Sodium Phenylbutyrate Ameliorates Inflammatory Response Induced by Staphylococcus aureus Lipoteichoic Acid via Suppressing TLR2/NF-κB/NLRP3 Pathways in MAC-T Cells
Abstract
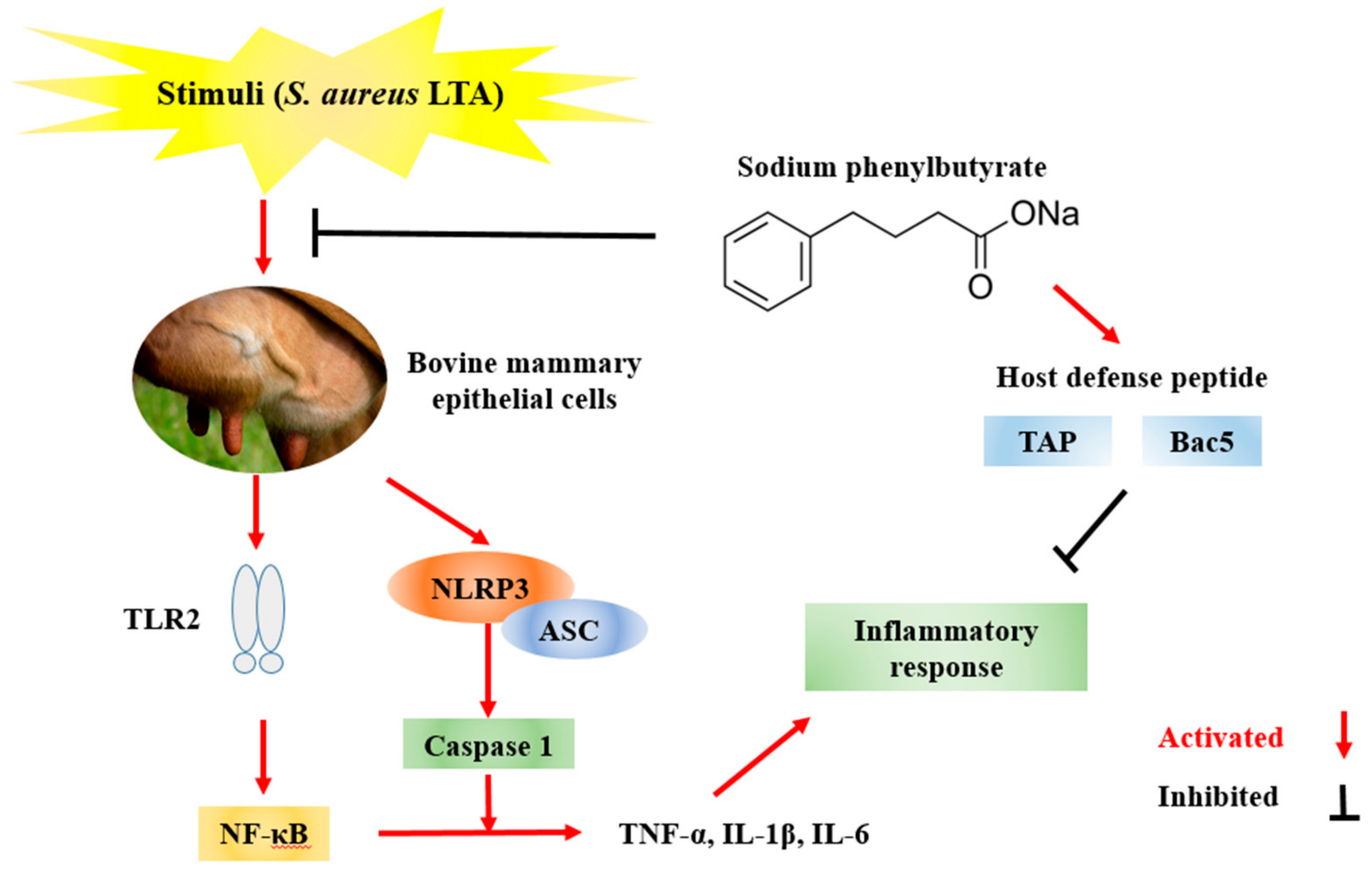
1. Introduction
2. Results
2.1. The Effect of SPB on Cell Viability
2.2. The Effect of SPB on Inflammatory Cytokines and HDP mRNA Expression in LTA-Stimulated MAC-T
2.3. The Effect of SPB on LTA-Induced TLR2 Expression
2.4. The Effect of SPB on NF-κB Signaling Pathway Activation
2.5. The Effect of SPB on NLRP3 Inflammasome Activation
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cell Culture and Treatment
4.3. Cell Viability Assay
4.4. Quantitative Real-Time PCR Analysis of Bovine Gene Expression
4.5. Western Blot Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ASC | apoptosis-associated speck-like protein containing CARD |
| Bac5 | bactenecin 5 |
| bMECs | bovine mammary epithelial cells |
| CARD | caspase recruitment domain |
| ER | endoplasmic reticulum |
| HDAC | histone deacetylase |
| HDP | host defense peptide |
| IκBα | inhibitor of NF-κB |
| IL-1β | interleukin 1 beta |
| IL-6 | interleukin 6 |
| LPS | lipopolysaccharide |
| LTA | lipoteichoic acid |
| MAC-T | bovine mammary alveolar cells |
| NOD | nucleotide-binding oligomerization domain-like |
| NLRP3 | NOD-like receptor protein 3 |
| NF-κB | nuclear factor kappa B |
| 4-PBA | 4-phenylbutyrate |
| qPCR | quantitative real-time PCR |
| SCFA | short-chain fatty acid |
| SB | sodium butyrate |
| SE | standard error |
| SPB | sodium phenylbutyrate |
| TLR2 | Toll-like receptor 2 |
| TAP | tracheal antimicrobial peptide |
| TNF-α | tumor necrosis factor alpha |
References
- Wei, Z.; Xiao, C.; Guo, C.; Zhang, X.; Wang, Y.; Wang, J.; Yang, Z.; Fu, Y. Sodium acetate inhibits Staphylococcus aureus internalization into bovine mammary epithelial cells by inhibiting NF-κB activation. Microb. Pathog. 2017, 107, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, S.; Meng, L.; Dong, L.; Zhao, S.; Lan, X.; Wang, J.; Zheng, N. Prevalence, antimicrobial susceptibility, and molecular characterization of Staphylococcus aureus isolated from dairy herds in northern China. J. Dairy Sci. 2017, 100, 8796–8803. [Google Scholar] [CrossRef] [PubMed]
- Bonsaglia, E.C.R.; Silva, N.C.C.; Rossi, B.F.; Camargo, C.H.; Dantas, S.T.A.; Langoni, H.; Guimarães, F.F.; Lima, F.S.; Fitzgerald, J.R.; et al. Molecular epidemiology of methicillin-susceptible Staphylococcus aureus (MSSA) isolated from milk of cows with subclinical mastitis. Microb. Pathog. 2018, 124, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Sadek, K.; Saleh, E.; Ayoub, M. Selective, reliable blood and milk bio-markers for diagnosing clinical and subclinical bovine mastitis. Trop. Anim. Health Prod. 2017, 49, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Kiku, Y.; Nagasawa, Y.; Tanabe, F.; Sugawara, K.; Watanabe, A.; Hata, E.; Ozawa, T.; Nakajima, K.; Arai, T.; Hayashi, T. The cell wall component lipoteichoic acid of Staphylococcus aureus induces chemokine gene expression in bovine mammary epithelial cells. J. Vet. Med. Sci. 2016, 78, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Park, Y. Anti-endotoxin mechanism of the KW4 peptide in inflammation in RAW 264.7 cells induced by LTA and drug-resistant Staphylococcus aureus 1630. Amino Acids 2018, 50, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Jiang, K.; Wu, H.; Qiu, C.; Deng, G.; Peng, X. Polydatin reduces Staphylococcus aureus lipoteichoic acid-induced injury by attenuating reactive oxygen species generation and TLR2-NFκB signalling. J. Cell Mol. Med. 2017, 21, 2796–2808. [Google Scholar] [CrossRef] [PubMed]
- Naganuma, Y.; Takakubo, Y.; Hirayama, T.; Tamaki, Y.; Pajarinen, J.; Sasaki, K.; Goodman, S.B.; Takagi, M. Lipoteichoic acid modulates inflammatory response in macrophages after phagocytosis of titanium particles through Toll-like receptor 2 cascade and inflammasomes. J. Biomed. Mater. Res. A 2016, 104, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jin, H.; Ye, D.; Wang, J.; Ao, X.; Dong, M.; Niu, W. Enterococcus faecalis Lipoteichoic Acid-induced NLRP3 Inflammasome via the Activation of the Nuclear Factor Kappa B Pathway. J. Endod. 2016, 42, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Callaway, J.B.; Ting, J.P. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Lacasse, P. Mammary tissue damage during bovine mastitis: Causes and control. J. Anim. Sci. 2008, 86, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Zhan, K.; Jiang, M.C.; Gong, X.; Zhao, G. Effect of short-chain fatty acids on the expression of genes involved in short-chain fatty acid transporters and inflammatory response in goat jejunum epithelial cells. In Vitro Cell Dev. Biol. Anim. 2018, 54, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, D.R.; Collins, L.B.; Wali, A.; Bigler, R.; Sun, W.; Bultman, S.J. The Warburg Effect Dictates the Mechanism of Butyrate Mediated Histone Acetylation and Cell Proliferation. Mol. Cell. 2012, 48, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.; Sun, L.; Zhou, G.; Ge, H.; Meng, Y.; Li, J.; Li, X.; Fang, X. Sodium Phenylbutyrate Inhibits Tumor Growth and the Epithelial-Mesenchymal Transition of Oral Squamous Cell Carcinoma In Vitro and In Vivo. Cancer Biother. Radiopharm. 2018, 33, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Van der Does, A.M.; Kenne, E.; Koppelaar, E.; Agerberth, B.; Lindbom, L. Vitamin D3 and phenylbutyrate promote development of a human dendritic cell subset displaying enhanced antimicrobial properties. J. Leukoc. Biol. 2014, 95, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Alva-Murillo, N.; Medina-Estrada, I.; Báez-Magaña, M.; Ochoa-Zarzosa, A.; López-Meza, J.E. The activation of the TLR2/p38 pathway by sodium butyrate in bovine mammary epithelial cells is involved in the reduction of Staphylococcus aureus internalization. Mol. Immunol. 2015, 68, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Carson, D.A.; Barkema, H.W.; Naushad, S.; De Buck, J. Bacteriocins of non-aureus Staphylococci isolated from bovine milk. Appl. Environ. Microbiol. 2017, 83, 01015–01017. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Jaiprakash, P.; Dave, A.; Pai, D. Idiopathic granulomatous mastitis: An institutional experience. Turk. J. Surg. 2017, 33, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Wall, S.K.; Hernández-Castellano, L.E.; Ahmadpour, A.; Bruckmaier, R.M.; Wellnitz, O. Differential glucocorticoid-induced closure of the blood-milk barrier during lipopolysaccharide- and lipoteichoic acid-induced mastitis in dairy cows. J. Dairy Sci. 2016, 99, 7544–7553. [Google Scholar] [CrossRef] [PubMed]
- Scali, F.; Camussone, C.; Calvinho, L.F.; Cipolla, M.; Zecconi, A. Which are important targets in development of S. aureus, mastitis vaccine? Res. Vet. Sci. 2015, 100, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Bougarn, S.; Cunha, P.; Harmache, A.; Fromageau, A.; Gilbert, F.B.; Rainard, P. Muramyl dipeptide synergizes with Staphylococcus aureus lipoteichoic acid to recruit neutrophils in the mammary gland and to stimulate mammary epithelial cells. Clin. Vaccine Immunol. 2010, 17, 1797–1809. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Zarzosa, A.; Villarreal-Fernández, E.; Cano-Camacho, H.; López-Meza, J.E. Sodium butyrate inhibits Staphylococcus aureus internalization in bovine mammary epithelial cells and induces the expression of antimicrobial peptide genes. Microb. Pathog. 2009, 47, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.P.; Deng, W.H.; Guo, W.Y.; Shi, Q.; Zhao, L.; You, Y.D.; Mei, F.C.; Zhou, Y.; Wang, C.Y.; Chen, C.; et al. Inhibition of endoplasmic reticulum stress by 4-phenylbutyric acid prevents vital organ injury in rat acute pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Sang, W.; Chen, S.; Chen, R.; Zhang, H.; Xue, F.; Li, Z.; Liu, Y.; Gong, Y.; Zhang, H.; et al. 4-PBA inhibits LPS-induced inflammation through regulating ER stress and autophagy in acute lung injury models. Toxicol. Lett. 2017, 271, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Zhou, L.; Zhang, X.; Wen, T.; Shi, H.; Xie, B.; Li, Z.; Chen, D.; Wang, Z.; Duan, Z. Endoplasmic reticulum stress-activated glycogen synthase kinase 3β aggravates liver inflammation and hepatotoxicity in mice with acute liver failure. Inflammation 2015, 38, 1151–1165. [Google Scholar] [CrossRef] [PubMed]
- Kościuczuk, E.M.; Lisowski, P.; Jarczak, J.; Krzyżewski, J.; Zwierzchowski, L.; Bagnicka, E. Expression patterns of β-defensin and cathelicidin genes in parenchyma of bovine mammary gland infected with coagulase-positive or coagulase-negative Staphylococci. BMC Vet. Res. 2014, 10, 246. [Google Scholar] [CrossRef] [PubMed]
- Taha-Abdelaziz, K.; Perez-Casal, J.; Schott, C.; Hsiao, J.; Attah-Poku, S.; Slavić, D.; Caswell, J.L. Bactericidal activity of tracheal antimicrobial peptide against respiratory pathogens of cattle. Vet. Immunol. Immunopathol. 2013, 152, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Mardirossian, M.; Barrière, Q.; Timchenko, T.; Müller, C.; Pacor, S.; Mergaert, P.; Scocchi, M.; Wilson, D.N. Fragments of the Nonlytic Proline-Rich Antimicrobial Peptide Bac5 Kill Escherichia coli Cells by Inhibiting Protein Synthesis. Antimicrob. Agents Chemother. 2018, 62, e00534-18. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Pang, X.L.; Shang, W.J.; Xie, H.C.; Wang, J.X.; Feng, G.W. Over-expressed microRNA-181a reduces glomerular sclerosis and renal tubular epithelial injury in rats with chronic kidney disease via down-regulation of the TLR/NF-κB pathway by binding to CRY1. Mol. Med. 2018, 24, 49. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Hayden, M.S. New regulators of NF-kappaB in inflammation. Nat. Rev. Immunol. 2008, 8, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Kim, H.J.; Kim, D.I.; Lee, K.B.; Park, H.J.; Jeong, J.S.; Cho, S.H.; Lee, Y.C. Blockade of Interplay between IL-17A and Endoplasmic Reticulum Stress Attenuates LPS-Induced Lung Injury. Theranostics 2015, 5, 1343–1362. [Google Scholar] [CrossRef] [PubMed]
- Oviedo-Boyso, J.; Barriga-Rivera, J.G.; Valdez-Alarcón, J.J.; Bravo-Patiño, A.; Cárabez-Trejo, A.; Cajero-Juárez, M.; Baizabal-Aguirre, V.M. Internalization of Staphylococcus aureus by bovine endothelial cells is associated with the activity state of NF-kappaB and modulated by the pro-inflammatory cytokines TNF-alpha and IL-1beta. Scand. J. Immunol. 2008, 67, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Mangan, M.S.J.; Olhava, E.J.; Roush, W.R.; Seidel, H.M.; Glick, G.D.; Latz, E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discov. 2018, 17, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Coussens, A.K.; Wilkinson, R.J.; Martineau, A.R. Phenylbutyrate Is Bacteriostatic against Mycobacterium tuberculosis and Regulates the Macrophage Response to Infection, Synergistically with 25-Hydroxy-Vitamin D3. PLoS Pathog. 2015, 11, e1005007. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Sodium phenylbutyrate is available from the authors. |





| Gene | Primer | Sequence (5′–3′) | Product Size (bp) |
|---|---|---|---|
| TNF-α | Forward | TGGGCTCCAAGCATCCAACT | 182 |
| Reverse | GGCCCTCACATTCTGGGTGT | ||
| IL-1β | Forward | TCGAAACGTCCTCCGACGAG | 131 |
| Reverse | TGAGAGGAGGTGGAGAGCCT | ||
| IL-6 | Forward | CAAGCGCCTTCACTCCATTC | 176 |
| Reverse | GATTTTGTCGACCATGCGCT | ||
| TAP | Forward | CGCTCCTCTTCCTGGTCCTG | 197 |
| Reverse | TGATCCCGGCTGTGTCTTGG | ||
| Bac5 | Forward | CAGTCACCCTGGACCCATCA | 124 |
| Reverse | GGGCGGAACGGTGGATAGAA | ||
| 18S rRNA | Forward | AGTGGAGCCTGCGGCTTAAT | 105 |
| Reverse | CACCACCCACGGAATCGAGA |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhang, M.; Jiang, N.; Zhang, A. Sodium Phenylbutyrate Ameliorates Inflammatory Response Induced by Staphylococcus aureus Lipoteichoic Acid via Suppressing TLR2/NF-κB/NLRP3 Pathways in MAC-T Cells. Molecules 2018, 23, 3056. https://doi.org/10.3390/molecules23123056
Wang X, Zhang M, Jiang N, Zhang A. Sodium Phenylbutyrate Ameliorates Inflammatory Response Induced by Staphylococcus aureus Lipoteichoic Acid via Suppressing TLR2/NF-κB/NLRP3 Pathways in MAC-T Cells. Molecules. 2018; 23(12):3056. https://doi.org/10.3390/molecules23123056
Chicago/Turabian StyleWang, Xin, Mengmeng Zhang, Ning Jiang, and Aizhong Zhang. 2018. "Sodium Phenylbutyrate Ameliorates Inflammatory Response Induced by Staphylococcus aureus Lipoteichoic Acid via Suppressing TLR2/NF-κB/NLRP3 Pathways in MAC-T Cells" Molecules 23, no. 12: 3056. https://doi.org/10.3390/molecules23123056
APA StyleWang, X., Zhang, M., Jiang, N., & Zhang, A. (2018). Sodium Phenylbutyrate Ameliorates Inflammatory Response Induced by Staphylococcus aureus Lipoteichoic Acid via Suppressing TLR2/NF-κB/NLRP3 Pathways in MAC-T Cells. Molecules, 23(12), 3056. https://doi.org/10.3390/molecules23123056





