Raman Spectroscopic Analysis to Detect Reduced Bone Quality after Sciatic Neurectomy in Mice
Abstract
:1. Introduction
2. Results
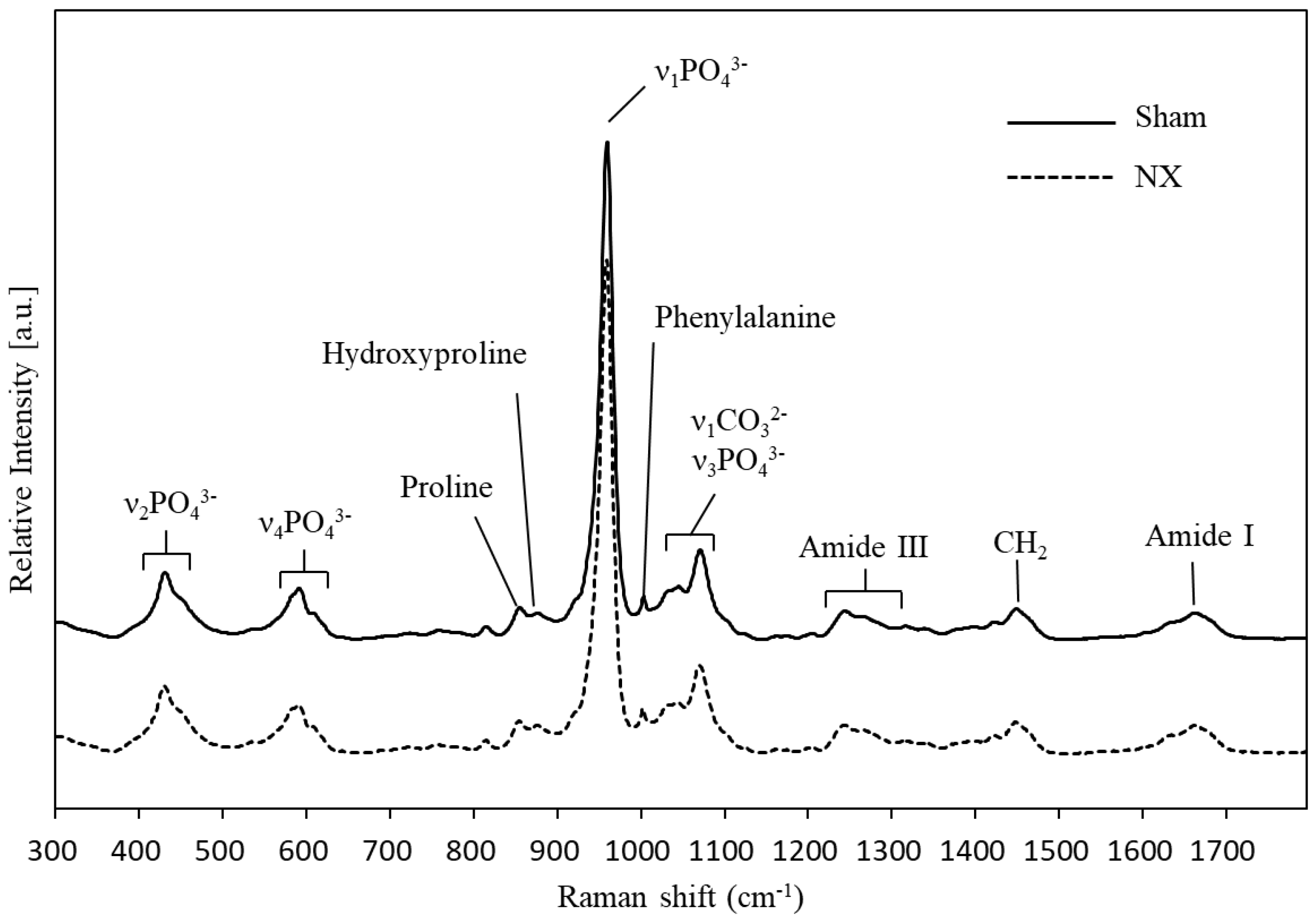
2.1. Raman Spectroscopy
2.2. Principal Component Analysis (PCA) of the Raman Spectral Data
2.3. Bone Mineral Density
2.4. Micro-CT
3. Discussion
4. Materials and Methods
4.1. Animals and Surgical Procedures
4.2. Raman Spectroscopy
4.3. Multivariate Analysis of Raman Spectral Data
4.4. BMD Measurement
4.5. Micro-CT Scanning
4.6. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DM | Diabetes mellitus |
| CKD | Chronic kidney disease |
| RA | Rheumatoid arthritis |
| BMD | Bone mineral density |
| DXA | Dual energy X-ray absorptiometry |
| CT | Computed tomography |
| MRI | Magnetic resonance imaging |
| FT-IR | Fourier transform infrared spectroscopy |
| OVX | Ovariectomy |
| PCA | Principal component analysis |
| PCs | Principal components |
| SCI | Spinal cord injury |
| NX | Neurectomy |
| BV/TV | Bone volume |
| SMI | Structural model index |
| EX | Exercise |
| Micro-CT | Micro computed tomography |
| ASBMR | American Society for Bone and Mineral Research |
References
- Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ. Tech. Rep. Ser. 1994, 843, 1–129.
- Ensrud, K.E.; Thompson, D.E.; Cauley, J.A.; Nevitt, M.C.; Kado, D.M.; Hochberg, M.C.; Santora, A.C., 2nd; Black, D.M. Prevalent vertebral deformities predict mortality and hospitalization in older women with low bone mass. J. Am. Geriatr. Soc. 2000, 48, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.D.; Center, J.R.; Eisman, J.A.; Nguyen, T.V. Bone loss, weight loss, and weight fluctuation predict mortality risk in elderly men and women. J. Bone Miner. Res. 2007, 22, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yoshida, H. Low bone mineral density at femoral neck is a predictor of increased mortality in elderly Japanese women. Osteoporosis Int. 2010, 21, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Klibanski, A.; Adams-Campbell, L.; Bassford, T.L.; Blair, S.N.; Boden, S.D.; Dickersin, K.; Gifford, D.R.; Glasse, L.; Goldring, S.R.; Hruska, K.; et al. Osteoporosis prevention, diagnosis, and therapy. J. Am. Med. Assoc. 2001, 285, 785–795. [Google Scholar]
- Cummings, S.R.; Bates, D.; Black, D.M. Clinical use of bone densitometry: Scientific review. J. Am. Med. Assoc. 2002, 288, 1889–1897. [Google Scholar] [CrossRef]
- Soen, S.; Fukunaga, M.; Sugimoto, T.; Sone, T.; Fujiwara, S.; Endo, N.; Gorai, I.; Shiraki, M.; Hagino, H.; Hosoi, T.; et al. Diagnostic criteria for primary osteoporosis: Year 2012 revision. J. Bone Miner. Metab. 2013, 31, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Blake, G.M.; Fogelman, I. How important are BMD accuracy errors for the clinical interpretation of DXA scans? J. Bone Miner. Res. 2008, 23, 457–462. [Google Scholar] [CrossRef] [PubMed]
- McCreadie, B.R.; Goldstein, S.A. Biomechanics of fracture: Is bone mineral density sufficient to assess risk? J. Bone Miner. Res. 2000, 15, 2305–2308. [Google Scholar] [CrossRef] [PubMed]
- Stone, K.L.; Seeley, D.G.; Lui, L.Y.; Cauley, J.A.; Ensrud, K.; Browner, W.S.; Nevitt, M.C.; Cummings, S.R. BMD at multiple sites and risk of fracture of multiple types: Long-term results from the Study of Osteoporotic Fractures. J. Bone Miner. Res. 2003, 18, 1947–1954. [Google Scholar] [CrossRef] [PubMed]
- Marshall, D.; Johnell, O.; Wedel, H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 1996, 312, 1254–1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, M.; Marumo, K. Collagen cross-links as a determinant of bone quality: A possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos. Int. 2010, 21, 195–214. [Google Scholar] [CrossRef] [PubMed]
- Seeman, E.; Delmas, P.D. Bone quality—The material and structural basis of bone strength and fragility. New Engl. J. Med. 2006, 354, 2250–2261. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, E. Methods for assessing bone quality: A review. Clin. Orthop. Relat. Res. 2011, 469, 2128–2138. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.; Mendelsohn, R. Infrared analysis of bone in health and disease. J. Biomed. Optics 2005, 10. [Google Scholar] [CrossRef] [PubMed]
- Chappard, D.; Basle, M.F.; Legrand, E.; Audran, M. New laboratory tools in the assessment of bone quality. Osteoporos. Int. 2011, 22, 2225–2240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paschalis, E.P.; Gamsjaeger, S.; Tatakis, D.N.; Hassler, N.; Robins, S.P.; Klaushofer, K. Fourier transform Infrared spectroscopic characterization of mineralizing type I collagen enzymatic trivalent cross-links. Calcif. Tissue Int. 2015, 96, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.D.; Finney, W.F. Recent developments in Raman and infrared spectroscopy and imaging of bone tissue. Spectroscopy 2004, 18, 155–159. [Google Scholar] [CrossRef] [Green Version]
- Morris, M.D.; Mandair, G.S. Raman assessment of bone quality. Clin. Orthop. Relat. Res. 2011, 469, 2160–2169. [Google Scholar] [CrossRef] [PubMed]
- Mandair, G.S.; Morris, M.D. Contributions of Raman spectroscopy to the understanding of bone strength. Bonekey Rep. 2015, 4, 620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCreadie, B.R.; Morris, M.D.; Chen, T.C.; Sudhaker Rao, D.; Finney, W.F.; Widjaja, E.; Goldstein, S.A. Bone tissue compositional differences in women with and without osteoporotic fracture. Bone 2006, 39, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- Akkus, O.; Adar, F.; Schaffler, M.B. Age-related changes in physicochemical properties of mineral crystals are related to impaired mechanical function of cortical bone. Bone 2004, 34, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Tarnowski, C.P.; Ignelzi, M.A., Jr.; Morris, M.D. Mineralization of developing mouse calvaria as revealed by Raman microspectroscopy. J. Bone Miner. Res. 2002, 17, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Chen, J.; Wu, D.; Du, Z.; Lei, Q.; Cai, Z.; Schultze-Mosgau, S. Effects of ovariectomy on rat mandibular cortical bone: A study using Raman spectroscopy and multivariate analysis. Anal. Chem. 2012, 84, 3318–3323. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Fan, L.; Yang, J.; Shen, A.G.; Hu, J.M. A longitudinal Raman microspectroscopic study of osteoporosis induced by spinal cord injury. Osteoporos. Int. 2010, 21, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.D.; Jiang, L.S.; Dai, L.Y. Mechanisms of osteoporosis in spinal cord injury. Clin. Endocrinol. 2006, 65, 555–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yonezu, H.; Ikata, T.; Takata, S.; Shibata, A. Effects of sciatic neurectomy on the femur in growing rats: Application of peripheral quantitative computed tomography and Fourier transform infrared spectroscopy. J. Bone Miner. Metab. 1999, 17, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.G.; Yan, W.H.; Wei, Z.X.; Liu, J.B. Difference in intraosseous blood vessel volume and number in osteoporotic model mice induced by spinal cord injury and sciatic nerve resection. J. Bone Miner. Metab. 2012, 30, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ishimoto, T.; Nakano, T. Unloading-Induced Degradation of the Anisotropic Arrangement of Collagen/Apatite in Rat Femurs. Calcif. Tissue Int. 2017, 100, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.Q.; Jee, W.S.; Bigornia, A.E.; King, J.G., Jr.; D’Souza, S.M.; Li, X.J.; Ma, Y.F.; Wechter, W.J. Time responses of cancellous and cortical bones to sciatic neurectomy in growing female rats. Bone 1996, 19, 13–21. [Google Scholar] [CrossRef]
- Ishimaru, Y.; Oshima, Y.; Imai, Y.; Iimura, T.; Takanezawa, S.; Hino, K.; Miura, H. Detection of changes in bone quality of osteoporotic model induced by sciatic nerve resection by using Raman spectroscopy. Proc. SPIE 2018, 10497. [Google Scholar] [CrossRef]
- Ishimaru, Y.; Oshima, Y.; Imai, Y.; Iimura, T.; Takanezawa, S.; Hino, K.; Miura, H. Evaluation of bone quality in osteoporosis model mice by Raman spectroscopy. Proc. SPIE 2017, 10251. [Google Scholar] [CrossRef]
- Penel, G.; Delfosse, C.; Descamps, M.; Leroy, G. Composition of bone and apatitic biomaterials as revealed by intravital Raman microspectroscopy. Bone 2005, 36, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Penel, G.; Leroy, G.; Rey, C.; Bres, E. MicroRaman spectral study of the PO4 and CO3 vibrational modes in synthetic and biological apatites. Calcif. Tissue Int. 1998, 63, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Sonju Clasen, A.B.; Ruyter, I.E. Quantitative determination of type A and type B carbonate in human deciduous and permanent enamel by means of Fourier transform infrared spectrometry. Adv. Dent. Res. 1997, 11, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Amer, M.S. Raman Spectroscopy for Soft Matter Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; p. 301. [Google Scholar]
- Takata, S.; Yasui, N. Disuse osteoporosis. J. Med. Invest. 2001, 48, 147–156. [Google Scholar] [PubMed]
- Jiang, S.D.; Jiang, L.S.; Dai, L.Y. Spinal cord injury causes more damage to bone mass, bone structure, biomechanical properties and bone metabolism than sciatic neurectomy in young rats. Osteoporos. Int. 2006, 17, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Paschalis, E.P.; Gamsjaeger, S.; Klaushofer, K. Vibrational spectroscopic techniques to assess bone quality. Osteoporos. Int. 2017, 28, 2275–2291. [Google Scholar] [CrossRef] [PubMed]
- Yerramshetty, J.S.; Lind, C.; Akkus, O. The compositional and physicochemical homogeneity of male femoral cortex increases after the sixth decade. Bone 2006, 39, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Kazama, J.J.; Yamato, H.; Fukagawa, M. Changes in chemical composition of cortical bone associated with bone fragility in rat model with chronic kidney disease. Bone 2011, 48, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Creecy, A.; Uppuganti, S.; Merkel, A.R.; O’Neal, D.; Makowski, A.J.; Granke, M.; Voziyan, P.; Nyman, J.S. Changes in the fracture resistance of bone with the progression of type 2 diabetes in the ZDSD rat. Calcif. Tissue Int. 2016, 99, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Kohn, D.H.; Sahar, N.D.; Wallace, J.M.; Golcuk, K.; Morris, M.D. Exercise alters mineral and matrix composition in the absence of adding new bone. Cells Tissues Organs 2009, 189, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Bala, Y.; Farlay, D.; Boivin, G. Bone mineralization: From tissue to crystal in normal and pathological contexts. Osteoporos. Int. 2013, 24, 2153–2166. [Google Scholar] [CrossRef] [PubMed]
- Cazalbou, S.; Combes, C.; Eichert, D.; Rey, C.; Glimcher, M.J. Poorly crystalline apatites: Evolution and maturation in vitro and in vivo. J. Bone Miner. Metab. 2004, 22, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Gourion-Arsiquaud, S.; Burket, J.C.; Havill, L.M.; DiCarlo, E.; Doty, S.B.; Mendelsohn, R.; van der Meulen, M.C.; Boskey, A.L. Spatial variation in osteonal bone properties relative to tissue and animal age. J. Bone Miner. Res. 2009, 24, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, O.; Bilgin, M.D.; Evis, Z.; Pleshko, N.; Severcan, F. Early Alterations in Bone Characteristics of Type I Diabetic Rat Femur: A Fourier Transform Infrared (FT-IR) Imaging Study. Appl. Spectrosc. 2016, 70, 2005–2015. [Google Scholar] [CrossRef] [PubMed]
- Oest, M.E.; Gong, B.; Esmonde-White, K.; Mann, K.A.; Zimmerman, N.D.; Damron, T.A.; Morris, M.D. Parathyroid hormone attenuates radiation-induced increases in collagen crosslink ratio at periosteal surfaces of mouse tibia. Bone 2016, 86, 91–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paschalis, E.P.; Verdelis, K.; Doty, S.B.; Boskey, A.L.; Mendelsohn, R.; Yamauchi, M. Spectroscopic characterization of collagen cross-links in bone. J. Bone Miner. Res. 2001, 16, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Imai, Y. Fatostatin, an SREBP inhibitor, prevented RANKL-induced bone loss by suppression of osteoclast differentiation. Biochim. Biophys. Acta 2015, 1852, 2432–2441. [Google Scholar] [CrossRef] [PubMed]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Muller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Not available. |





| Raman Shift, cm−1 | Assignment | Comments |
|---|---|---|
| 430 | ν2 PO43− | Strong band |
| 450 | ν2 PO43− | Shoulder on 430 cm−1 band |
| 584–590 | ν4 PO43− | Multiple partially resolved components |
| 609 | ν4 PO43− | Shoulder on 590 cm−1 band |
| 853 | ν(C-C) | Collagen proline, may include δ(C-C-H) contribution from tyrosine |
| 872 | ν(C-C) | Mostly collagen hydroxyproline |
| 955 | ν1 PO43− | Transient bone mineral (P-O) phase, usually seen in immature bone |
| 957 | ν1 PO43− | Bone mineral containing extensive HPO42−, usually immature |
| 959–962 | ν1 PO43− | Bone mineral, mature |
| 1003 | ν(C-C) | Phenylalanine |
| 1035 | ν3 PO43− | Overlaps with proline ν(C-C) component |
| 1048 | ν3 PO43− | |
| 1070 | ν1 CO32− | Overlaps with component of ν3 PO43− |
| 1076 | ν3 PO43− | Overlaps with component of ν1 CO32− |
| 1242 | Amide III | Protein β-sheet and random coils |
| 1272 | Amide III | Protein α-helix |
| 1340 | Amide III | Protein α-helix, sometimes called CH2CH2 wag |
| 1446 | δ(CH2) | Protein CH2 deformation |
| 1660 | Amide I | Strongest amide I ν(C=O) component, polarization sensitive |
| 1690 | Amide I | Shoulder, prominent with immature cross-links |
| The band also relates to β-sheet or disordered secondary structure |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishimaru, Y.; Oshima, Y.; Imai, Y.; Iimura, T.; Takanezawa, S.; Hino, K.; Miura, H. Raman Spectroscopic Analysis to Detect Reduced Bone Quality after Sciatic Neurectomy in Mice. Molecules 2018, 23, 3081. https://doi.org/10.3390/molecules23123081
Ishimaru Y, Oshima Y, Imai Y, Iimura T, Takanezawa S, Hino K, Miura H. Raman Spectroscopic Analysis to Detect Reduced Bone Quality after Sciatic Neurectomy in Mice. Molecules. 2018; 23(12):3081. https://doi.org/10.3390/molecules23123081
Chicago/Turabian StyleIshimaru, Yasumitsu, Yusuke Oshima, Yuuki Imai, Tadahiro Iimura, Sota Takanezawa, Kazunori Hino, and Hiromasa Miura. 2018. "Raman Spectroscopic Analysis to Detect Reduced Bone Quality after Sciatic Neurectomy in Mice" Molecules 23, no. 12: 3081. https://doi.org/10.3390/molecules23123081
APA StyleIshimaru, Y., Oshima, Y., Imai, Y., Iimura, T., Takanezawa, S., Hino, K., & Miura, H. (2018). Raman Spectroscopic Analysis to Detect Reduced Bone Quality after Sciatic Neurectomy in Mice. Molecules, 23(12), 3081. https://doi.org/10.3390/molecules23123081






