An Efficient Synthesis of Acenaphtho[1,2-b]indole Derivatives via Domino Reaction
Abstract
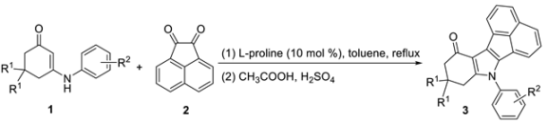
:1. Introduction
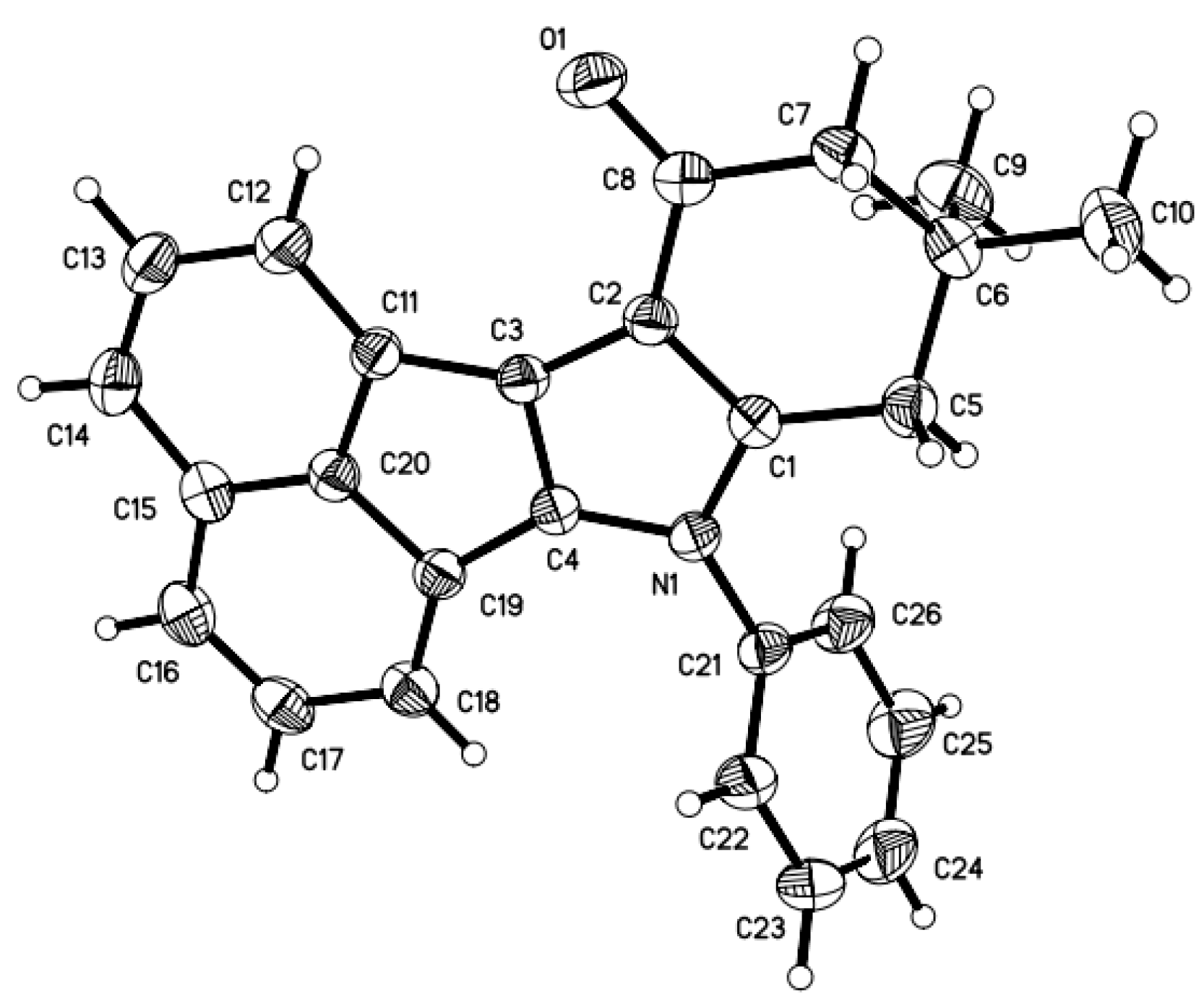
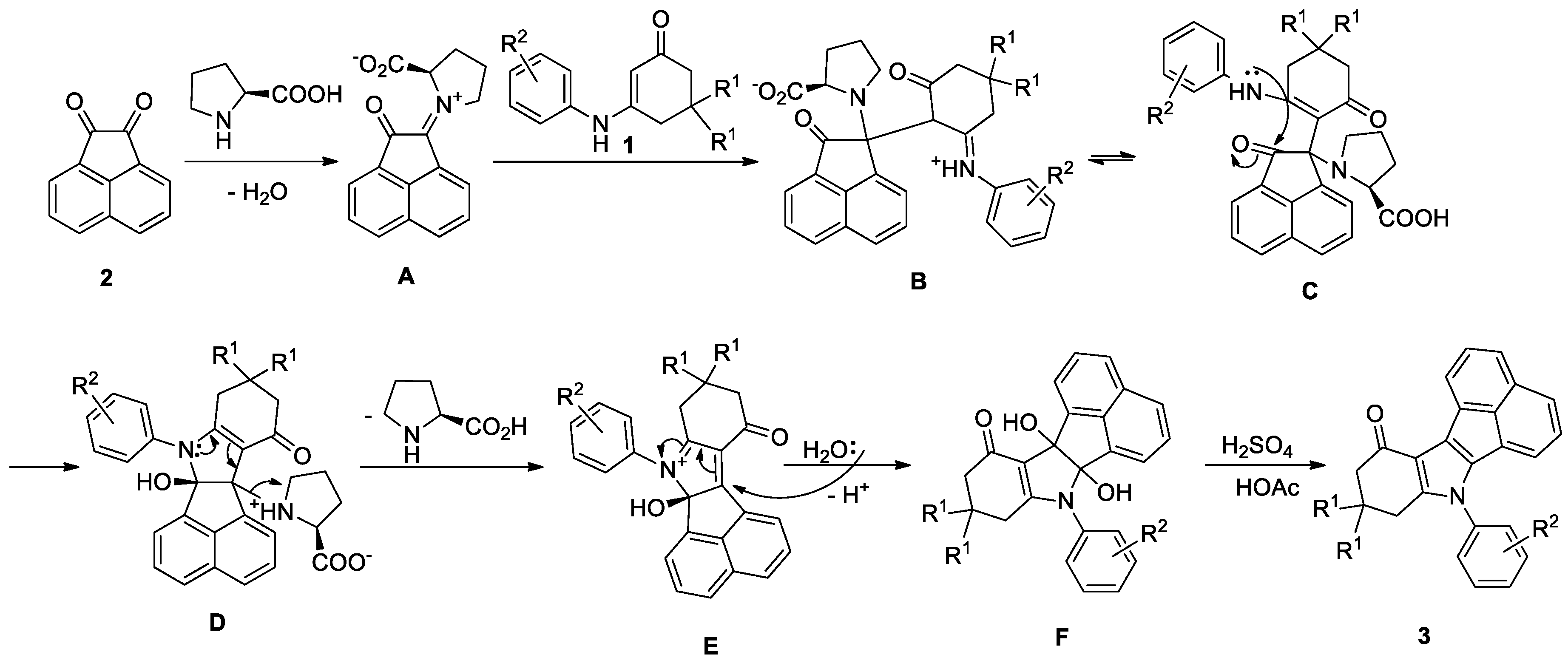
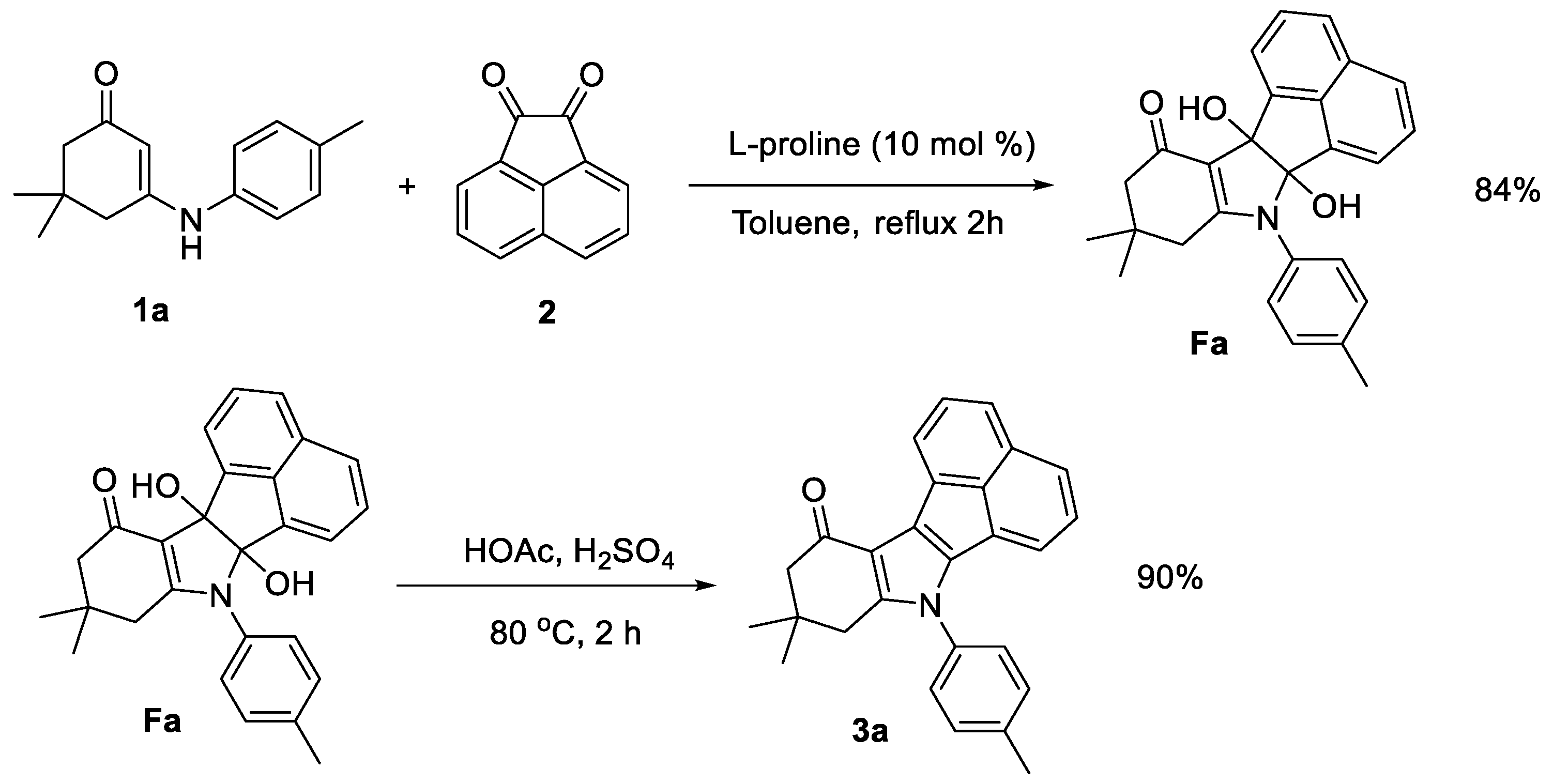
2. Results and Discussion
3. Experimental
3.1. General Information
3.2. General Procedure for the Synthesis of Acenaphtho[1,2-b]indole Derivatives 3
3.3. General Procedure for the Synthesis of Tetrahydroacenaphtho[1,2-b]indole Derivatives Fa
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Somei, M.; Yamada, F. Simple indole alkaloids and those with a nonrearranged monoterpenoid unit. Nat. Prod. Rep. 2004, 21, 278–311. [Google Scholar] [CrossRef] [PubMed]
- Bandidni, M.; Eichholzer, A. Catalytic functionalization of indoles in a new dimension. Angew. Chem. Int. Ed. 2009, 48, 9608–9644. [Google Scholar] [CrossRef] [PubMed]
- Kochanowska-Karamya, A.J.; Hamann, M.J. Marine indole alkaloids: Potential new drug leads for the control of depression and anxiety. Chem. Rev. 2010, 110, 4480–4497. [Google Scholar] [CrossRef]
- Chen, I.; Safe, S.; Bjeldanes, L. Indole-3-carbinol and diindolymethane as aryl hydrocarbon (Ah) receptor agonists and antagonists in T47D human breast cancer. Biochem. Pharmacol. 1996, 51, 1069–1076. [Google Scholar] [CrossRef]
- Suzen, S.; Buyukbingol, E. Anti-cancer activity studies of indolalthiohydantoin (PTT) on certain cancer cell lines. Farmaco 2000, 55, 246–248. [Google Scholar] [CrossRef]
- Giagoudakis, G.; Markantonis, S.L. Relationships between the concentrations of prostaglandins and the nonsteroidal anti-inflammatory drugs indomethacin, diclofenac, and ibuprofen. Pharmacotherapy 2005, 25, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Suzen, S.; Buyukbingol, E. Evaluation of anti-HIV activity of 5-(2-phenyl-3’-indolal)-2-thiohydantoin. Farmaco 1998, 53, 525–527. [Google Scholar] [CrossRef]
- Walter, G.; Liebl, R.; voa Angerer, E. 2-Phenylindole sulfamates: Inhibitors of steroid sulfatase with antiproliferative activity in MCF-7 breast cancer cells. J. Steroid Biochem. Mol. Biol. 2004, 88, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Yannai, S.; Rennert, G.; Gruener, N.; Fares, F.A. 3,3’-Diindolymethane induces apoptosisin human cancer cells. Biochem. Biophys. Res. Commun. 1996, 228, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Horton, D.A.; Bourne, G.T.; Smythe, M.L. The combinational synthesis of bicyclic privileged structures or privileged substructures. Chem. Rev. 2003, 103, 893–930. [Google Scholar] [CrossRef] [PubMed]
- Shiri, M. Indoles in multicomponent process (MCPs). Chem. Rev. 2012, 112, 3508–3549. [Google Scholar] [CrossRef] [PubMed]
- Lescot, E.; Muzard, G.; Markovits, J.; Belleney, J.; Roques, B.P.; Lepecq, J.B. Synthesis of 11H-pyridocarbazoles and derivatives. Comparison of their DNA binding and antitumor activity with those of 6H- and 7H-pyridocarbazoles. J. Med. Chem. 1986, 29, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Thevissen, K.; Marchand, A.; Chaltin, P.; Meert, E.M.K.; Cammue, B.P.A. Antifungal carbazoles. Curr. Med. Chem. 2009, 16, 2205–2211. [Google Scholar] [CrossRef] [PubMed]
- Scopton, A.; Kelly, T.R. Synthesis of HKI 0231B. J. Org. Chem. 2005, 70, 10004–10012. [Google Scholar] [CrossRef] [PubMed]
- Kraus, A.; Wu, T. A concise synthesis of 5-demethyl-HKI 0231A and 5-demethyl-HKI 0231B. Tetrahedron Lett. 2006, 47, 7801–7804. [Google Scholar] [CrossRef]
- Chen, X.B.; Luo, T.B.; Gou, G.Z.; Wang, J.; Liu, W.; Lin, J. Selective synthesis of acenaphtho[1,2-b]indole derivatives via tandem regioselective aza-ene addition/N-cycliaztion/ SN1 type reaction. Asian J. Org. Chem. 2015, 4, 921–928. [Google Scholar] [CrossRef]
- Shan, D.; Gao, Y.; Jia, Y. Intramolecular larock indole synthesis: Preparation of 3,4-fused tricyclic indoles and total synthesis of fargesine. Angew. Chem. Int. Ed. 2013, 52, 4902–4905. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Wang, H.; Li, X.; Xin, X.; Wang, C.; Wan, B. Rhodium-catalyzed C-H annulation of nitrones with alkynes: A regiospecific route to unsymmetrical 2,3-disryl-substituted indoles. Angew. Chem. Int. Ed. 2015, 54, 10613–10617. [Google Scholar] [CrossRef] [PubMed]
- Narayanaiyer, V.; Ninadnamdeo, R.; Dilip, H.G. Reaction of dimedone enamines with α-ketoacids. J. Chem. Res. (S) 1985, 244–245. [Google Scholar] [CrossRef]
- Shibata, N.; Fujimoto, H.; Mizuta, S.; Ogawa, S.; Ishiuchi, Y.; Nakamura, S.; Toru, T. Efficient synthesis of bicyclic α-hydroxy-α-trifluoromethyl-γ-lactams. Synlett 2006, 3484–3488. [Google Scholar] [CrossRef]
- Pei, Q.L.; Cui, B.D.; Han, W.Y.; Wu, Z.J.; Zhang, X.M. A facile synthesis of 3-hydroxy-3-(trifluoromethyl)- 1H-pyrrol-2(3H)—Ones with BrØnsted acid-catalyzed condensation-cyclization reactions of β-enamino esters and ethyl trifluoropyruvate. Tetrahedron 2014, 70, 4595–4601. [Google Scholar] [CrossRef]
- Tietze, L.F. Domino reactions in organic synthesis. Chem. Rev. 1996, 96, 115–136. [Google Scholar] [CrossRef] [PubMed]
- Zubarev, A.A.; Larionova, N.A.; Rodinovskaya, L.A.; Mortikov, V.Y.; Shestopalov, A.M. Synthesis of 2,5-asymmetrically substituted 3,4-diaminothieno[2,3-b]thiophenes by domino reaction. ACS Comb. Sci. 2013, 15, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wang, J.J.; Zhang, J.J.; Cao, C.P.; Huang, Z.B.; Shi, D.Q. Regioselective synthesis of functionalized [1,8]naphthyridine derivatives via three-component domino reaction under catalyst-free conditions. Green Chem. 2015, 17, 973–981. [Google Scholar] [CrossRef]
- Fu, L.; Feng, X.; Zhang, J.J.; Hu, J.D.; Xun, Z.; Wang, J.J.; Huang, Z.B.; Shi, D.Q. Highly efficient construction of a bridged pentacyclic skeleton via a six-component domino reaction under microwave irradiation. Green Chem. 2015, 17, 1535–1545. [Google Scholar] [CrossRef]
- Xun, Z.; Feng, X.; Wang, J.J.; Shi, D.Q.; Huang, Z.B. Multicomponent strategy for the preparation of pyrrolo[1,2-a]pyrimidine derivatives under catalyst-free and microwave irradiation conditions. Chin. J. Chem. 2016, 34, 696–702. [Google Scholar] [CrossRef]
- Lin, W.; Hu, X.X.; Wang, Y.Z.; Song, S.; Zhang, M.Y.; Shi, D.Q. Microwave-assisted synthesis of 3-substituted indole derivatives via three-component domino reaction. Chin. J. Org. Chem. 2018, 38, 855–862. [Google Scholar] [CrossRef]
- Hu, J.D.; Cao, C.P.; Lin, W.; Hu, M.H.; Huang, Z.B.; Shi, D.Q. Selective synthesis of polyfunctionalized pyrido[2,3-b]indoles by multicomponent domino reactions. J. Org. Chem. 2014, 79, 7935–7944. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.P.; Xu, C.L.; Lin, W.; Li, X.M.; Hu, M.H.; Wang, J.X.; Huang, Z.B.; Shi, D.Q.; Wang, Y.C. Microwave-assisted improved synthesis of pyrrolo[2,3,4-kl]acridine and dihydropyrrolo[2,3,4-kl]acridine derivatives catalyzed by silica sulfuric acid. Molecules 2013, 18, 1613–1625. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.M.; Li, Q.; Zhang, J.J.; Lin, W.; Wang, J.X.; Wang, Y.C.; Huang, Z.B.; Shi, D.Q. Ultrasound-promoted one-pot, four-component synthesis of pyridine-2(1H)-one derivatives. Molecules 2013, 18, 14519–14528. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Zhang, J.J.; Zhao, Y.; Zhang, G.N.; Wang, Y.C.; Shi, D.Q. An efficient and multi-component synthesis of functionalized pyrazole derivatives. Heterocycles 2017, 94, 531–540. [Google Scholar] [CrossRef]
- Wang, J.X.; Gao, Y.; Zhang, J.J.; Zhang, G.N.; Ren, J.F.; Zhao, Y.; Wang, Y.C.; Shi, D.Q. An efficient and multi-component synthesis of 5-imino-3,5-dihydro-2H-chromeno[3,4-c] pyridine-2-one derivatives. Heterocycles 2017, 94, 1143–1151. [Google Scholar] [CrossRef]
Sample Availability: Samples of compounds 3a–3n are available from the authors. |
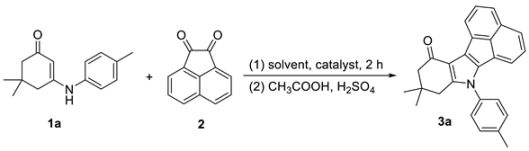
| Entry | Solvent | Catalyst (mol %) | Temperature (°C) | Yield (%) |
|---|---|---|---|---|
| 1 | Ethanol | No | Reflux | 19 |
| 2 | Ethanol | L-Proline (10) | Reflux | 41 |
| 3 | Chloroform | L-Proline (10) | Reflux | 28 |
| 4 | THF | L-Proline (10) | Reflux | 40 |
| 5 | 1,4-Dioxane | L-Proline (10) | Reflux | 23 |
| 6 | DMF | L-Proline (10) | 80 | 42 |
| 7 | Water | L-Proline (10) | 80 | 20 |
| 8 | Toluene | L-Proline (10) | 80 | 65 |
| 9 | Toluene | p-TSA (10) | 80 | 46 |
| 10 | Toluene | S-Phenylalanine (10) | 80 | 18 |
| 11 | Toluene | Phenylamine (10) | 80 | 30 |
| 12 | Toluene | Pyrrolidine(10) | 80 | 56 |
| 13 | Toluene | Piperidine(10) | 80 | 60 |
| 14 | Toluene | Benzylamine(10) | 80 | 40 |
| 15 | Toluene | Dibenzylamine(10) | 80 | 63 |
| 16 | Toluene | L-Proline (5) | 80 | 45 |
| 17 | Toluene | L-Proline (15) | 80 | 59 |
| 18 | Toluene | L-Proline (20) | 80 | 45 |
| 19 | Toluene | L-Proline (10) | 40 | Trace |
| 20 | Toluene | L-Proline (10) | 60 | 25 |
| 21 | Toluene | L-Proline (10) | Reflux | 80 |
| Entry | R1 | R2 | Product | Yield (%) |
|---|---|---|---|---|
| 1 | CH3 | 4-CH3 | 3a | 80 |
| 2 | CH3 | 3-Cl-4-F | 3b | 82 |
| 3 | CH3 | 4-CH3O | 3c | 85 |
| 4 | CH3 | 4-Br | 3d | 76 |
| 5 | CH3 | 4-NO2 | 3e | 70 |
| 6 | CH3 | 3,5-(CH3)2 | 3f | 78 |
| 7 | CH3 | H | 3g | 80 |
| 8 | CH3 | 2-Cl | 3h | 82 |
| 9 | CH3 | 4-t-Bu | 3i | 80 |
| 10 | H | H | 3j | 84 |
| 11 | H | 2-Cl | 3k | 79 |
| 12 | H | 2,4-(CH3)2 | 3l | 80 |
| 13 | H | 4-t-Bu | 3m | 81 |
| 14 | H | 3,5-(CH3)2 | 3n | 83 |
| 15 | CH3 | 2-t-Bu | 3o | trace |
| 16 | CH3 | 2,6-(t-Bu)2 | 3p | trace |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.-N.; Yuan, X.; Niu, W.; Zhu, M.; Wang, J.; Wang, Y. An Efficient Synthesis of Acenaphtho[1,2-b]indole Derivatives via Domino Reaction. Molecules 2018, 23, 3045. https://doi.org/10.3390/molecules23113045
Zhang G-N, Yuan X, Niu W, Zhu M, Wang J, Wang Y. An Efficient Synthesis of Acenaphtho[1,2-b]indole Derivatives via Domino Reaction. Molecules. 2018; 23(11):3045. https://doi.org/10.3390/molecules23113045
Chicago/Turabian StyleZhang, Guo-Ning, Xia Yuan, Weiping Niu, Mei Zhu, Juxian Wang, and Yucheng Wang. 2018. "An Efficient Synthesis of Acenaphtho[1,2-b]indole Derivatives via Domino Reaction" Molecules 23, no. 11: 3045. https://doi.org/10.3390/molecules23113045
APA StyleZhang, G.-N., Yuan, X., Niu, W., Zhu, M., Wang, J., & Wang, Y. (2018). An Efficient Synthesis of Acenaphtho[1,2-b]indole Derivatives via Domino Reaction. Molecules, 23(11), 3045. https://doi.org/10.3390/molecules23113045






