Development and In Vitro Evaluation of Linear PEI-Shelled Heparin/Berberine Nanoparticles in Human Osteosarcoma U-2 OS Cells
Abstract
:1. Introduction
2. Results
2.1. Characterizations of Heparin/Berberine (HP/BBR) and Heparin/Berberine/Linear Polyethylenimine (HP/BBR/LPEI) Nanoparticles
2.2. The Release Profiles of Berberine and Berberine Nanoparticles
2.3. The Effects of Berberine and Berberine Nanoparticles on Cell Viability of Osteosarcoma U-2 OS Cells
2.4. The Cellular Uptake of Berberine and Berberine Nanoparticles
2.5. Cell Cycle Distribution of U-2 OS Cells Treated With Berberine and Berberine Nanoparticles
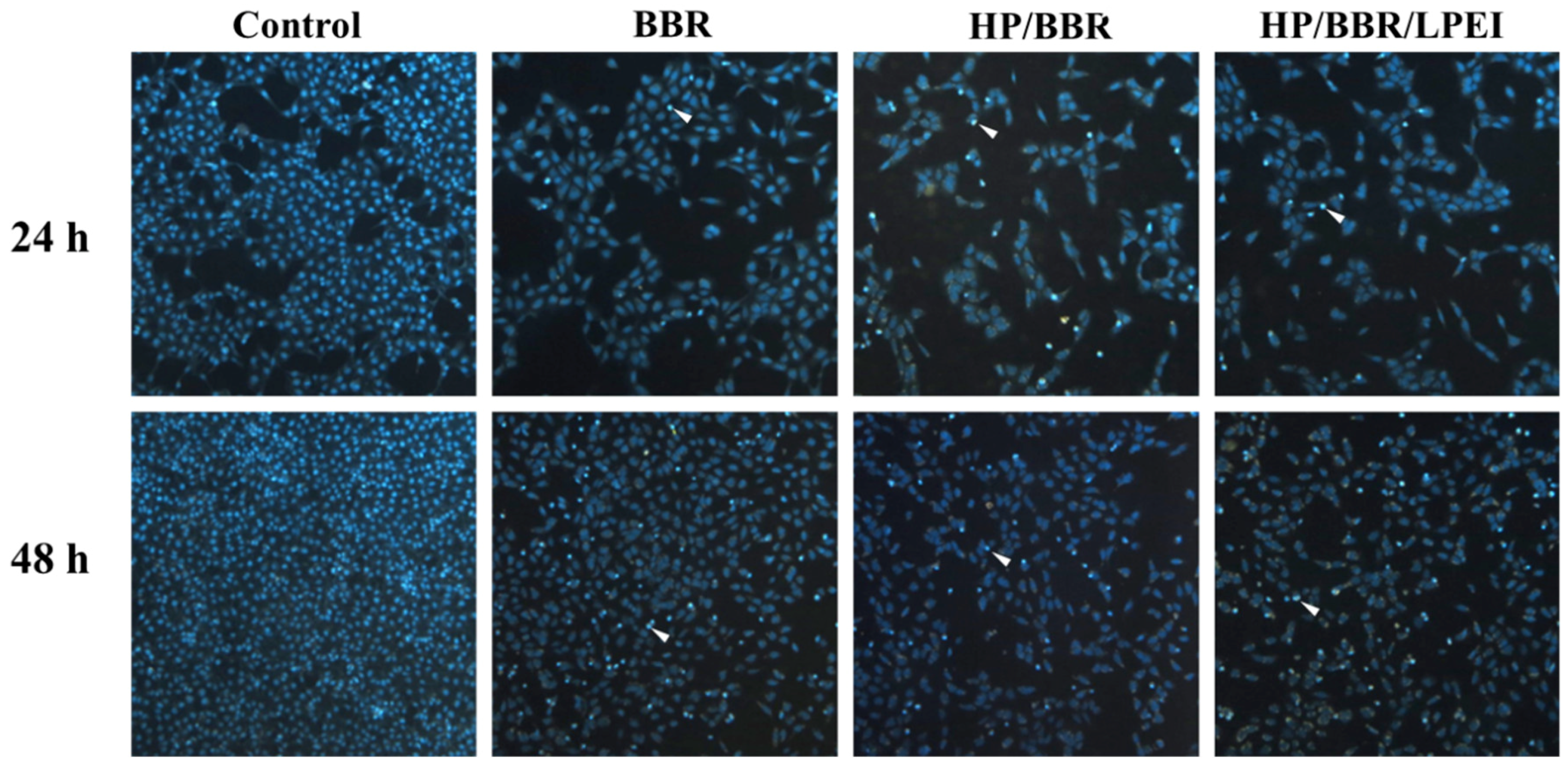
2.6. BBR and BBR NPs Induce DNA Condensation
2.7. Berberine and Berberine Nanoparticles Induce the Expression of p53 and Apoptosis-Associated Proteins
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of HP/BBR or HP/BBR/LPEI Nanoparticles
4.3. Particle Size and Zeta Potential Measurements
4.4. Encapsulation Efficiency Assay of BBR
4.5. Release Profile
4.6. Cell Culture
4.7. Cell Viability Assay and Morphological Observation
4.8. Cellular Uptake of Berberine and Berberine Nanoparticles
4.9. Cell Cycle Assay
4.10. DAPI Staining
4.11. Western Blotting
4.12. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tan, W.; Li, Y.; Chen, M.; Wang, Y. Berberine hydrochloride: anticancer activity and nanoparticulate delivery system. Int. J. Nanomedicine 2011, 6, 1773–1777. [Google Scholar] [CrossRef] [PubMed]
- Budeyri Gokgoz, N.; Avci, F.G.; Yoneten, K.K.; Alaybeyoglu, B.; Ozkirimli, E.; Sayar, N.A.; Kazan, D.; Sariyar Akbulut, B. Response of Escherichia coli to Prolonged Berberine Exposure. Microb. Drug Resist. 2017, 23, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Catapano, A.L. Berberine, a plant alkaloid with lipid- and glucose-lowering properties: From in vitro evidence to clinical studies. Atherosclerosis 2015, 243, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.W.; Yao, X.Q.; Chen, Z.Y.; Ko, W.H.; Huang, Y. Cardiovascular actions of berberine. Cardiovasc. Drug Rev. 2001, 19, 234–244. [Google Scholar] [CrossRef]
- Zhu, Y.; Ma, N.; Li, H.X.; Tian, L.; Ba, Y.F.; Hao, B. Berberine induces apoptosis and DNA damage in MG63 human osteosarcoma cells. Mol. Med. Rep. 2014, 10, 1734–1738. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Kao, S.T.; Chen, G.W.; Ho, H.C.; Chung, J.G. Apoptosis of human leukemia HL-60 cells and murine leukemia WEHI-3 cells induced by berberine through the activation of caspase-3. Anticancer Res. 2006, 26, 227–242. [Google Scholar] [PubMed]
- Liu, Z.; Liu, Q.; Xu, B.; Wu, J.; Guo, C.; Zhu, F.; Yang, Q.; Gao, G.; Gong, Y.; Shao, C. Berberine induces p53-dependent cell cycle arrest and apoptosis of human osteosarcoma cells by inflicting DNA damage. Mutat. Res. 2009, 662, 75–83. [Google Scholar] [CrossRef]
- Mittal, A.; Tabasum, S.; Singh, R.P. Berberine in combination with doxorubicin suppresses growth of murine melanoma B16F10 cells in culture and xenograft. Phytomedicine 2014, 21, 340–347. [Google Scholar] [CrossRef]
- Choi, M.S.; Oh, J.H.; Kim, S.M.; Jung, H.Y.; Yoo, H.S.; Lee, Y.M.; Moon, D.C.; Han, S.B.; Hong, J.T. Berberine inhibits p53-dependent cell growth through induction of apoptosis of prostate cancer cells. Int. J. Oncol. 2009, 34, 1221–1230. [Google Scholar] [Green Version]
- Park, K.S.; Kim, J.B.; Lee, S.J.; Bae, J. Berberine-induced growth inhibition of epithelial ovarian carcinoma cell lines. J. Obstet. Gynaecol. Res. 2012, 38, 535–540. [Google Scholar] [CrossRef]
- Li, L.; Wang, X.; Sharvan, R.; Gao, J.; Qu, S. Berberine could inhibit thyroid carcinoma cells by inducing mitochondrial apoptosis, G0/G1 cell cycle arrest and suppressing migration via PI3K-AKT and MAPK signaling pathways. Biomed. Pharmacother. 2017, 95, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.P.; Chuang, T.C.; Yeh, M.H.; Hsu, S.C.; Way, T.D.; Chen, P.Y.; Wang, S.S.; Chang, Y.H.; Kao, M.C.; Liu, J.Y. Growth suppression of HER2-overexpressing breast cancer cells by berberine via modulation of the HER2/PI3K/Akt signaling pathway. J. Agric. Food Chem. 2011, 59, 8216–8224. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Q.; Liu, Z.; Li, B.; Sun, Z.; Zhou, H.; Zhang, X.; Gong, Y.; Shao, C. Berberine, a genotoxic alkaloid, induces ATM-Chk1 mediated G2 arrest in prostate cancer cells. Mut. Res. 2012, 734, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Zhang, C.; Feng, J.; Hou, L.; Yan, L.; Zhou, Z.; Liu, Z.; Liu, C.; Fan, Y.; Zheng, B.; Xu, Z. Induction of G1 cell cycle arrest and apoptosis by berberine in bladder cancer cells. Eur. J. Pharmacol. 2011, 661, 1–7. [Google Scholar] [CrossRef]
- Puthdee, N.; Seubwai, W.; Vaeteewoottacharn, K.; Boonmars, T.; Cha’on, U.; Phoomak, C.; Wongkham, S. Berberine Induces Cell Cycle Arrest in Cholangiocarcinoma Cell Lines via Inhibition of NF-kappaB and STAT3 Pathways. Biol. Pharm. Bull. 2017, 40, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.T.; Lu, C.C.; Yang, J.S.; Chiang, J.H.; Li, T.C.; Ip, S.W.; Hsia, T.C.; Liao, C.L.; Lin, J.G.; Wood, W.G.; et al. Berberine induced apoptosis via promoting the expression of caspase-8, -9 and -3, apoptosis-inducing factor and endonuclease G in SCC-4 human tongue squamous carcinoma cancer cells. Anticancer Res. 2009, 29, 4063–4070. [Google Scholar] [PubMed]
- Pund, S.; Borade, G.; Rasve, G. Improvement of anti-inflammatory and anti-angiogenic activity of berberine by novel rapid dissolving nanoemulsifying technique. Phytomedicine 2014, 21, 307–314. [Google Scholar] [CrossRef]
- Zuo, F.; Nakamura, N.; Akao, T.; Hattori, M. Pharmacokinetics of berberine and its main metabolites in conventional and pseudo germ-free rats determined by liquid chromatography/ion trap mass spectrometry. Drug Metab. Disp. 2006, 34, 2064–2072. [Google Scholar] [CrossRef]
- Wang, L.; Li, H.; Wang, S.; Liu, R.; Wu, Z.; Wang, C.; Wang, Y.; Chen, M. Enhancing the antitumor activity of berberine hydrochloride by solid lipid nanoparticle encapsulation. AAPS PharmSciTech 2014, 15, 834–844. [Google Scholar] [CrossRef]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv. Drug Deliv. Rev. 2001, 47, 113–131. [Google Scholar] [CrossRef]
- Torchilin, V.P. Multifunctional nanocarriers. Adv. Drug Deliv. Rev. 2006, 58, 1532–1555. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, R.; Singh, S.; Tripathi, M.; Bhatnagar, P.; Kakkar, P.; Gupta, K.C. O-hexadecyl-dextran entrapped berberine nanoparticles abrogate high glucose stress induced apoptosis in primary rat hepatocytes. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Halimani, M.; Chandran, S.P.; Kashyap, S.; Jadhav, V.M.; Prasad, B.L.; Hotha, S.; Maiti, S. Dendritic effect of ligand-coated nanoparticles: enhanced apoptotic activity of silica-berberine nanoconjugates. Langmuir 2009, 25, 2339–2347. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Huang, W.Y.; Lai, C.H.; Hsu, Y.M.; Yao, Y.H.; Chen, T.Y.; Wu, J.Y.; Peng, S.F.; Lin, Y.H. Development of novel nanoparticles shelled with heparin for berberine delivery to treat Helicobacter pylori. Acta Biomater. 2011, 7, 593–603. [Google Scholar] [CrossRef]
- Wu, S.J.; Don, T.M.; Lin, C.W.; Mi, F.L. Delivery of berberine using chitosan/fucoidan-taurine conjugate nanoparticles for treatment of defective intestinal epithelial tight junction barrier. Mar. Drugs 2014, 12, 5677–5697. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Sugahara, K.; Ozbek, S. Evolution of glycosaminoglycans: Comparative biochemical study. Commun. Integr. Biol. 2011, 4, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Sasisekharan, R.; Shriver, Z.; Venkataraman, G.; Narayanasami, U. Roles of heparan-sulphate glycosaminoglycans in cancer. Nat. Rev. Cancer 2002, 2, 521–528. [Google Scholar] [CrossRef]
- Sun, W.; Saldana, M.D.; Fan, L.; Zhao, Y.; Dong, T.; Jin, Y.; Zhang, J. Sulfated polysaccharide heparin used as carrier to load hydrophobic lappaconitine. Int. J. Biol. Macromol. 2016, 84, 275–280. [Google Scholar] [CrossRef]
- Mei, L.; Liu, Y.; Xia, C.; Zhou, Y.; Zhang, Z.; He, Q. Polymer-Drug Nanoparticles Combine Doxorubicin Carrier and Heparin Bioactivity Functionalities for Primary and Metastatic Cancer Treatment. Mol. Pharm. 2017, 14, 513–522. [Google Scholar] [CrossRef]
- Xue, M.; Zhang, L.; Yang, M.X.; Zhang, W.; Li, X.M.; Ou, Z.M.; Li, Z.P.; Liu, S.H.; Li, X.J.; Yang, S.Y. Berberine-loaded solid lipid nanoparticles are concentrated in the liver and ameliorate hepatosteatosis in db/db mice. Int. J. Nanomedicine 2015, 10, 5049–5057. [Google Scholar] [CrossRef]
- Hainaut, P.; Hollstein, M. p53 and human cancer: the first ten thousand mutations. Adv. Cancer Res. 2000, 77, 81–137. [Google Scholar] [PubMed]
- Rivlin, N.; Brosh, R.; Oren, M.; Rotter, V. Mutations in the p53 Tumor Suppressor Gene: Important Milestones at the Various Steps of Tumorigenesis. Genes Cancer 2011, 2, 466–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katiyar, S.K.; Meeran, S.M.; Katiyar, N.; Akhtar, S. p53 Cooperates berberine-induced growth inhibition and apoptosis of non-small cell human lung cancer cells in vitro and tumor xenograft growth in vivo. Mol. Carcinog. 2009, 48, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ji, Q.; Ye, N.; Sui, H.; Zhou, L.; Zhu, H.; Fan, Z.; Cai, J.; Li, Q. Berberine Inhibits Invasion and Metastasis of Colorectal Cancer Cells via COX-2/PGE2 Mediated JAK2/STAT3 Signaling Pathway. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.F.; Hsu, H.K.; Lin, C.C.; Cheng, Y.M.; Hsu, K.H. Novel PEI/Poly-gamma-Gutamic Acid Nanoparticles for High Efficient siRNA and Plasmid DNA Co-Delivery. Molecules 2017, 22. [Google Scholar] [CrossRef] [PubMed]
- Chou, G.L.; Peng, S.F.; Liao, C.L.; Ho, H.C.; Lu, K.W.; Lien, J.C.; Fan, M.J.; La, K.C.; Chung, J.G. Casticin impairs cell growth and induces cell apoptosis via cell cycle arrest in human oral cancer SCC-4 cells. Environ. Toxicol. 2017, 33, 127–141. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |






| Weight Ratio | Size (nm) | Polydispersity Index (PDI) | Zeta Potential (mV) | Loading Efficiency (%) |
|---|---|---|---|---|
| HP/BBR = 24/100 | 282.0 ± 5.1 | 0.20 ± 0.02 | −35.7 ± 0.4 | 49.9 ± 1.3 |
| HP/BBR = 48/100 | 272.0 ± 5.7 | 0.18 ± 0.02 | −40.7 ± 0.7 | 91.4 ± 0.4 |
| HP/BBR = 72/100 | 231.1 ± 6.4 | 0.17 ± 0.01 | −45.7 ± 0.2 | 78.3 ± 0.3 |
| HP/BBR = 96/100 | 232.7 ± 1.9 | 0.17 ± 0.03 | −48.0 ± 1.0 | 73.8 ± 0.3 |
| HP/BBR = 120/100 | 218.4 ± 3.9 | 0.20 ± 0.01 | −44.8 ± 0.6 | 73.4 ± 1.3 |
| HP/BBR = 144/100 | 232.9 ± 11.9 | 0.19 ± 0.00 | −51.9 ± 1.8 | 77.4 ± 0.3 |
| Weight Ratio | Size (nm) | Polydispersity Index (PDI) | Zeta Potential (mV) | Loading Efficiency (%) |
|---|---|---|---|---|
| HP/BBR/LPEI = 72/100/0 | 226.3 ± 3.0 | 0.19 ± 0.01 | −46.5 ± 0.3 | 82.0 ± 0.4 |
| HP/BBR/LPEI = 72/100/4 | 227.1 ± 4.2 | 0.22 ± 0.01 | −44.9 ± 0.7 | 82.2 ± 1.7 |
| HP/BBR/LPEI = 72/100/8 | 234.8 ± 6.3 | 0.32 ± 0.03 | −41.9 ± 1.0 | 86.2 ± 0.3 |
| HP/BBR/LPEI = 72/100/12 | 405.7 ± 85.2 | 0.56 ± 0.12 | −36.1 ± 1.2 | 87.4 ± 1.8 |
| HP/BBR/LPEI = 72/100/16 | 402.3 ± 10.4 | 0.78 ± 0.02 | −35.6 ± 0.5 | 81.1 ± 0.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, H.-K.; Hsu, K.-H.; Cheng, Y.-M.; Suen, H.-Y.; Peng, S.-F. Development and In Vitro Evaluation of Linear PEI-Shelled Heparin/Berberine Nanoparticles in Human Osteosarcoma U-2 OS Cells. Molecules 2018, 23, 3121. https://doi.org/10.3390/molecules23123121
Hsu H-K, Hsu K-H, Cheng Y-M, Suen H-Y, Peng S-F. Development and In Vitro Evaluation of Linear PEI-Shelled Heparin/Berberine Nanoparticles in Human Osteosarcoma U-2 OS Cells. Molecules. 2018; 23(12):3121. https://doi.org/10.3390/molecules23123121
Chicago/Turabian StyleHsu, Hung-Kun, Kuang-Hsing Hsu, Ya-Ming Cheng, Hao-Yi Suen, and Shu-Fen Peng. 2018. "Development and In Vitro Evaluation of Linear PEI-Shelled Heparin/Berberine Nanoparticles in Human Osteosarcoma U-2 OS Cells" Molecules 23, no. 12: 3121. https://doi.org/10.3390/molecules23123121




