Effects of Kisspeptin-10 on Hypothalamic Neuropeptides and Neurotransmitters Involved in Appetite Control
Abstract
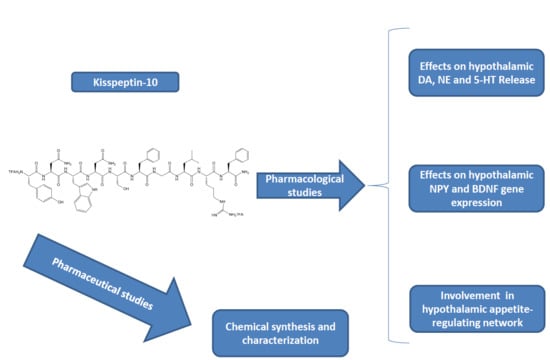
:1. Introduction
2. Results
Pharmacological Studies
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.2. Synthesis and Characterization
4.3. Cell Cultures and Viability Test
4.4. RNA Extraction, Reverse Transcription, and Real-Time Reverse Transcription Polymerase Chain Reaction (Real-Time RT PCR)
4.5. Neurotransmitter Extraction and High Performance Liquid Chromatography (HPLC) Determination
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lee, J.H.; Miele, M.E.; Hicks, D.J.; Phillips, K.K.; Trent, J.M.; Weissman, B.E.; Welch, D.R. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J. Natl. Cancer Inst. 1996, 88, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Kotani, M.; Detheux, M.; Vandenbogaerde, A.; Communi, D.; Vanderwinden, J.M.; Le Poul, E.; Brézillon, S.; Tyldesley, R.; Suarez-Huerta, N.; Vandeput, F.; et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J. Biol. Chem. 2001, 276, 34631–34636. [Google Scholar] [CrossRef] [PubMed]
- Ohtaki, T.; Shintani, Y.; Honda, S.; Matsumoto, H.; Hori, A.; Kanehashi, K.; Terao, Y.; Kumano, S.; Takatsu, Y.; Masuda, Y.; et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 2001, 411, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Kirby, H.R.; Maguire, J.J.; Colledge, W.H.; Davenport, A.P. International Union of Basic and Clinical Pharmacology. LXXVII. Kisspeptin receptor nomenclature, distribution, and function. Pharmacol. Rev. 2010, 62, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Stengel, A.; Wang, L.; Goebel-Stengel, M.; Taché, Y. Centrally injected kisspeptin reduces food intake by increasing meal intervals in mice. Neuroreport 2011, 22, 253–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arai, A.C. The role of kisspeptin and GPR54 in the hippocampus. Peptides 2009, 30, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Pineda, R.; Plaisier, F.; Millar, R.P.; Ludwig, M. Amygdala Kisspeptin Neurons: Putative Mediators of Olfactory Control of the Gonadotropic Axis. Neuroendocrinology 2017, 104, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Gresham, R.; Li, S.; Adekunbi, D.A.; Hu, M.; Li, X.F.; O’Byrne, K.T. Kisspeptin in the medial amygdala and sexual behavior in male rats. Neurosci. Lett. 2016, 627, 13–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottsch, M.L.; Cunningham, M.J.; Smith, J.T.; Popa, S.M.; Acohido, B.V.; Crowley, W.F.; Seminara, S.; Clifton, D.K.; Steiner, R.A. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 2004, 145, 4073–4077. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, J.; Herbison, A.E. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 2006, 147, 5817–5825. [Google Scholar] [CrossRef] [PubMed]
- Irwig, M.S.; Fraley, G.S.; Smith, J.T.; Acohido, B.V.; Popa, S.M.; Cunningham, M.J.; Gottsch, M.L.; Clifton, D.K.; Steiner, R.A. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology 2004, 80, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.W.; Woods, S.C.; Porte, D., Jr.; Seeley, R.J.; Baskin, D.G. Central nervous system control of food intake. Nature 2000, 404, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Gottsch, M.L.; Clifton, D.K.; Steiner, R.A. Kisspepeptin-GPR54 signaling in the neuroendocrine reproductive axis. Mol. Cell. Endocrinol. 2006, 254–255, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.M.; Bentsen, A.H.; Mikkelsen, J.D.; Tena-Sempere, M. Kisspeptins: Bridging energy homeostasis and reproduction. Brain Res. 2010, 1364, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Rahayu, L.P.; Behiry, M.E.; Endo, N.; Tanaka, T. Effect of investigational kisspeptin/metastin analog, TAK-683, on luteinizing hormone secretion at different stages of the luteal phase in goats. J. Reprod. Dev. 2017, 63, 221–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, E.L.; Patterson, M.; Murphy, K.G.; Smith, K.L.; Dhillo, W.S.; Todd, J.F.; Ghatei, M.A.; Bloom, S.R. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J. Neuroendocrinol. 2004, 16, 850–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellano, J.M.; Navarro, V.M.; Fernández-Fernández, R.; Nogueiras, R.; Tovar, S.; Roa, J.; Vazquez, M.J.; Vigo, E.; Casanueva, F.F.; Aguilar, E.; et al. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology 2005, 146, 3917–3925. [Google Scholar] [CrossRef] [PubMed]
- Clarke, I.J.; Henry, B.A. Leptin and reproduction. Rev. Reprod. 1999, 4, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.; Ashford, M.L. Leptin in the CNS: Much more than a satiety signal. Neuropharmacology 2003, 44, 845–854. [Google Scholar] [CrossRef]
- Lazzarino, G.P.; Andreoli, M.F.; Rossetti, M.F.; Stoker, C.; Tschopp, M.V.; Luque, E.H.; Ramos, J.G. Cafeteria diet differentially alters the expression of feeding-related genes through DNA methylation mechanisms in individual hypothalamic nuclei. Mol. Cell. Endocrinol. 2017, 450, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Roa, J.; Garcia-Galiano, D.; Varela, L.; Sánchez-Garrido, M.A.; Pineda, R.; Castellano, J.M.; Ruiz-Pino, F.; Romero, M.; Aguilar, E.; López, M.; et al. The mammalian target of rapamycin as novel central regulator of puberty onset via modulation of hypothalamic Kiss1 system. Endocrinology 2009, 150, 5016–5026. [Google Scholar] [CrossRef] [PubMed]
- McShane, T.M.; May, T.; Miner, J.L.; Keisler, D.H. Central actions of neuropeptide-Y may provide a neuromodulatory link between nutrition and reproduction. Biol. Reprod. 1992, 46, 1151–1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaillou, E.; Baumont, R.; Chilliard, Y.; Tillet, Y. Several subpopulations of neuropeptide Y-containing neurons exist in the infundibular nucleus of sheep: An immunohistochemical study of animals on different diets. J. Comp. Neurol. 2002, 444, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Foradori, C.D.; Whitlock, B.K.; Daniel, J.A.; Zimmerman, A.D.; Jones, M.A.; Read, C.C.; Steele, B.P.; Smith, J.T.; Clarke, I.J.; Elsasser, T.H.; et al. Kisspeptin stimulates growth hormone release by utilizing neuropeptide Y pathways and is dependent on the presence of ghrelin in the ewe. Endocrinology 2017, 158, 3526–3539. [Google Scholar] [CrossRef] [PubMed]
- Conner, J.M.; Lauterborn, J.C.; Yan, Q.; Gall, C.M.; Varon, S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: Evidence for anterograde axonal transport. J. Neurosci. 1997, 17, 2295–2313. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, L.; Di Nisio, C.; Recinella, L.; Orlando, G.; Ferrante, C.; Chiavaroli, A.; Leone, S.; Di Michele, P.; Shohreh, R.; Vacca, M. Obestatin inhibits dopamine release in rat hypothalamus. Eur. J. Pharmacol. 2010, 641, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, L.; Orlando, G.; Ferrante, C.; Recinella, L.; Leone, S.; Chiavaroli, A.; Di Nisio, C.; Shohreh, R.; Manippa, F.; Ricciuti, A.; et al. Peripheral chemerin administration modulates hypothalamic control of feeding. Peptides 2014, 51, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, C.; Orlando, G.; Recinella, L.; Leone, S.; Chiavaroli, A.; Di Nisio, C.; Shohreh, R.; Manippa, F.; Ricciuti, A.; Vacca, M.; et al. Central apelin-13 administration modulates hypothalamic control of feeding. J. Biol. Regul. Homeost. Agents 2016, 30, 883–888. [Google Scholar] [PubMed]
- Ferrante, C.; Orlando, G.; Recinella, L.; Leone, S.; Chiavaroli, A.; Di Nisio, C.; Shohreh, R.; Manippa, F.; Ricciuti, A.; Vacca, M.; et al. Central inhibitory effects on feeding induced by the adipo-myokine irisin. Eur. J. Pharmacol. 2016, 791, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Backholer, K.; Smith, J.T.; Rao, A.; Pereira, A.; Iqbal, J.; Ogawa, S.; Clarke, I.J. Kisspeptin cells in the ewe brain respond to leptin and communicate with neuropeptide Y and proopiomelanocortin cells. Endocrinology 2010, 151, 2233–2243. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.L.; Dhillon, S.S.; Belsham, D.D. Kisspeptin directly regulates neuropeptide Y synthesis and secretion via the ERK1/2 and p38 mitogen-activated protein kinase signaling pathways in NPY-secreting hypothalamic neurons. Endocrinology 2010, 151, 5038–5047. [Google Scholar] [CrossRef] [PubMed]
- Luque, R.M.; Kineman, R.D.; Tena-Sempere, M. Regulation of hypothalamic expression of KiSS-1 and GPR54 genes by metabolic factors: Analyses using mouse models and a cell line. Endocrinology 2007, 148, 4601–4611. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.Y.; van den Pol, A.N. Kisspeptin directly excites anorexigenic proopiomelanocortin neurons but inhibits orexigenic neuropeptide Y cells by an indirect synaptic mechanism. J. Neurosci. 2010, 30, 10205–10219. [Google Scholar] [CrossRef] [PubMed]
- Cowley, M.A.; Smart, J.L.; Rubinstein, M.; Cerdán, M.G.; Diano, S.; Horvath, T.L.; Cone, R.D.; Low, M.J. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 2001, 411, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Bomberg, E.; Billington, C.; Levine, A.; Kotz, C.M. Brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus reduces energy intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R1003–R1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Bomberg, E.; Billington, C.; Levine, A.; Kotz, C.M. Brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus increases energy expenditure by elevating metabolic rate. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R992–R1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, B.; Goulding, E.H.; Zang, K.; Cepoi, D.; Cone, R.D.; Jones, K.R.; Tecott, L.H.; Reichardt, L.F. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat. Neurosci. 2003, 6, 736–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.J.; Chang, C.K.; Liu, I.M.; Chi, T.C.; Yu, H.J.; Cheng, J.T. Changes in endogenous monoamines in aged rats. Clin. Exp. Pharmacol. Physiol. 2001, 28, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Szawka, R.E.; Ribeiro, A.B.; Leite, C.M.; Helena, C.V.; Franci, C.R.; Anderson, G.M.; Hoffman, G.E.; Anselmo-Franci, J.A. Kisspeptin regulates prolactin release through hypothalamic dopaminergic neurons. Endocrinology 2010, 151, 3247–3257. [Google Scholar] [CrossRef] [PubMed]
- Gillard, E.R.; Dang, D.Q.; Stanley, B.G. Evidence that neuropeptide Y and dopamine in the perifornical hypothalamus interact antagonistically in the control of food intake. Brain Res. 1993, 628, 128–136. [Google Scholar] [CrossRef]
- Yang, Z.J.; Meguid, M.M. LHA dopaminergic activity in obese and lean Zucker rats. Neuroreport 1995, 6, 1191–1194. [Google Scholar] [CrossRef] [PubMed]
- Leibowitz, S.F.; Alexander, J.T. Hypothalamic serotonin in control of eating behavior, meal size, and body weight. Biol. Psychiatry 1998, 44, 851–864. [Google Scholar] [CrossRef]
- Recinella, L.; Leone, S.; Ferrante, C.; Chiavaroli, A.; Shohreh, R.; Di Nisio, C.; Vacca, M.; Orlando, G.; Salvatori, R.; Brunetti, L. Effects of growth hormone-releasing hormone gene targeted ablation on ghrelin-induced feeding. Growth Horm. IGF Res. 2017, 37, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Ke, R.; Ma, X.; Lee, L.T.O. Understanding the functions of kisspeptin and kisspeptin receptor (Kiss1R) from clinical case studies. Peptides 2018. [Google Scholar] [CrossRef] [PubMed]
- Munkboel, C.H.; Larsen, L.W.; Weisser, J.J.; Møbjerg Kristensen, D.; Styrishave, B. Sertraline Suppresses Testis and Adrenal Steroid Production and Steroidogenic Gene Expression While Increasing LH in Plasma of Male Rats Resulting in Compensatory Hypogonadism. Toxicol. Sci. 2018, 163, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Mollica, A.; Pinnen, F.; Stefanucci, A.; Costante, R. The evolution of peptide synthesis: From early days to small molecular machines. Curr. Bioact. Compd. 2013, 9, 184–202. [Google Scholar] [CrossRef]
- Mollica, A.; Costante, R.; Akdemir, A.; Carradori, S.; Stefanucci, A.; Macedonio, G.; Ceruso, M.; Supuran, C.T. Exploring new Probenecid-based carbonic anhydrase inhibitors: Synthesis, biological evaluation and docking studies. Bioorg. Med. Chem. 2015, 23, 5311–5318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mollica, A.; Costante, R.; Stefanucci, A.; Novellino, E. Cyclotides: A natural combinatorial peptide library or a bioactive sequence player? J. Enzyme Inhib. Med. Chem. 2015, 30, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Mollica, A.; Costante, R.; Novellino, E.; Stefanucci, A.; Pieretti, S.; Zador, F.; Samavati, R.; Borsodi, A.; Benyhe, S.; Vetter, I.; et al. Design, synthesis and biological evaluation of two opioid agonist and Cav2.2 blocker multitarget ligands. Chem. Biol. Drug Des. 2014, 86, 156–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sintiprungrat, K.; Singhto, N.; Sinchaikul, S.; Chen, S.T.; Thongboonkerd, V. Alterations in cellular proteome and secretome upon differentiation from monocyte to macrophage by treatment with phorbol myristate acetate: Insights into biological processes. J. Proteom. 2010, 73, 602–618. [Google Scholar] [CrossRef] [PubMed]
- Kroll, H.; Bolsover, S.; Hsu, J.; Kim, S.H.; Bouloux, P.M. Kisspeptin-evoked calcium signals in isolated primary rat gonadotropin- releasing hormone neurones. Neuroendocrinology 2011, 93, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Schwetz, T.A.; Reissaus, C.A.; Piston, D.W. Differential stimulation of insulin secretion by GLP-1 and Kisspeptin-10. PLoS ONE 2014, 9, e113020. [Google Scholar] [CrossRef] [PubMed]
- Kadokawa, H.; Suzuki, S.; Hashizume, T. Kisspeptin-10 stimulates the secretion of growth hormone and prolactin directly from cultured bovine anterior pituitary cells. Anim. Reprod. Sci. 2008, 105, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Pruszyńska-Oszmałek, E.; Kołodziejski, P.A.; Sassek, M.; Sliwowska, J.H. Kisspeptin-10 inhibits proliferation and regulates lipolysis and lipogenesis processes in 3T3-L1 cells and isolated rat adipocytes. Endocrine 2017, 56, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Menghini, L.; Leporini, L.; Scanu, N.; Pintore, G.; La Rovere, R.; Di Filippo, E.S.; Pietrangelo, T.; Fulle, S. Effect of phytochemical concentrations on biological activities of cranberry extracts. J. Biol. Regul. Homeost. Agents 2011, 25, 27–35. [Google Scholar] [PubMed]
- Brunetti, L.; Leone, S.; Orlando, G.; Ferrante, C.; Recinella, L.; Chiavaroli, A.; Di Nisio, C.; Shohreh, R.; Manippa, F.; Ricciuti, A.; et al. Hypotensive effects of omentin-1 related to increased adiponectin and decreased interleukin-6 in intra-thoracic pericardial adipose tissue. Pharmacol. Rep. 2014, 66, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, C.; Recinella, L.; Locatelli, M.; Guglielmi, P.; Secci, D.; Leporini, L.; Chiavaroli, A.; Leone, S.; Martinotti, S.; Brunetti, L.; et al. Protective Effects Induced by Microwave-Assisted Aqueous Harpagophytum Extract on Rat Cortex Synaptosomes Challenged with Amyloid β-Peptide. Phytother. Res. 2017, 31, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Kisspeptin-10 (H-Tyr-Asn-Trp-Asn-Ser-Phe-Gly-Leu-Arg-Phe-NH2, YNWNSFGLRF-NH2) has been prepared from the authors. |




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlando, G.; Leone, S.; Ferrante, C.; Chiavaroli, A.; Mollica, A.; Stefanucci, A.; Macedonio, G.; Dimmito, M.P.; Leporini, L.; Menghini, L.; et al. Effects of Kisspeptin-10 on Hypothalamic Neuropeptides and Neurotransmitters Involved in Appetite Control. Molecules 2018, 23, 3071. https://doi.org/10.3390/molecules23123071
Orlando G, Leone S, Ferrante C, Chiavaroli A, Mollica A, Stefanucci A, Macedonio G, Dimmito MP, Leporini L, Menghini L, et al. Effects of Kisspeptin-10 on Hypothalamic Neuropeptides and Neurotransmitters Involved in Appetite Control. Molecules. 2018; 23(12):3071. https://doi.org/10.3390/molecules23123071
Chicago/Turabian StyleOrlando, Giustino, Sheila Leone, Claudio Ferrante, Annalisa Chiavaroli, Adriano Mollica, Azzurra Stefanucci, Giorgia Macedonio, Marilisa Pia Dimmito, Lidia Leporini, Luigi Menghini, and et al. 2018. "Effects of Kisspeptin-10 on Hypothalamic Neuropeptides and Neurotransmitters Involved in Appetite Control" Molecules 23, no. 12: 3071. https://doi.org/10.3390/molecules23123071
APA StyleOrlando, G., Leone, S., Ferrante, C., Chiavaroli, A., Mollica, A., Stefanucci, A., Macedonio, G., Dimmito, M. P., Leporini, L., Menghini, L., Brunetti, L., & Recinella, L. (2018). Effects of Kisspeptin-10 on Hypothalamic Neuropeptides and Neurotransmitters Involved in Appetite Control. Molecules, 23(12), 3071. https://doi.org/10.3390/molecules23123071