Comparison of Phenols Content and Antioxidant Activity of Fruits from Different Maturity Stages of Ribes stenocarpum Maxim
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of the Extraction Condition
2.2. HPLC Separation
2.3. Validation of the Method
2.4. Comparison of Phenols Content of RSM Fruits from Different Stages of Maturity
2.5. Analysis of Antioxidant Activities
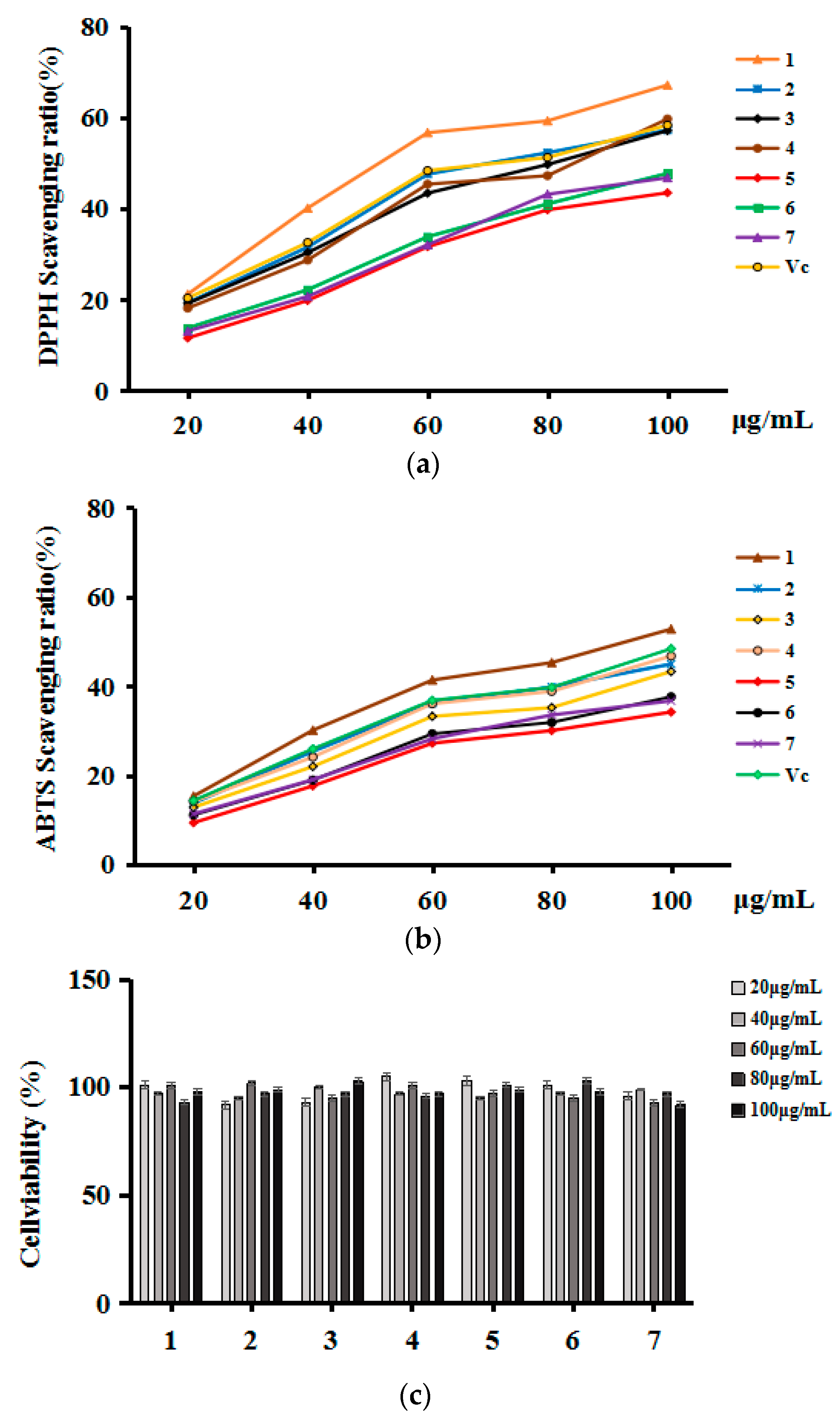
2.5.1. The DPPH and ABTS Free Radical Scavenging Activities
2.5.2. Scavenging Abilities against Intracellular ROS
3. Experimental Section
3.1. Materials
3.2. Reagents
3.3. Preparation of Standard Solutions
3.4. Extraction
3.5. HPLC Analysis
3.6. Experimental Design and Data Analysis
3.7. Biochemical Assays
3.7.1. DPPH and ABTS Free Radical Scavenging Activity Assays
3.7.2. Assay of Intracellular Activities of Phenols
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, Y.J. Woody Plants of Qinghai Province; Qinghai People’s Publishing House: Xining, China, 1987; pp. 223–237. [Google Scholar]
- Yang, Y.C.; He, T.N.; Lu, S.L. Tibetan Medicine; Qinghai People’s Publishing House: Xining, China, 1991; pp. 404–406. [Google Scholar]
- Yang, X.L. Studies on distribution, development and utilization of Ribe resources in Qinghai province. Sci. Technol. Qinghai Agric. For. 2011, 4, 68–70. [Google Scholar]
- Kähkönen, M.; Heinämäki, J.; Ollilainen, V.; Heinonen, M. Berry anthocyanins: Isolation, identification and antioxidant activities. J. Sci. Food Agric. 2003, 83, 1403–1411. [Google Scholar] [CrossRef]
- Krisch, J.; Ördögh, L.; Galgóczy, L.; Papp, T.; Vágvölgyi, C. Anticandidal effect of berry juices and extracts from Ribes species. Cent. Eur. J. Biol. 2009, 4, 86–89. [Google Scholar] [CrossRef] [Green Version]
- Nanashima, N.; Horie, K.; Maeda, H.; Tomisawa, T.; Kitajima, M.; Nakamura, T. Blackcurrant anthocyanins increase the levels of collagen, elastin, and hyaluronic acid in human skin fibroblasts and ovariectomized rats. Nutrients 2018, 10, 495. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yang, Y.Q.; Hu, F.Z.; Shi, Z.X. Analysis on the seed fatty acids of Ribes stenocarpum maxim. Chin. J. Anal. Lab. 2004, 23, 36–38. [Google Scholar]
- S1imestad, R.; Solheim, H. Anthocyanins from black currants (Ribes nigrum L.). J. Agric. Food Chem. 2002, 50, 3228–3231. [Google Scholar] [CrossRef]
- Sandell, M.; Laaksonen, O.; Järvinen, R.; Rostiala, N.; Pohjanheimo, T.; Tiitinen, K.; Kallio, H. Orosensory profiles and chemical composition of black currant (Ribes nigrum) juice and fractions of press residue. J. Agric. Food Chem. 2009, 57, 3718–3728. [Google Scholar] [CrossRef] [PubMed]
- Nowak, R.; Zgorka, G. Phenolic acids in fruits and leaves of Ribes nigrum L.and Ribes grossularia L. Acta Pol. Pharm. 1997, 54, 155–160. [Google Scholar]
- Blech, S.; Budzikiewicz, H. β-Carboline alkaloids from Ribes nigrum L. Z. Naturforsch. C 1994, 49, 540–544. [Google Scholar] [CrossRef]
- Li, G.L.; Zhang, X.L.; You, J.M.; Sun, Z.W.; Suo, Y.R. Highly sensitive and selective pre-column derivatization high-performance liquid chromatography approach for rapid determination of triterpenes acids and application to Swertia species. Anal. Chim. Acta 2011, 688, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, L.; Wen, M.J.; Zeng, F.L.; Xie, C.X.; Kang, T.G. Study on the content measurement and quality evaluation of ginsenoside constituent from different habitats with UPLC analysis method. Chin. J. Tradit. Chin. Med. Pharm. 2015, 30, 1963–1968. [Google Scholar]
- Yang, B.; Kallio, H.P. Fatty acid composition of lipids in sea buckthorn (Hippophae rhamnoides L.) berries of different origins. J. Agric. Food Chem. 2001, 49, 1939–1947. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Li, X.H.; Hu, J.W.; Ji, Q.R. Study on synchronous extraction and isolation of bioactive compounds from Ampelopsis Grossedentata. Food Res. Dev. 2011, 32, 183–186. [Google Scholar]
- Reen, R.K.; Karan, M.; Singh, K.; Karan, V.; Johri, R.K.; Singh, J. Screening of various Swertia species extracts in primary monolayer cultures of rat hepatocytes against carbon tetrachloride and paracetamol-induced toxicity. J. Ethnopharmacol. 2001, 75, 239–247. [Google Scholar] [CrossRef]
- Ruan, Z.Y.; Wang, E.M. Characterization on the polysaccharide concentration and its immune activity of lycium barbarum from different area. Acad. Period. Farm Prod. Process. 2008, 8, 37–38. [Google Scholar]
- Lafka, T.I.; Sinanoglou, V.; Lazos, E.S. On the extraction and antioxidant activity of phenolic compounds from winery wastes. Food Chem. 2007, 104, 1206–1214. [Google Scholar] [CrossRef]
- Gao, N.X.; Wang, Y.H.; Jiao, X.Y.; Chou, S.R.; Li, E.H.; Li, B. Preparative purification of polyphenols from Aronia melanocarpa (Chokeberry) with cellular antioxidant and antiproliferative activity. Molecules 2018, 23, 139. [Google Scholar] [CrossRef] [PubMed]
- Mirosława, T.; Aneta, W. Comparison of phenolic compounds and antioxidant potential between selected edible fruits and their leaves. J. Funct. Foods 2015, 14, 736–746. [Google Scholar]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer efficacy of polyphenols and their combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef] [PubMed]
- Lall, R.K.; Syed, D.N.; Adhami, V.M.; Khan, M.I.; Mukhtar, H. Dietary polyphenols in prevention and treatment of prostate cancer. Int. J. Mol. Sci. 2015, 16, 3350–3376. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K. Emerging phytochemicals for the prevention and treatment of head and neck cancer. Molecules 2016, 21, 1610. [Google Scholar] [CrossRef] [PubMed]
- Bisignano, C.; Mandalari, G.; Smeriglio, A.; Trombetta, D.; Pizzo, M.M.; Pennisi, R.; Sciortino, M.T. Almond skin extracts abrogate HSV-1 replication by blocking virus binding to the cell. Viruses 2017, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- Krakauer, T. The polyphenol chlorogenic acid inhibits staphy-lococcal exotoxin-induced inflammatory cytokines and chemokines. Immunopharmacol. Immunotoxicol. 2002, 24, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.J.; Sun, K.X.; Wang, L.; Xie, L.P. Inhibited effect of chloro-genic acid on virus in vitro. J. Harbin Med. Univ. 2001, 35, 430–432. [Google Scholar]
- Ferrazzano, G.F.; Cantile, T.; Coda, M.; Alcidi, B.; Sangianantoni, G.; Ingenito, A.; Stasio, M.; Volpe, M.G. In vivo release kinetics and antibacterial activity of novel polyphenols-enriched chewing gums. Molecules 2016, 21, 1008. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Tangney, C.C.; Rasmussen, H.E. Polyphenols, inflammation, and cardiovascular disease. Curr. Atheroscler. Rep. 2013, 15, 324. [Google Scholar] [CrossRef] [PubMed]
- Jurikova, T.; Sochor, J.; Rop, O.; Mlcek, J.; Balla, S.; Szekeres, L.; Adam, V.; Kizek, R. Polyphenolic profile and biological activity of Chinese hawthorn (Crataegus pinnatifida BUNGE) fruits. Molecules 2012, 17, 14490–14509. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Song, S.H.; Zhao, L.; Xu, G.H. Progress in plant polyphenols and their physiological functions. Acta. Agric. Jiangxi. 2007, 19, 105–107. [Google Scholar]
- Ky, I.; Crozier, A.; Cros, G.; Teissedre, P.-L. Polyphenols composition of wine and grape sub-products and potential effects on chronic diseases. Nutr. Aging 2014, 2, 165–177. [Google Scholar]
- Prakash, M.J.; Manikandan, S.; Mekala, V. Modeling and optimization of betalain extraction from Opuntiaficus-indica using Box-Behnken design with desirability function. Ind. Crop. Prod. 2013, 49, 304–311. [Google Scholar] [CrossRef]
- Qiao, D.L.; Hu, B.; Gan, D.; Sun, Y.; Ye, H.; Zeng, X.X. Extraction optimized by using response surface methodology, purification and preliminary characterization of polysaccharides from Hyriopisis cumingii. Carbohydr. Polym. 2009, 76, 422–429. [Google Scholar] [CrossRef]
- Paradiso, V.M.; Clemente, A.; Summo, C.; Pasqualone, A.; Caponio, F. Towards green analysis of virgin olive oil phenolic compounds: Extraction by a natural deep eutectic solvent and direct spectrophotometric detection. Food Chem. 2016, 212, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Luo, S.-W. The mechanism for enhancing extraction of ferulic acid from Radix Angelica sinensis by high hydrostatic pressure. Sep. Purif. Technol. 2016, 165, 208–213. [Google Scholar] [CrossRef]
- Belwal, T.; Dhyani, P.; Bhatt, I.D.; Rawal, R.S.; Pande, V. Optimization extraction conditions for improving phenolic content and antioxidant activity in Berberis asiatica fruits using response surface methodology (RSM). Food Chem. 2016, 207, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Du, H.; Wang, L.; Shu, Q.; Zheng, Y.; Xu, Y.; Zhang, J.; Yang, R.; Ge, Y. Flavonoid composition and antioxidant activity of Tree Peony (Paeonia section Moutan) yellow flowers. J. Agric. Food Chem. 2009, 57, 8496–8503. [Google Scholar] [CrossRef] [PubMed]
- Leite, A.V.; Malta, L.G.; Riccio, M.F.; Eberlin, M.N.; Pastore, G.M.; Marostica Junior, M.R. Antioxidant potential of rat plasma by administration of freeze-dried jaboticaba peel (Myrciaria jaboticaba Vell Berg). J. Agric. Food Chem. 2011, 59, 2277–2283. [Google Scholar] [CrossRef] [PubMed]
- Luan, G.-X.; Wang, Y.-W.; Wang, Z.-H.; Zhou, W.-N.; Hu, N.; Li, G.; Wang, H.-L. Flavonoid glycosides from fenugreek seeds regulate glycolipid metabolism by improving mitochondrial function in 3T3-L1 adipocytes in vitro. J. Agric. Food Chem. 2018, 66, 3169–3178. [Google Scholar] [CrossRef] [PubMed]
- Rafiei, H.; Omidian, K.; Bandy, B. Protection by different classes of dietary polyphenols against palmitic acidinduced steatosis, nitro-oxidative stress and endoplasmic reticulum stress in HepG2 hepatocytes. J. Funct. Foods 2018, 44, 173–182. [Google Scholar] [CrossRef]
Sample Availability: Samples of the RSM fruits are available from the authors. |

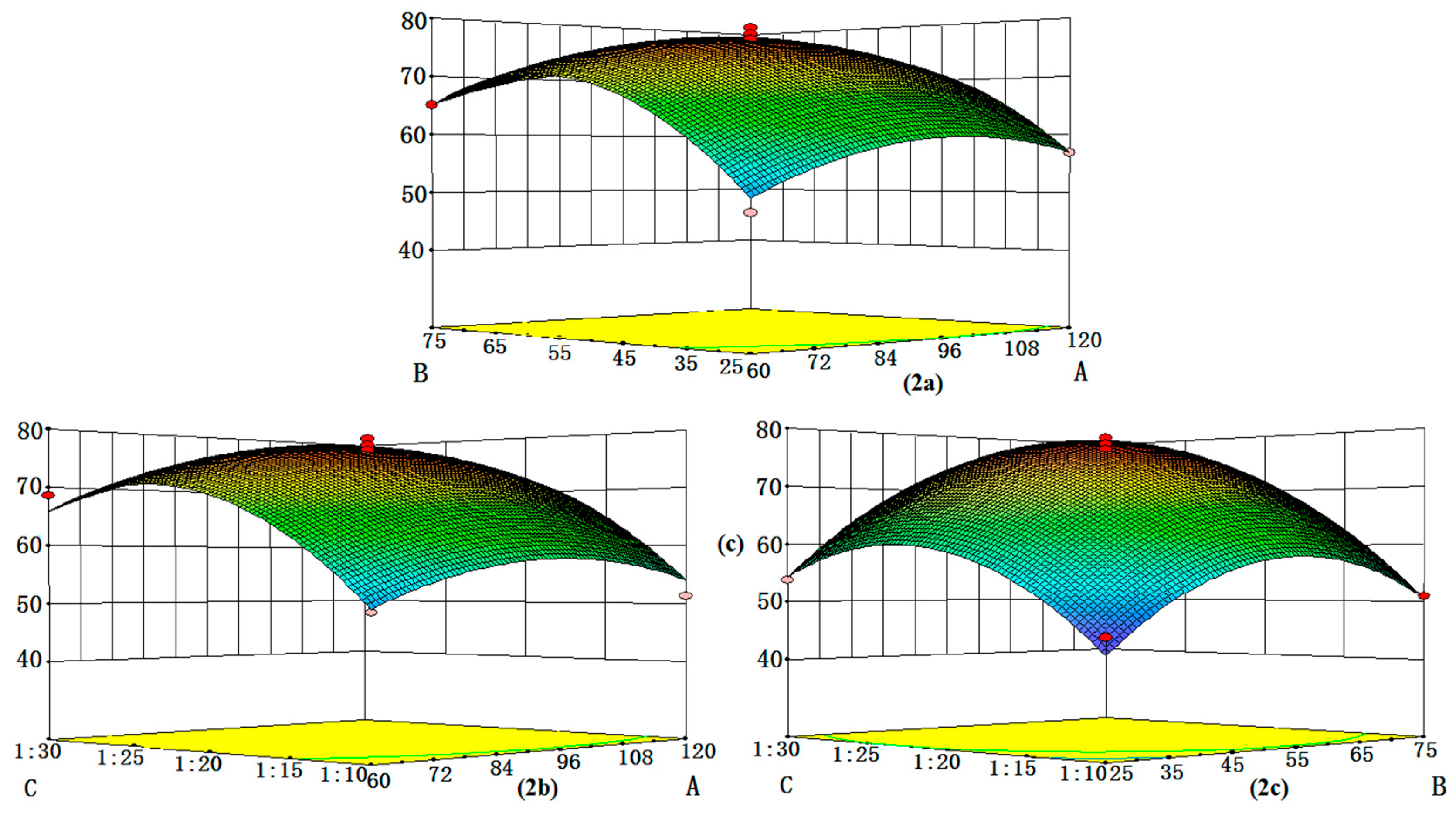



| Run | Extraction Time (min) | Solvent Concentration (%) | Ratio of Sample to Solvent | Total Content of Nine Phenols (mg/g) |
|---|---|---|---|---|
| 1 | 120.00 | 75.00 | 1:20 | 69.32 |
| 2 | 90.00 | 50.00 | 1:20 | 76.39 |
| 3 | 90.00 | 25.00 | 1:10 | 45.32 |
| 4 | 90.00 | 50.00 | 1:20 | 77.12 |
| 5 | 60.00 | 25.00 | 1:20 | 47.68 |
| 6 | 90.00 | 75.00 | 1:30 | 68.73 |
| 7 | 60.00 | 75.00 | 1:20 | 65.12 |
| 8 | 90.00 | 25.00 | 1:30 | 53.88 |
| 9 | 60.00 | 50.00 | 1:10 | 49.37 |
| 10 | 60.00 | 50.00 | 1:30 | 68.59 |
| 11 | 120.00 | 25.00 | 1:20 | 56.92 |
| 12 | 90.00 | 50.00 | 1:20 | 78.31 |
| 13 | 90.00 | 75.00 | 1:10 | 51.12 |
| 14 | 90.00 | 50.00 | 1:20 | 71.87 |
| 15 | 120.00 | 50.00 | 1:10 | 51.33 |
| 16 | 90.00 | 50.00 | 1:20 | 77.32 |
| 17 | 120.00 | 50.00 | 1:30 | 71.32 |
| Phenolic Acids | Regression Equationa | r | LOD | LOQ | Instrument Precision (n = 6) | Method Precision (n = 3) | ||
|---|---|---|---|---|---|---|---|---|
| (μg/L) | (μg/L) | Intra-Day | Inter-Day | Intra-Day | Inter-Day | |||
| Gallic acid | y = 0.853x − 0.042 | 0.9973 | 0.27 | 0.98 | 0.7 | 1.2 | 1.3 | 2.8 |
| Catechin | y = 4.017x − 0.315 | 0.9981 | 0.32 | 0.95 | 0.6 | 0.9 | 1.4 | 2.7 |
| Chlorogenic acid | y = 1.823x − 0.032 | 0.9978 | 0.28 | 1.08 | 0.6 | 1.1 | 1.9 | 3.7 |
| Vanillic acid | y = 4.107x − 0.057 | 0.9992 | 0.27 | 0.77 | 0.8 | 1.1 | 1.3 | 2.4 |
| Syringic acid | y = 7.154x − 0.127 | 0.9983 | 0.29 | 0.83 | 0.8 | 1.0 | 1.6 | 3.2 |
| Coumaric acid | y = 9.987x − 0.141 | 0.9976 | 0.16 | 0.61 | 0.9 | 1.4 | 1.7 | 3.5 |
| Ferulic acid | y = 7.357x − 0.068 | 0.9985 | 0.21 | 0.72 | 0.8 | 1.3 | 1.6 | 3.2 |
| Rosemary acid | y = 0.669x − 0.013 | 0.9969 | 0.30 | 1.01 | 0.6 | 1.1 | 1.3 | 2.5 |
| Quercetin acid | y = 6.830x − 0.063 | 0.9982 | 0.10 | 0.32 | 0.7 | 1.1 | 1.3 | 2.7 |
| Phenols | Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | Sample 6 | Sample 7 |
|---|---|---|---|---|---|---|---|
| Gallic acid | 9.76 ± 1.20 | 8.53 ± 1.56 | 14.77 ± 1.98** | 11.52 ± 1.88 | 10.13 ± 1.71 | 10.32 ± 1.80 | 7.02 ± 1.05 |
| Catechin | 19.71 ± 2.35 | 17.62 ± 2.13 | 16.15 ± 2.01* | 14.10 ± 1.97** | 13.33 ± 1.92** | 11.09 ± 1.73** | 8.59 ± 1.30** |
| Chlorogenic acid | 14.21 ± 1.97 | 13.77 ± 1.93 | 12.17 ± 1.82 | 13.76 ± 1.95 | 9.49 ± 1.59** | 5.32 ± 1.07** | 2.15 ± 0.59** |
| Vanillic acid | 1.35 ± 0.23 | 1.97 ± 0.28 | 3.11 ± 0.47** | 4.53 ± 0.60** | 5.18 ± 0.81** | 5.03 ± 0.79** | 4.81 ± 0.71** |
| Syringic acid | 2.15 ± 0.46 | 5.19 ± 0.98** | 7.17 ± 1.21** | 11.36 ± 1.72** | 7.30 ± 1.38** | 6.09 ± 1.18** | 3.12 ± 0.46 |
| Coumaric acid | 23.07 ± 2.98 | 18.65 ± 1.92* | 14.73 ± 1.61** | 12.90 ± 1.42** | 10.00 ± 1.18** | 13.19 ± 1.47** | 15.77 ± 1.72** |
| Ferulic acid | 24.17 ± 2.73 | 16.98 ± 1.77** | 10.52 ± 0.95** | 5.65 ± 0.56** | 3.53 ± 0.65** | 3.10 ± 0.43** | 1.72 ± 0.29** |
| Rosemary acid | - | 1.03 ± 0.27 | 1.79 ± 0.38 | 3.39 ± 0.76** | 5.48 ± 0.89** | 9.16 ± 1.32** | 9.55 ± 1.28** |
| Quercetin acid | - | 2.15 ± 0.31 | 2.97 ± 0.60 | 4.13 ± 0.87 | 6.40 ± 0.94** | 7.11 ± 1.54** | 11.39 ± 1.83** |
| Codes | Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | Sample 6 | Sample 7 |
|---|---|---|---|---|---|---|---|
| Picking time | August 10, 2017 | August 20, 2017 | September 1, 2017 | September 10, 2017 | September 20, 2017 | October 1, 2017 | October 10, 2017 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Qi, D.; Wang, S.; Cao, X.; Ye, Y.; Suo, Y. Comparison of Phenols Content and Antioxidant Activity of Fruits from Different Maturity Stages of Ribes stenocarpum Maxim. Molecules 2018, 23, 3148. https://doi.org/10.3390/molecules23123148
Wang Y, Qi D, Wang S, Cao X, Ye Y, Suo Y. Comparison of Phenols Content and Antioxidant Activity of Fruits from Different Maturity Stages of Ribes stenocarpum Maxim. Molecules. 2018; 23(12):3148. https://doi.org/10.3390/molecules23123148
Chicago/Turabian StyleWang, Yuwei, Delin Qi, Shulin Wang, Xiaohai Cao, Ying Ye, and Yourui Suo. 2018. "Comparison of Phenols Content and Antioxidant Activity of Fruits from Different Maturity Stages of Ribes stenocarpum Maxim" Molecules 23, no. 12: 3148. https://doi.org/10.3390/molecules23123148





