Chromatographic Analyses, In Vitro Biological Activities, and Cytotoxicity of Cannabis sativa L. Essential Oil: A Multidisciplinary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Farm and Plant Extraction
2.2. Color Analysis
2.3. Gas Chromatography/Mass Spectrometry (GC/MS) Analysis
2.4. Total Phenolic Acid Content
2.5. Total Phenolic Content
2.6. Total Flavonoid Content
2.7. HPLC Analysis
3. Biological Activities
3.1. Radical Scavenging and Chelating Activity
3.1.1. Free-Radical Scavenging Activity
3.1.2. Radical Cation Scavenging Activity
3.1.3. Evaluation of Total Antioxidant Capacity Using Phosphomolybdenum Assay
3.1.4. Cupric Ion Reducing (CUPRAC) Method
3.1.5. Ferric Reducing Antioxidant Power (FRAP) Method
3.1.6. Metal Chelating Activity on Ferrous Ions
3.2. Antimicrobial Activity
3.2.1. Staphylococcus aureus
3.2.1.1. Bacterial Strains and Culture Conditions
3.2.1.2. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
3.2.1.3. AB Planktonic Susceptibility Assay
3.2.1.4. Determination of Minimum Biofilm Eradication Concentration
3.2.1.5. AB Biofilm Eradication Assay
3.2.1.6. Biofilm Eradication Evaluation by Crystal Violet Assay and Live/Dead Cell Viability Staining
3.2.1.7. Cell Viability Evaluation through Colony-Forming Unit Count
3.2.2. Helicobacter pylori
3.2.2.1. Bacterial Strains and Culture Conditions
3.2.2.2. Minimum Inhibitory Concentration and Minimum Bactericidal Concentration Determination
3.2.3. Candida and Malassezia Strain Growth Inhibition
3.3. Cytotoxicity
3.3.1. Cell Lines
3.3.2. Treatment Protocol
3.3.3. Cytotoxicity Assay
3.4. Enzyme Inhibitory Activity
3.4.1. Cholinesterase Inhibition
3.4.2. α-Amylase Inhibition
3.4.3. α-Glucosidase Inhibition
3.4.4. Tyrosinase Inhibition
3.4.5. Lipase Inhibition
3.5. G. mellonella Injection Procedure
3.6. Statistical Analysis
4. Results and Discussion
4.1. Phytochemical Analyses
4.1.1. Color Analysis
4.1.2. GC Flame Ionization Detector Analysis of the Tetrahydrocannabinol (THC) Content
4.1.3. GC/MS Analysis of the Volatile Components of the EO
4.1.4. Total Phenolic, Flavonoid, and Phenolic Acid Content
4.1.5. HPLC-PDA Analysis of the Phenolic Fraction
4.2. Antioxidant Properties
4.3. Antimicrobial and Antibiofilm Activity of the Hemp EO versus Staphylococcus aureus
4.4. Anti-Helicobacter Pylori Activity
4.5. Antifungal Activity of Hemp EO
4.6. Cytotoxicity
4.7. Enzyme Inhibition
4.8. In Vivo Toxicity Studies
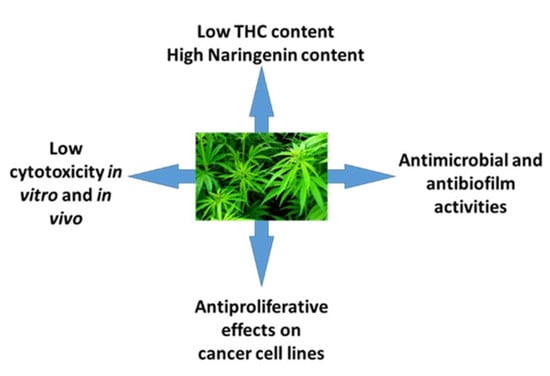
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gazzetta Ufficiale della Repubblica Italiana. LEGGE 2 Dicembre 2016, n. 242 Disposizioni Per La Promozione Della Coltivazione e Della Filiera Agroindustriale Della Canapa. Available online: http://www.gazzettaufficiale.it/eli/id/2016/12/30/16G00258/sg (accessed on 08 December 2018).
- Fernández-Ruiz, J.; Sagredo, O.; Pazos, M.R.; García, C.; Pertwee, R.; Mechoulam, R.; Martínez-Orgado, J. Cannabidiol for neurodegenerative disorders: Important new clinical applications for this phytocannabinoid? Br. J. Clin. Pharmacol. 2013, 75, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Amaducci, M.T. Ricerche sulla tecnica colturale delle canape monoiche utilizzate per fabbricazione di carte pregiate. Sementi Elette 1969, 3, 166–179. [Google Scholar]
- Benelli, G.; Pavela, R.; Lupidi, G.; Nabissi, M.; Petrelli, R.; Ngahang Kamte, S.L.; Cappellacci, L.; Fiorini, D.; Sut, S.; Dall’Acqua, S.; et al. The crop-residue of fiber hemp cv. Futura 75: From a waste product to a source of botanical insecticides. Environ. Sci. Pollut. Res. Int. 2018, 25, 10515–10525. [Google Scholar] [CrossRef] [PubMed]
- Nissen, L.; Zatta, A.; Stefanini, I.; Grandi, S.; Sgorbati, B.; Biavati, B.; Monti, A. Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.). Fitoterapia 2010, 81, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.; Zitterl-Eglseer, K.; Deans, S.G.; Franz, C.M. Essential oils of different cultivars of Cannabis sativa L. and their antimicrobial activity. Flavour Fragr. J. 2001, 16, 259–262. [Google Scholar] [CrossRef]
- Gorski, R.; Szklarz, M.; Kaniewski, R. Efficacy of hemp essential oil in the control of rosy apple aphid (Dysaphis plantaginea Pass.) occurring on apple tree. Prog. Plant Prot. 2009, 49, 2013–2016. [Google Scholar]
- Górskirgorski, R.; Sobieralski, K.; Siwulski, M. The effect of Hemp essential oil on mortality Aulacorthum Solani Kalt. and Tetranychus urticae Koch. Ecol. Chem. Engineer. S. 2016, 23, 505–511. [Google Scholar] [CrossRef]
- Bedini, S.; Flamini, G.; Cosci, F.; Conti, B. Cannabis sativa and Humulus lupulus essential oils as novel control tools against the invasive mosquito Aedes albopictus and fresh water snail Physella acuta. Ind. Crops Prod. 2016, 85, 318–323. [Google Scholar] [CrossRef]
- Gulluni, N.; Re, T.; Loiacono, I.; Lanzo, G.; Gori, L.; Macchi, C.; Epifani, F.; Bragazzi, N.; Firenzuoli, F. Cannabis essential oil: A preliminary study for the evaluation of the brain effects. Evid. Based Complement. Alternat. Med. 2018, 2018, 1709182. [Google Scholar] [CrossRef] [PubMed]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential oils in insect control: Low-risk products in a high-stakes world. Annu. Rev. Entomol. 2012, 57, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Boutaoui, N.; Zaiter, L.; Benayache, F.; Benayache, S.; Cacciagrano, F.; Cesa, S.; Secci, D.; Carradori, S.; Giusti, A.M.; Campestre, C.; et al. Atriplex mollis Desf. aerial parts: Extraction procedures, secondary metabolites and color analysis. Molecules 2018, 23, 1962. [Google Scholar] [CrossRef] [PubMed]
- Scazzocchio, F.; Mondì, L.; Ammendolia, M.G.; Goldoni, P.; Comanducci, A.; Marazzato, M.; Conte, M.P.; Rinaldi, F.; Crestoni, M.E.; Fraschetti, C.; et al. Coriander (Coriander sativum) essential oil: Effect on multidrug resistant uropathogenic Escherichia coli. Nat. Prod. Commun. 2017, 12, 623–626. [Google Scholar] [PubMed]
- Vladimir-Knežević, S.; Blažeković, B.; Štefan, M.B.; Alegro, A.; Kőszegi, T.; Petrik, J. Antioxidant activities and polyphenolic contents of three selected Micromeria species from Croatia. Molecules 2011, 16, 1454–1470. [Google Scholar] [CrossRef] [PubMed]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- Berk, S.; Tepe, B.; Arslan, S.; Sarikurkcu, C. Screening of the antioxidant, antimicrobial and DNA damage protection potentials of the aqueous extract of Asplenium ceterach DC. Afr. J. Biotechnol. 2011, 10, 8902–8908. [Google Scholar]
- Zengin, G.; Ceylan, R.; Guler, G.O.; Carradori, S.; Uysal, S.; Aktumsek, A. Enzyme inhibitory effect and antioxidant properties of Astragalus lagurus extracts. Curr. Enzyme Inhib. 2016, 12, 177–182. [Google Scholar] [CrossRef]
- Locatelli, M.; Zengin, G.; Uysal, A.; Carradori, S.; De Luca, E.; Bellagamba, G.; Aktumsek, A.; Lazarova, I. Multicomponent pattern and biological activities of seven Asphodeline taxa: Potential sources of natural-functional ingredients for bioactive formulations. J. Enzyme Inhib. Med. Chem. 2017, 32, 60–67. [Google Scholar] [CrossRef]
- Zengin, G.; Locatelli, M.; Carradori, S.; Mocan, A.; Aktumsek, A. Total phenolics, flavonoids, condensed tannins content of eight Centaurea species and their broad inhibitory activities against cholinesterase, tyrosinase, α-amylase and α-glucosidase. Not. Bot. Horti Agrobo. 2016, 44, 195–200. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Chiavaroli, A.; Recinella, L.; Ferrante, C.; Locatelli, M.; Carradori, S.; Macchione, N.; Zengin, G.; Leporini, L.; Leone, S.; Martinotti, S.; et al. Crocus sativus, Serenoa repens and Pinus massoniana extracts modulate inflammatory response in isolated rat prostate challenged with LPS. J. Biol. Regul. Homeostat. Agents 2017, 31, 531–541. [Google Scholar]
- Melucci, D.; Locatelli, M.; Locatelli, C.; Zappi, A.; De Laurentiis, F.; Carradori, S.; Campestre, C.; Leporini, L.; Zengin, G.; Picot, C.M.N.; et al. A comparative assessment of biological effects and chemical profile of Italian Asphodeline lutea extracts. Molecules 2018, 23, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- National Committee for Clinical Laboratory Standards (NCCLS). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 8th ed.; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2009. [Google Scholar]
- Pettit, R.K.; Weber, C.A.; Kean, M.J.; Hoffmann, H.; Pettit, G.R.; Tan, R.; Franks, K.S.; Horton, M.L. Microplate Alamar blue assay for Staphylococcus epidermidis biofilm susceptibility testing. Antimicrob. Agents Chemother. 2005, 49, 2612–2617. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.S.; Chen, C.C.; Chuang, Y.C.; Su, B.A.; Chiu, Y.H.; Hsu, H.J.; Ko, W.C.; Tang, H.J. Efficacy of combination oral antimicrobial agents against biofilm-embedded methicillin-resistant Staphylococcus aureus. J. Microbiol. Immunol. Infect. 2013, 46, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Grande, R.; Di Giulio, M.; Bessa, L.J.; Di Campli, E.; Baffoni, M.; Guarnieri, S.; Cellini, L. Extracellular DNA in Helicobacter pylori biofilm: A backstairs rumour. J. Appl. Microbiol. 2011, 110, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Sisto, F.; Scaltrito, M.M.; Russello, G.; Bonomi, A.; Dubini, F. Antimicrobial susceptibility testing of Helicobacter pylori determined by microdilution method using a new medium. Curr. Microbiol. 2009, 58, 559–563. [Google Scholar] [CrossRef] [PubMed]
- National Committee for Clinical Laboratory Standards (NCCLS). Methods for Broth Dilution Antifungal Susceptibility Testing of Yeast, 30th ed.; National Committee for Clinical Laboratory Standards: Villanova, PA, USA, 2008. [Google Scholar]
- Rojas, F.D.; Sosa Mde, L.; Fernández, M.S.; Cattana, M.E.; Córdoba, S.B.; Giusiano, G.E. Antifungal susceptibility of M. furfur, M. sympodialis, and M. globosa to azole drugs and amphotericin B evaluated using a broth microdilution method. Med. Mycol. 2014, 52, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Kanno, N.; Glaser, S.; Chowdhury, U.; Phinizy, J.L.; Baiocchi, L.; Francis, H.; LeSage, G.; Alpini, G. Gastrin inhibits cholangiocarcinoma growth through increased apoptosis by activation of Ca2+-dependent protein kinase C-alpha. J. Hepatol. 2001, 34, 284–291. [Google Scholar] [CrossRef]
- Di Giacomo, S.; Di Sotto, A.; El-Readi, M.Z.; Mazzanti, G.; Wink, M. α-Hexylcinnamaldehyde synergistically increases doxorubicin cytotoxicity towards human cancer cell lines. Anticancer Res. 2016, 36, 3347–3351. [Google Scholar]
- Picot, M.C.N.; Zengin, G.; Mollica, A.; Stefanucci, A.; Carradori, S.; Mahomoodally, M.F. In vitro and in silico studies of mangiferin from Aphloia theiformis on key enzymes linked to diabetes type 2 and associated complications. Med. Chem. 2017, 13, 633–640. [Google Scholar] [CrossRef]
- Zengin, G.; Locatelli, M.; Stefanucci, A.; Macedonio, G.; Novellino, E.; Mirzaie, S.; Carradori, S.; Brunetti, L.; Orlando, G.; Menghini, L.; et al. Chemical characterization, antioxidant properties, anti-inflammatory activity and enzyme inhibition of Ipomoea batatas L. leaf extracts. Int. J. Food Prop. 2017, 20, 1907–1919. [Google Scholar] [CrossRef]
- Roh, C.; Jung, U. Screening of crude plant extracts with anti-obesity activity. Int. J. Mol. Sci. 2012, 13, 1710–1719. [Google Scholar] [CrossRef]
- Harding, C.R.; Schroeder, G.N.; Collins, J.W.; Frankel, G. Use of Galleria mellonella as a model organism to study Legionella pneumophila infection. J. Vis. Exp. 2013, 81, e50964. [Google Scholar] [CrossRef]
- Cesa, S.; Carradori, S.; Bellagamba, G.; Locatelli, M.; Casadei, M.A.; Masci, A.; Paolicelli, P. Evaluation of processing effects on anthocyanin content and colour modifications of blueberry (Vaccinium spp.) extracts: Comparison between HPLC-DAD and CIELAB analyses. Food Chem. 2017, 232, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Andre, C.M.; Hausman, J.F.; Guerriero, G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 2016, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Ur-Rehman, T.; Mirza, B.; Ul-Haq, I.; Zia, M. Antioxidant, antimicrobial, cytotoxic and protein kinase inhibition activities of fifteen traditional medicinal plants from Pakistan. Pharm. Chem. J. 2017, 51, 391–398. [Google Scholar] [CrossRef]
- Naz, S.; Hanif, M.A.; Bhatti, H.N.; Shahid, M. Partition, fractionation, antioxidant potential and phenolics profiling of Cannabis sativa growing in Pakistan. Oxid. Commun. 2016, 39, 2946–2960. [Google Scholar]
- Rovellini, P.; Folegatti, L.; Baglio, D.; De Cesarei, S.; Fusari, P.; Venturini, S.; Cavalieri, A. Chemical characterization of oil obtained by the cold pressing of Cannabis sativa L. seeds. Riv. Ital. Sostanze Gr. 2013, 90, 139–152. [Google Scholar]
- Yan, X.; Tang, J.; dos Santos Passos, C.; Nurisso, A.; Simões-Pires, C.A.; Ji, M.; Lou, H.; Fan, P. Characterization of lignanamides from hemp (Cannabis sativa L.) seed and their antioxidant and acetylcholinesterase inhibitory activities. J. Agric. Food Chem. 2015, 63, 10611–10619. [Google Scholar] [CrossRef]
- Mollica, A.; Locatelli, M.; Macedonio, G.; Carradori, S.; Sobolev, A.P.; De Salvador, R.F.; Monti, S.M.; Buonanno, M.; Zengin, G.; Angeli, A.; et al. Microwave-assisted extraction, HPLC analysis, and inhibitory effects on carbonic anhydrase I, II, VA, and VII isoforms of 14 blueberry Italian cultivars. J. Enzyme Inhib. Med. Chem. 2016, 31 (Suppl. 4), 1–6. [Google Scholar] [CrossRef]
- Patel, K.; Singh, G.K.; Patel, D.K. A review on pharmacological and analytical aspects of naringenin. Chin. J. Integr. Med. 2018, 24, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Mira, L.; Fernandez, M.T.; Santos, M.; Rocha, R.; Florêncio, M.H.; Jennings, K.R. Interactions of flavonoids with iron and copper ions: A mechanism for their antioxidant activity. Free Radic. Res. 2002, 36, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Cherrak, S.A.; Mokhtari-Soulimane, N.; Berroukeche, F.; Bensenane, B.; Cherbonnel, A.; Merzouk, H.; Elhabiri, M. In vitro antioxidant versus metal ion chelating properties of flavonoids: A structure-activity investigation. PLoS ONE 2016, 11, e0165575. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Galati, E.M.; Monforte, M.T.; Lanuzza, F.; D’angelo, V.; Circosta, C. Polyphenolic compounds and antioxidant activity of cold-pressed seed oil from Finola cultivar of Cannabis sativa L. Phytother. Res. 2016, 30, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, I.; Khan, A.U.; Asghar, M.N.; Ashfaq, M.; Shahid, S.; Ahmed, D. In vitro total antioxidant and radical scavenging activities of organic extracts from leaves, stem and inflorescence of Cannabis sativa L. Asian J. Chem. 2012, 24, 5067–5072. [Google Scholar]
- Dastgheyb, S.S.; Otto, M. Staphylococcal adaptation to diverse physiologic niches: An overview of transcriptomic and phenotypic changes in different biological environments. Future Microbiol. 2015, 10, 1981–1995. [Google Scholar] [CrossRef] [PubMed]
- Grande, R.; Nistico, L.; Sambanthamoorthy, K.; Longwell, M.; Iannitelli, A.; Cellini, L.; Di Stefano, A.; Hall Stoodley, L.; Stoodley, P. 2014 Temporal expression of agrB, cidA, and alsS in the early development of Staphylococcus aureus UAMS-1 biofilm formation and the structural role of extracellular DNA and carbohydrates. Pathog. Dis. 2014, 70, 414–422. [Google Scholar] [CrossRef]
- Gordon, R.J.; Lowy, F.D. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin. Infect. Dis. 2008, 46 (Suppl. 5), S350–S359. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Stoodley, P.; Kathju, S.; Høiby, N.; Moser, C.; Costerton, J.W.; Moter, A.; Bjarnsholt, T. Towards diagnostic guidelines for biofilm-associated infections. FEMS Immunol. Med. Microbiol. 2012, 65, 127–145. [Google Scholar] [CrossRef]
- Yue, J.; Yang, H.; Liu, S.; Song, F.; Guo, J.; Huang, C. Influence of naringenin on the biofilm formation of Streptococcus mutans. J. Dent. 2018, 76, 24–31. [Google Scholar] [CrossRef]
- Wang, L.-H.; Wang, M.-S.; Zeng, X.-A.; Xu, X.-M.; Brennan, C.S. Membrane and genomic DNA dual-targeting of citrus flavonoid naringenin against Staphylococcus aureus. Integr. Biol. 2017, 9, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-H.; Zeng, X.-A.; Wang, M.-S.; Brennan, C.S.; Gong, D. Modification of membrane properties and fatty acids biosynthesis-related genes in Escherichia coli and Staphylococcus aureus: Implications for the antibacterial mechanism of naringenin. BBA-Biomembranes 2018, 1860, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Sisto, F.; Scaltrito, M.M.; Masia, C.; Bonomi, A.; Cocce, V.; Marano, G.; Haynes, R.K.; Miani, A.; Farronato, G.; Taramelli, D. In vitro activity of artemisone and artemisinin derivatives against extracellular and intracellular Helicobacter pylori. Int. J. Antimicrob. Agents 2016, 48, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Levison, M.E.; Levison, J.H. Pharmacokinetics and pharmacodynamics of antibacterial agents. Infect. Dis. Clin. North Am. 2009, 23, 791–815. [Google Scholar] [CrossRef] [PubMed]
- Nariman, F.; Eftekhar, F.; Habibi, Z.; Massarrat, S.; Malekzadeh, R. Antibacterial activity of twenty Iranian plant extracts against clinical isolates of Helicobacter pylori. Iran. J. Basic Med. Sci. 2009, 12, 105–111. [Google Scholar]
- Kuete, V. Potential of Cameroonian plants and derived products against microbial infections: A review. Planta Med. 2010, 76, 1479–1491. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, D.; Esposito, G.; Cirillo, C.; Cipriano, M.; De Winter, B.Y.; Scuderi, C.; Sarnelli, G.; Cuomo, R.; Steardo, L.; De Man, J.G.; et al. Cannabidiol reduces intestinal inflammation through the control of neuroimmune axis. PLoS ONE 2011, 6, e28159. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, C.A.; Rodrigues Marques, C.; dos Santos Costa, R.; da Silva, H.B.F.; Alcantara-Neves, N.M. Cytokines, cytokine gene polymorphisms and Helicobacter pylori infection: Friend or foe? World J. Gastroenterol. 2014, 20, 5235–5243. [Google Scholar] [CrossRef] [PubMed]
- Lampronti, I.; Saab, A.M.; Gambari, R. Antiproliferative activity of essential oils derived from plants belonging to the Magnoliophyta division. Int. J. Oncol. 2006, 29, 989–995. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Menichini, F.; Saab, A.M.; Statti, G.A.; Menichini, F. Antiproliferative effects of essential oils and their major constituents in human renal adenocarcinoma and amelanotic melanoma cells. Cell Prolif. 2008, 41, 1002–1012. [Google Scholar] [CrossRef]
- Di Giacomo, S.; Di Sotto, A.; Mazzanti, G.; Wink, M. Chemosensitizing properties of β-caryophyllene and β-caryophyllene oxide in combination with doxorubicin in human cancer cells. Anticancer Res. 2017, 37, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Di Giacomo, S.; Abete, L.; Cocchiola, R.; Mazzanti, G.; Eufemi, M.; Di Sotto, A. Caryophyllane sesquiterpenes inhibit DNA-damage by tobacco smoke in bacterial and mammalian cells. Food Chem. Toxicol. 2018, 111, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Lee, K.; Lee, D.H.; Shin, S.Y.; Yong, Y.; Lee, Y.H. Anti-invasive effect of β-myrcene, a component of the essential oil from Pinus koraiensis cones, in metastatic MDA-MB-231 human breast cancer cells. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 563–569. [Google Scholar] [CrossRef]
- Kozioł, A.; Stryjewska, A.; Librowski, T.; Sałat, K.; Gaweł, M.; Moniczewski, A.; Lochyński, S. An overview of the pharmacological properties and potential applications of natural monoterpenes. Mini Rev. Med. Chem. 2014, 14, 1156–1168. [Google Scholar] [CrossRef] [PubMed]
- Raha, S.; Yumnam, S.; Hong, G.E.; Lee, H.J.; Saralamma, V.V.; Park, H.; Heo, J.D.; Lee, S.J.; Kim, E.H.; Kim, J.; et al. 2015 Naringin induces autophagy-mediated growth inhibition by downregulating the PI3K/Akt/mTOR cascade via activation of MAPK pathways in AGS cancer cells. Int. J. Oncol. 2015, 47, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Arul, D.; Subramanian, P. Naringenin (citrus flavonone) induces growth inhibition, cell cycle arrest and apoptosis in human hepatocellular carcinoma cells. Pathol. Oncol. Res. 2013, 19, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Song, H.M.; Park, G.H.; Eo, H.J.; Lee, J.W.; Kim, M.K.; Lee, J.R.; Lee, M.H.; Koo, J.S.; Jeong, J.B. Anti-proliferative effect of naringenin through p38-dependent downregulation of cyclin D1 in human colorectal cancer cells. Biomol. Ther. 2015, 23, 339–344. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.P.; Silva, N.F.; Andrade, E.H.A.; Gratieri, T.; Setzer, W.N.; Maia, J.G.S.; da Silva, J.K.R. Tyrosinase inhibitory activity, molecular docking studies and antioxidant potential of chemotypes of Lippia origanoides (Verbenaceae) essential oils. PLoS ONE 2017, 12, e0175598. [Google Scholar] [CrossRef]
- Priscilla, D.H.; Roy, D.; Suresh, A.; Kumar, V.; Thirumurugan, K. Naringenin inhibits α-glucosidase activity: A promising strategy for the regulation of postprandial hyperglycemia in high fat diet fed streptozotocin induced diabetic rats. Chem.-Biol. Interact. 2014, 210, 77–85. [Google Scholar] [CrossRef]
- Guo, X.; Liu, J.; Cai, S.; Wang, O.; Ji, B. Synergistic interactions of apigenin, naringin, quercetin and emodin on inhibition of 3T3-L1 preadipocyte differentiation and pancreas lipase activity. Obes. Res. Clin. Pract. 2016, 10, 327–339. [Google Scholar] [CrossRef]
- Abdel-Salam, O.M.; Youness, E.R.; Khadrawy, Y.A.; Sleem, A.A. Acetylcholinesterase, butyrylcholinesterase and paraoxonase 1 activities in rats treated with cannabis, tramadol or both. Asian Pac. J. Trop. Med. 2016, 9, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Sharma, R.K.; Singh, N.; Sharma, B. Acetylcholinesterase from human erythrocytes membrane: A screen for evaluating the activity of some traditional plant extracts. Cell. Mol. Biol. 2012, 58, 160–169. [Google Scholar] [PubMed]
- Cook, S.M.; McArthur, J.D. Developing Galleria mellonella as a model host for human pathogens. Virulence 2013, 4, 350–353. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |





| CIELAB Parameters * | Hemp Essential Oil Mean Value ± SD | Aromatic Water Mean Value ± SD |
|---|---|---|
| L* | 57.26 ± 1.66 | 60.46 ± 2.54 |
| a* | −5.68 ± 0.33 | −1.22 ± 0.15 |
| b* | 24.36 ± 1.63 | 2.23 ± 0.24 |
| C*ab | 25.01 ± 1.66 | 2.54 ± 0.28 |
| hab | 103.13 ± 0.11 | 118.66 ± 0.72 |
| Compound | Area % | KI b | KIlit c |
|---|---|---|---|
| α-Pinene a | 8 | 936 | 936 |
| β-Pinene | 3 | 975 | 978 |
| β-Myrcene a | 11 | 984 | 987 |
| d-Limonene a | 2 | 1030 | 1027 |
| β-Ocimene | 3 | 1051 | 1050 |
| α-Terpinolene | 6 | 1089 | 1084 |
| (E)-Caryophyllene a | 28 | 1427 | 1427 |
| trans-α-Bergamotene | 4 | 1439 | 1437 |
| Humulene | 13 | 1459 | 1459 |
| β-Selinene | 4 | 1492 | 1486 |
| α-Selinene | 3 | 1500 | 1497 |
| Caryophyllene oxide a | 15 | 1592 | 1589 |
| Test Sample | Total Phenolic Content (mg GAE/g Extract) * | Total Flavonoid Content (mg RE/g Extract) * | Total Phenolic Acid Content (mg CE/g Extract) * |
|---|---|---|---|
| Hemp EO | nt | nt | nt |
| Aromatic water | 28.04 ± 0.23 | 4.04 ± 0.03 | 1.76 ± 0.14 |
| Compound | Hemp EO | Aromatic Water |
|---|---|---|
| Gallic acid | 0.23 ± 0.03 | 0.62 ± 0.08 |
| Catechin | 60 ± 4 | 7.5 ± 0.2 |
| p-OH Benzoic acid | 0.35 ± 0.02 | |
| Epicatechin | 56 ± 5 | |
| Syringic acid | 7.8 ± 1.3 | |
| 3-OH Benzoic acid | 4.6 ± 0.4 | |
| Rutin | 0.18 ± 0.03 | |
| t-Ferulic acid | 0.37 ± 0.04 | |
| Naringin | 83 ± 15 | 0.63 ± 0.09 |
| 2,3-DiMeO benzoic acid | 10.4 ± 0.3 | |
| Benzoic acid | 31.9 ± 0.9 | |
| Quercetin | 1.7 ± 0.1 | |
| Naringenin | 706 ± 62 | 0.16 ± 0.02 |
| Total | 962.35 | 9.09 |
| Test Sample | Phosphomolybdenum (mmol TE/g Extract or Oil) | DPPH (mg TE/g Sample) | ABTS (mg TE/g Sample) | CUPRAC (mg TE/g Sample) | FRAP (mg TE/g Sample) | Metal Chelating Activity (mg EDTAE/g Sample) |
|---|---|---|---|---|---|---|
| Hemp EO | 35.12 ± 1.63 | 5.56 ± 0.42 | na | 141.15 ± 2.74 | 57.02 ± 0.69 | 19.27 ± 1.23 |
| Aromatic water | 1.42 ± 0.05 | 39.71 ± 1.54 | 103.38 ± 0.07 | 109.15 ± 1.78 | 82.93 ± 1.70 | 3.79 ± 0.25 |
| Bacterial Strain | Clinical Isolation | MIC (mg/mL) | MBC (mg/mL) | MBC/MIC | MBEC (mg/mL) |
|---|---|---|---|---|---|
| S. aureus ATCC 29213 | Wound | 8 | 16 | 2 | 24 |
| S. aureus 101 | Vaginal swab of a pregnant woman | 8 | 16 | 2 | 24 |
| S. aureus 104 | Pharyngeal swab of a male patient | 8 | 16 | 2 | 24 |
| S. aureus 105 | Urinary specimen of a male patient | 8 | 16 | 2 | 16 |
| H. Pylori Strains | Hemp EO MIC/MBC (µg/mL) | Naringenin MIC/MBC (µg/mL) | Antimicrobial Susceptibility (µg/mL) |
|---|---|---|---|
| F4 | 16/16 | 16/16 | MNZ 32; CLR > 256; AMX 0.064 |
| E34 | 16/32 | 16/16 | MNZ 1; CLR 0.064; AMX 0.016 |
| Ro1 | 16/16 | 16/16 | MNZ 1; CLR 64; AMX 0.016 |
| Ra2 | 16/16 | 16/16 | MNZ 1; CLR 128; AMX 0.016 |
| E17 | 16/16 | 16/16 | MNZ 2; CLR 256; AMX 0.064 |
| 68 | 16/32 | 16/16 | MNZ 32; CLR 0.0019; AMX 0.016 |
| ATCC 43629 | 8/8 | 16/16 | MNZ 2; CLR 0.032; AMX 0.064 |
| 23 | 32/32 | 32/32 | MNZ 1; CLR 0.064; AMX 0.032 |
| Ro5 | 32/32 | 16/16 | MNZ 128; CLR 16; AMX 0.125 |
| 110R | 64/64 | 16/16 | MNZ 128; CLR 0.03; AMX 0.016 |
| F1 | 32/32 | 32/32 | MNZ 2; CLR 4; AMX 0.064 |
| 190 | 32/32 | 32/32 | MNZ 1; CLR 0.032; AMX 0.032 |
| F40/499 | 32/32 | 16/16 | MNZ 32; CLR 8; AMX 0.016 |
| F40/442 | 32/64 | 8/8 | MNZ 64; CLR 0.015; AMX 0.015 |
| F34/497 | 64/64 | 16/16 | MNZ 128; CLR 4; AMX 0.064 |
| Cell Line | Hemp EO | Doxorubicin |
|---|---|---|
| IC50 (CL) μg/mL | ||
| MCF7 | 83.2 (72.7–95.2) | 7.6 (4.5–12.9) |
| MDA-MB-468 | 53.0 (44.2–64.3) | 3.1 (2.1–4.6) |
| Caco-2 | 28.7 (17.9–45.8) | 23.3 (11.1–48.3) |
| Mz-ChA-1 | 22.3 (8.3–42.5) | 15.7 (6.0–31.5) |
| H69 | nd | 13.7 (7.3–25.5) |
| Test Sample | AChE Inhibition (mg GALAE/g Extract or Oil) | BChE Inhibition (mg GALAE/g Extract or Oil) | Tyrosinase Inhibition (mg KAE/g Extract or Oil) | α-Amylase Inhibition (mmol ACAE/g Extract or Oil) | α-Glucosidase Inhibition (mmol ACAE/g Extract or Oil) | Lipase Inhibition (mg OE/g Extract or Oil) |
|---|---|---|---|---|---|---|
| Hemp EO | na | 3.40 ± 0.14 | 35.95 ± 3.19 | na | 3.77 ± 0.03 | 70.14 ± 2.40 |
| Aromatic water | 2.56 ± 0.02 | 3.48 ± 0.02 | 28.24 ± 1.94 | 0.10 ± 0.01 | 0.17 ± 0.04 | na |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zengin, G.; Menghini, L.; Di Sotto, A.; Mancinelli, R.; Sisto, F.; Carradori, S.; Cesa, S.; Fraschetti, C.; Filippi, A.; Angiolella, L.; et al. Chromatographic Analyses, In Vitro Biological Activities, and Cytotoxicity of Cannabis sativa L. Essential Oil: A Multidisciplinary Study. Molecules 2018, 23, 3266. https://doi.org/10.3390/molecules23123266
Zengin G, Menghini L, Di Sotto A, Mancinelli R, Sisto F, Carradori S, Cesa S, Fraschetti C, Filippi A, Angiolella L, et al. Chromatographic Analyses, In Vitro Biological Activities, and Cytotoxicity of Cannabis sativa L. Essential Oil: A Multidisciplinary Study. Molecules. 2018; 23(12):3266. https://doi.org/10.3390/molecules23123266
Chicago/Turabian StyleZengin, Gokhan, Luigi Menghini, Antonella Di Sotto, Romina Mancinelli, Francesca Sisto, Simone Carradori, Stefania Cesa, Caterina Fraschetti, Antonello Filippi, Letizia Angiolella, and et al. 2018. "Chromatographic Analyses, In Vitro Biological Activities, and Cytotoxicity of Cannabis sativa L. Essential Oil: A Multidisciplinary Study" Molecules 23, no. 12: 3266. https://doi.org/10.3390/molecules23123266
APA StyleZengin, G., Menghini, L., Di Sotto, A., Mancinelli, R., Sisto, F., Carradori, S., Cesa, S., Fraschetti, C., Filippi, A., Angiolella, L., Locatelli, M., Mannina, L., Ingallina, C., Puca, V., D’Antonio, M., & Grande, R. (2018). Chromatographic Analyses, In Vitro Biological Activities, and Cytotoxicity of Cannabis sativa L. Essential Oil: A Multidisciplinary Study. Molecules, 23(12), 3266. https://doi.org/10.3390/molecules23123266