | 1.000 | 0.998 | 0.995 | 0.886 | 0.906 | 1.000 | 1.000 | 0.998 | 0.995 | 1.000 | 0.991 | 0.991 | 0.986 | |
| DMSO | r2 | |||||||||||||
| linear | 0.818 | 0.886 | 0.799 | 0.821 | 1.000 | 0.969 | 0.953 | 0.933 | 0.735 | 0.994 | 0.905 | 0.800 | 0.896 | |
| log | 0.978 | 0.998 | 0.963 | 0.977 | 1.000 | 0.980 | 0.968 | 0.982 | 0.920 | 0.951 | 0.990 | 0.971 | 0.996 | |
| Polynom. | 0.994 | 0.994 | 1.000 | 0.999 | 1.000 | 0.990 | 0.993 | 0.997 | 0.996 | 1.000 | 1.000 | 0.975 | 0.999 |
| EXTRACTION MEDIUM | RT | 65 °C | 121 °C |
|---|---|---|---|
| W1 | LT | HT | HT |
| W2 | LT | HT | HT |
| W3 | LT | LT | LT |
| W4 | LT | LT | LT |
| AC1 | HT | HT | HT |
| AC2 | HT | HT | HT |
| AC3 | HT | HT | HT |
| AC4 | HT | LT | HT |
| Et1 | HT | HT | HT |
| Et2 | LT | LT | LT |
| Et3 | LT | LT | LT |
| Et4 | LT | LT | LT |
| DMSO1 | HT | LT | HT |
| DMSO2 | HT | LT | HT |
| DMSO3 | LT | LT | LT |
| DMSO4 | LT | LT | LT |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudłak, B.; Szczepańska, N.; Nedyalkova, M.; Simeonov, V.; Namieśnik, J. Chemometric Assessment and Best-Fit Function Modelling of the Toxic Potential of Selected Food Packaging Extracts. Molecules 2018, 23, 3028. https://doi.org/10.3390/molecules23113028
Kudłak B, Szczepańska N, Nedyalkova M, Simeonov V, Namieśnik J. Chemometric Assessment and Best-Fit Function Modelling of the Toxic Potential of Selected Food Packaging Extracts. Molecules. 2018; 23(11):3028. https://doi.org/10.3390/molecules23113028
Chicago/Turabian StyleKudłak, Błażej, Natalia Szczepańska, Miroslava Nedyalkova, Vasil Simeonov, and Jacek Namieśnik. 2018. "Chemometric Assessment and Best-Fit Function Modelling of the Toxic Potential of Selected Food Packaging Extracts" Molecules 23, no. 11: 3028. https://doi.org/10.3390/molecules23113028
APA StyleKudłak, B., Szczepańska, N., Nedyalkova, M., Simeonov, V., & Namieśnik, J. (2018). Chemometric Assessment and Best-Fit Function Modelling of the Toxic Potential of Selected Food Packaging Extracts. Molecules, 23(11), 3028. https://doi.org/10.3390/molecules23113028








