23-Hydroxyursolic Acid Isolated from the Stem Bark of Cussonia bancoensis Induces Apoptosis through Fas/Caspase-8-Dependent Pathway in HL-60 Human Promyelocytic Leukemia Cells
Abstract
1. Introduction
2. Results
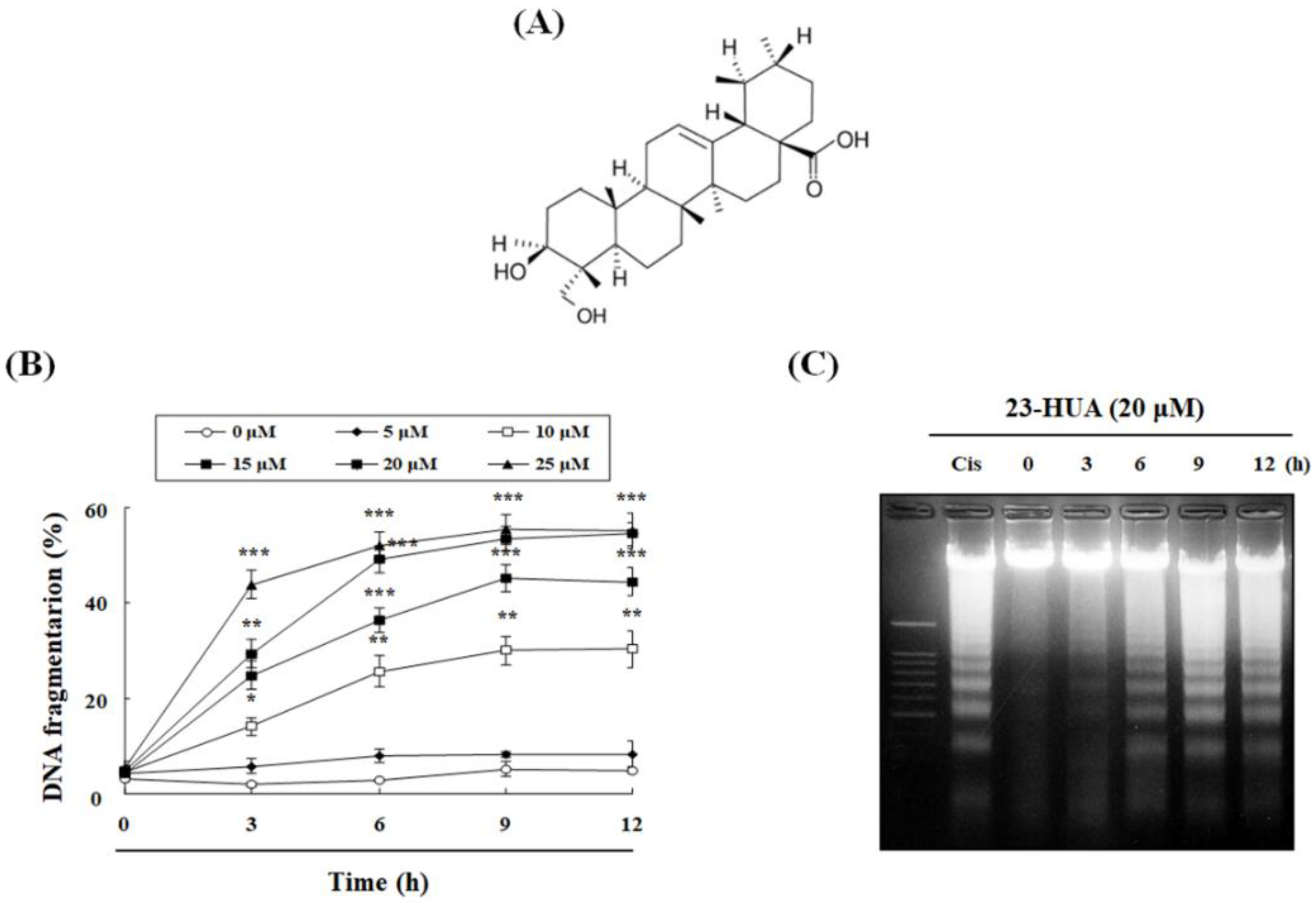
2.1. 23-HUA Causes Apoptosis in HL-60 Cells
2.2. 23-HUA Disrupts Mitochondrial Membrane Potential and Regulates Translocation of Mitochondria-Related Bcl-2 Family Proteins in HL-60 Cells
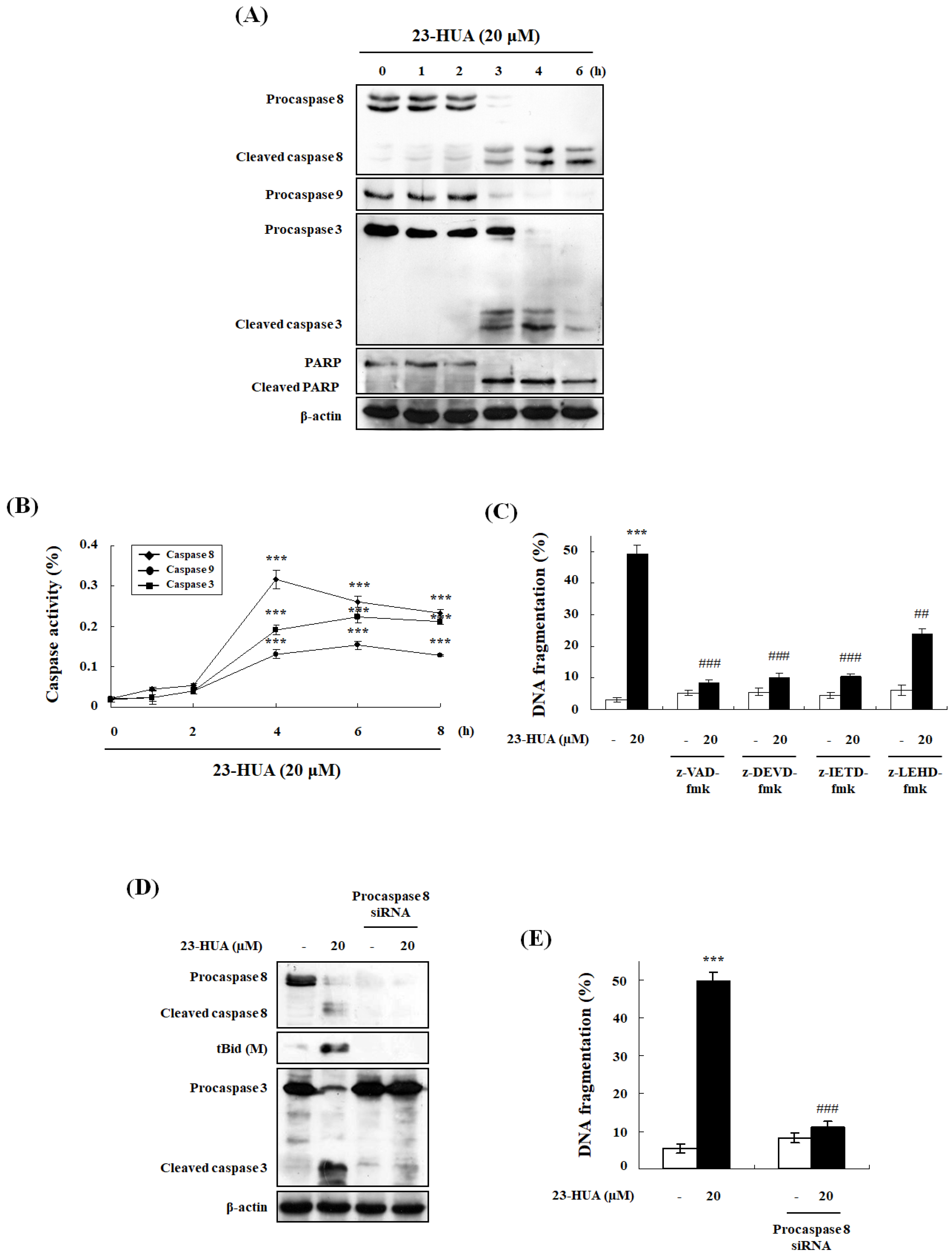
2.3. Caspase-Dependent Pathway Is Involved in 23-HUA-Induced Apoptosis
2.4. 23-HUA-Induced Apoptosis Requires DISC Formation in HL-60 Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Cultures
4.3. MTT Assay
4.4. DNA Fragmentation Assay
4.5. PI and Annexin V Double Staining
4.6. Determination of Mitochondria Membrane Potential (ΔΨm)
4.7. Preparation of Cytosolic and Mitochondrial Fractions
4.8. Western Blot and Immunoprecipitation Assay
4.9. Determination of Caspase Activity
4.10. Gene Knockdown Using siRNA
4.11. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Roberts, A.W.; Seymour, J.F.; Brown, J.R.; Wierda, W.G.; Kipps, T.J.; Khaw, S.L.; Carney, D.A.; He, S.Z.; Huang, D.C.; Xiong, H.; Cui, Y.; et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL-2 inhibition: Results of a phase I study of navitoclax in patients with relapsed or refractory disease. J. Clin. Oncol. 2012, 30, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Parrish, A.B.; Freel, C.D.; Kornbluth, S. Cellular mechanisms controlling caspase activation and function. Cold Spring Harb. Perspect. Biol. 2013, 5, 1–24. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.; Lu, M.; Petersen, S.; Ashkenazi, A. Apoptosis initiation through the cell-extrinsic pathway. Methods Enzymol. 2014, 544, 99–128. [Google Scholar] [PubMed]
- Kantari, C.; Walczak, H. Caspase-8 and bid: Caught in the act between death receptors and mitochondria. Biochim. Biophys. Acta 2011, 1813, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; Ahmed, S.; Brankov, N.; Perloff, M. Triterpenoids as potential agents for the chemoprevention and therapy of breast cancer. Front. Biosci. 2011, 16, 980–996. [Google Scholar] [CrossRef]
- Patlolla, J.M.; Rao, C.V. Triterpenoids for cancer prevention and treatment: Current status and future prospects. Curr. Pharm. Biotechnol. 2012, 13, 147–155. [Google Scholar] [CrossRef]
- Sultana, N.; Ata, A. Oleanolic acid and related derivatives as medicinally important compounds. J. Enzyme Inhib. Med. Chem. 2008, 23, 739–756. [Google Scholar] [CrossRef]
- Shah, B.A.; Qazi, G.N.; Taneja, S.C. Boswellic acids: A group of medicinally important compounds. Nat. Prod. Rep. 2009, 26, 72–89. [Google Scholar] [CrossRef]
- Setzer, W.N.; Setzer, M.C. Plant-derived triterpenoids as potential antineoplastic agents. Mini Rev. Med. Chem. 2003, 3, 540–556. [Google Scholar] [CrossRef]
- Laszczyk, M.N. Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. Planta Med. 2009, 75, 1549–1560. [Google Scholar] [CrossRef] [PubMed]
- Petronelli, A.; Pannitteri, G.; Testa, U. Triterpenoids as new promising anticancer drugs. Anticancer Drugs 2009, 20, 880–892. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Nguyen, A.H.; Kumar, A.P.; Tan, B.K.; Sethi, G. Targeted inhibition of tumor proliferation, survival, and metastasis by pentacyclic triterpenoids: Potential role in prevention and therapy of cancer. Cancer Lett. 2012, 320, 158–170. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Kuo, P.L.; Lin, C.C. Proliferative inhibition, cell-cycle dysregulation, and induction of apoptosis by ursolic acid in human non-small cell lung cancer A549 cells. Life Sci. 2004, 75, 2303–2316. [Google Scholar] [CrossRef]
- Yan, S.L.; Huang, C.Y.; Wu, S.T.; Yin, M.C. Oleanolic acid and ursolic acid induce apoptosis in four human liver cancer cell lines. Toxicol. In Vitro 2010, 24, 842–848. [Google Scholar] [CrossRef]
- Lee, K.T.; Sohn, I.C.; Park, H.J.; Kim, D.W.; Jung, G.O.; Park, K.Y. Essential moiety for antimutagenic and cytotoxic activity of hederagenin monodesmosides and bisdesmosides isolated from the stem bark of Kalopanax pictus. Planta Med. 2000, 66, 329–332. [Google Scholar] [CrossRef]
- Park, H.J.; Kwon, S.H.; Lee, J.H.; Lee, K.H.; Miyamoto, K.; Lee, K.T. Kalopanaxsaponin A is a basic saponin structure for the anti-tumor activity of hederagenin monodesmosides. Planta Med. 2001, 67, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Tapondjou, L.A.; Lontsi, D.; Sondengam, B.L.; Shaheen, F.; Choudhary, M.I.; van Heerden, F.R.; Park, H.J.; Lee, K.T. Saponins from Cussonia bancoensis and their inhibitory effects on nitric oxide production. J. Nat. Prod. 2003, 66, 1266–1269. [Google Scholar] [CrossRef]
- Takaya, M.; Nomura, M.; Takahashi, T.; Kondo, Y.; Lee, K.T.; Kobayashi, S. 23-hydroxyursolic acid causes cell growth-inhibition by inducing caspase-dependent apoptosis in human cervical squamous carcinoma HeLa cells. Anticancer Res. 2009, 29, 995–1000. [Google Scholar]
- Prenek, L.; Boldizsar, F.; Kugyelka, R.; Ugor, E.; Berta, G.; Nemeth, P.; Berki, T. The regulation of the mitochondrial apoptotic pathway by glucocorticoid receptor in collaboration with BCL-2 family proteins in developing T cells. Apoptosis 2017, 22, 239–253. [Google Scholar] [CrossRef]
- Paul, A.; Krelin, Y.; Arif, T.; Jeger, R.; Shoshan-Barmatz, V. A new role for the mitochondrial pro-apoptotic protein SMAC/Diablo in phospholipid synthesis associated with tumorigenesis. Mol. Ther. 2018, 26, 680–694. [Google Scholar] [CrossRef] [PubMed]
- Juarez-Rojas, A.L.; Garcia-Lorenzana, M.; Aragon-Martinez, A.; Gomez-Quiroz, L.E.; Retana-Marquez Mdel, S. Intrinsic and extrinsic apoptotic pathways are involved in rat testis by cold water immersion-induced acute and chronic stress. Syst. Biol. Reprod. Med. 2015, 61, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.L.; Kuo, P.L.; Lin, L.T.; Lin, C.C. Asiatic acid, a triterpene, induces apoptosis and cell cycle arrest through activation of extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways in human breast cancer cells. J. Pharmacol. Exp. Ther. 2005, 313, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.F.; Houng, J.Y.; Chang, C.L.; Wu, C.C.; Chang, F.R.; Wu, Y.C. Antioxidant activity, cytotoxicity, and DNA information of Glossogyne tenuifolia. J. Agric. Food Chem. 2005, 53, 6117–6125. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Jin, D.Q.; Kwon, E.J.; Park, S.H.; Lee, E.S.; Jeong, T.C.; Nam, D.H.; Huh, K.; Kim, J.A. Asiatic acid, a triterpene, induces apoptosis through intracellular Ca2+ release and enhanced expression of p53 in HepG2 human hepatoma cells. Cancer Lett. 2002, 186, 83–91. [Google Scholar] [CrossRef]
- Wu, Q.; Lv, T.; Chen, Y.; Wen, L.; Zhang, J.; Jiang, X.; Liu, F. Apoptosis of HL-60 human leukemia cells induced by Asiatic acid through modulation of B-cell lymphoma 2 family proteins and the mitogen-activated protein kinase signaling pathway. Mol. Med. Rep. 2015, 12, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.J.; Tait, S.W.G. Targeting BCL-2 regulated apoptosis in cancer. Open Biol. 2018, 8, 180002. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Gueydan, C.; Han, J. Plasma membrane changes during programmed cell deaths. Cell Res. 2018, 28, 9–21. [Google Scholar] [CrossRef]
- Los, M.; Mozoluk, M.; Ferrari, D.; Stepczynska, A.; Stroh, C.; Renz, A.; Herceg, Z.; Wang, Z.Q.; Schulze-Osthoff, K. Activation and caspase-mediated inhibition of PARP: A molecular switch between fibroblast necrosis and apoptosis in death receptor signaling. Mol. Biol. Cell 2002, 13, 978–988. [Google Scholar] [CrossRef]
- Chaitanya, G.V.; Steven, A.J.; Babu, P.P. PARP-1 cleavage fragments: Signatures of cell-death proteases in neurodegeneration. Cell Commun. Signal. 2010, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a008656. [Google Scholar] [CrossRef]
- Martinou, J.C.; Youle, R.J. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev. Cell 2011, 21, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Shamas-Din, A.; Kale, J.; Leber, B.; Andrews, D.W. Mechanisms of action of BCL-2 family proteins. Cold Spring Harb. Perspect. Biol. 2013, 5, a008714. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Leber, B.; Andrews, D.W. Interactions of pro-apoptotic BH3 proteins with anti-apoptotic BCL-2 family proteins measured in live MCF-7 cells using FILM FRET. Cell Cycle 2012, 11, 3536–3542. [Google Scholar] [CrossRef]
- Lee, K.W.; Chung, K.S.; Seo, J.H.; Yim, S.V.; Park, H.J.; Choi, J.H.; Lee, K.T. Sulfuretin from heartwood of Rhus verniciflua triggers apoptosis through activation of Fas, Caspase-8, and the mitochondrial death pathway in HL-60 human leukemia cells. J. Cell. Biochem. 2012, 113, 2835–2844. [Google Scholar] [CrossRef] [PubMed]
- Park, E.Y.; Kim, J.I.; Leem, D.G.; Shin, J.S.; Kim, K.T.; Choi, S.Y.; Lee, M.H.; Choi, J.H.; Lee, Y.S.; Lee, K.T. Resveratrol analogue (e)-8-acetoxy-2-[2-(3,4-diacetoxyphenyl)ethenyl]-quinazoline induces apoptosis via Fas-mediated pathway in HL-60 human leukemia cells. Oncol. Rep. 2016, 36, 3577–3587. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |





| Cell Line | Origin | IC50 (μM) a |
|---|---|---|
| HL-60 | Human promyelocytic leukemia | 11.07 ± 1.29 |
| U937 | Human histiocytic lymphoma | 12.13 ± 1.55 |
| HeLa | Human negroid cervix epitheloid carcinoma | 16.16 ± 1.77 |
| HepG2 | Human hepatoblastoma | 22.20 ± 1.07 |
| P388D1 | Mouse lymphoblast | 13.42 ± 1.13 |
| A172 | Human glioblastoma | 21.02 ± 1.14 |
| A431 | Human epidermoid carcinoma | 24.93 ± 2.92 |
| A549 | Human lung adenocarcinoma | 20.94 ± 2.37 |
| L132 | Human lung epithelial cell | 32.57 ± 2.91 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Won, J.-H.; Chung, K.-S.; Park, E.-Y.; Lee, J.-H.; Choi, J.-H.; Tapondjou, L.A.; Park, H.-J.; Nomura, M.; Hassan, A.H.E.; Lee, K.-T. 23-Hydroxyursolic Acid Isolated from the Stem Bark of Cussonia bancoensis Induces Apoptosis through Fas/Caspase-8-Dependent Pathway in HL-60 Human Promyelocytic Leukemia Cells. Molecules 2018, 23, 3306. https://doi.org/10.3390/molecules23123306
Won J-H, Chung K-S, Park E-Y, Lee J-H, Choi J-H, Tapondjou LA, Park H-J, Nomura M, Hassan AHE, Lee K-T. 23-Hydroxyursolic Acid Isolated from the Stem Bark of Cussonia bancoensis Induces Apoptosis through Fas/Caspase-8-Dependent Pathway in HL-60 Human Promyelocytic Leukemia Cells. Molecules. 2018; 23(12):3306. https://doi.org/10.3390/molecules23123306
Chicago/Turabian StyleWon, Jong-Heon, Kyung-Sook Chung, Eun-Young Park, Jeong-Hun Lee, Jung-Hye Choi, Leon Azefack Tapondjou, Hee-Juhn Park, Masaaki Nomura, Ahmed H.E. Hassan, and Kyung-Tae Lee. 2018. "23-Hydroxyursolic Acid Isolated from the Stem Bark of Cussonia bancoensis Induces Apoptosis through Fas/Caspase-8-Dependent Pathway in HL-60 Human Promyelocytic Leukemia Cells" Molecules 23, no. 12: 3306. https://doi.org/10.3390/molecules23123306
APA StyleWon, J.-H., Chung, K.-S., Park, E.-Y., Lee, J.-H., Choi, J.-H., Tapondjou, L. A., Park, H.-J., Nomura, M., Hassan, A. H. E., & Lee, K.-T. (2018). 23-Hydroxyursolic Acid Isolated from the Stem Bark of Cussonia bancoensis Induces Apoptosis through Fas/Caspase-8-Dependent Pathway in HL-60 Human Promyelocytic Leukemia Cells. Molecules, 23(12), 3306. https://doi.org/10.3390/molecules23123306







