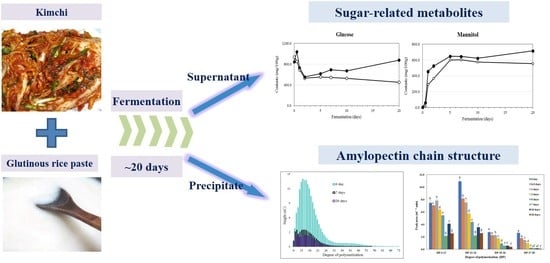
Analysis of Targeted Metabolites and Molecular Structure of Starch to Understand the Effect of Glutinous Rice Paste on Kimchi Fermentation
Abstract
:1. Introduction
2. Results and Discussion
2.1. pH of Kimchi during Fermentation
2.2. Count of Microorganisms during Fermentation
2.3. Total Starch Content in Kimchi during Fermentation
2.4. Sugars and Sugar Alcohols in Kimchi during Fermentation
2.5. Structural Analysis of Starch in Kimchi during Fermentation
3. Materials and Methods
3.1. Preparation of Kimchi, Fermentation, and Sampling
3.2. pH and Count of Microorganisms in Kimchi
3.3. Total Starch Content in Kimchi
3.4. Sugars and Sugar Alcohols Analysis
3.5. Structural Analysis of Starch in Kimchi
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cho, E.J.; Rhee, S.H.; Park, K.Y. Standardization of materials of Baechu kimchi recipe. J. Korean Soc. Food Sci. Nutr. 1999, 30, 1456–1463. [Google Scholar]
- Mheen, T.I.; Kwon, T.W. Effect of temperature and salt concentration on kimchi fermentation. Korean J. Food Sci. Technol. 1984, 16, 443–450. [Google Scholar]
- Choi, H.; Islam, M.S. Antidiabetic effect of Korean traditional Baechu (Chinese cabbage) kimchi in a type 2 diabeted model of rats. J. Med. Food 2009, 12, 292–297. [Google Scholar]
- Cho, J.; Lee, D.; Yang, C.; Jeon, J.; Kim, J.; Han, H. Microbial population dynamics of kimchi, a fermented cabbage product. FEMS Microbiol. Lett. 2006, 257, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Lee, S.H.; Lee, H.J.; Seo, H.Y.; Park, W.S.; Jeon, C.O. Effects of Leuconostoc mesenteroides starter cultures on microbial communities and metabolites during kimchi fermentation. Int. J. Food Microbiol. 2012, 153, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Song, J.H.; Jung, M.Y.; Lee, S.H.; Chang, J.Y. Large-scale targeted metagenomics analysis of bacterial ecological changes in 88 kimchi samples during fermentation. Food Microbiol. 2017, 66, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Park, K.Y.; Jeong, J.K.; Lee, Y.E.; Daily, J.W. Health benefits of kimchi (Korean fermented vegetables) as a probiotics foods. J. Med. Food 2014, 17, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Grosu-Tudor, S.S.; Zamfir, M. Probiotic potential of some lactic acid bacteria isolated from Romanian fermented vegetables. Ann. Rom. Soc. Cell Biol. 2012, 17, 234–239. [Google Scholar]
- Khan, I.; Kang, S.C. Probiotic potential of nutritionally improved Lactobacillus plantarum DGK-17 isolated from Kimchi e A traditional Korean fermented food. Food Control 2016, 88–94. [Google Scholar] [CrossRef]
- Jeong, S.H.; Lee, S.H.; Jung, J.Y.; Choi, E.J.; Jeon, C.O. Microbial succession and metabolite changes during long-term storage of Kimchi. J. Food Sci. 2013, 78, 763–769. [Google Scholar] [CrossRef]
- Ji, Y.; Kim, H.; Park, H.; Lee, J.; Lee, H.; Shin, H.; Kim, B.; Franz, C.M.A.P.; Holzapfel, W.H. Functionality and safety of lactic bacterial strains from Korean kimchi. Food Control 2013, 31, 467–473. [Google Scholar] [CrossRef]
- Shim, S.M.; Kim, J.Y.; Lee, S.M.; Park, J.B.; Oh, S.K.; Kim, Y.S. Profiling of fermentative metabolites in kimchi: Volatile and non-volatile organic acids. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 463–469. [Google Scholar] [CrossRef]
- Jang, M.S.; Park, M.O. Effect of glutinous rice paste on the fermentation of Puchukimchi. Korean J. Food Cook. Sci. 1998, 14, 421–429. [Google Scholar]
- Noomhorm, A.; Kongseree, N.; Apintanapong, M. Effect of aging on the quality of glutinous rice crackers. Cereal Chem. 1997, 74, 12–15. [Google Scholar] [CrossRef]
- Lee, G.C.; Han, J.A. Changes in the contents of total vitamin C and reducing sugars of starchy pastes added Kimchi during fermentation. Korean J. Food Cook. Sci. 1998, 14, 201–206. [Google Scholar]
- Park, W.P. Effect of starch sources on Yulmoo kimchi fermentation. Food Sci. Biotechnol. 1995, 4, 98–100. [Google Scholar]
- Lee, G.C.; Han, J.A. Changes in physical and microbial properties of starchy paste added Kimchi during fermentation. Korean J. Food Cook. Sci. 1998, 14, 195–200. [Google Scholar]
- Seo, S.H.; Park, S.E.; Kim, E.J.; Lee, K.I.; Na, C.S.; Son, H.S. A GC-MS based metabolomics approach to determine the effect of salinity on Kimchi. Food Res. Int. 2018, 105, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.Y.; Jeong, J.K.; Moon, S.H.; Park, K.Y. Effects of brined baechu cabbage and seasoning on fermentation of kimchi. J. Korean Soc. Food Sci. Nutr. 2014, 43, 1081–1087. [Google Scholar] [CrossRef]
- Zabat, M.A.; Sano, W.H.; Cabral, D.J.; Wurster, J.I.; Belenky, P. The impact of vegan product on the kimchi microbiome. Food Microbiol. 2018, 74, 171–178. [Google Scholar] [CrossRef]
- Hahn, Y.S.; Oh, J.Y.; Song, J.E. The study on amylolytic enzyme and protease activities of Kimchi. Korean J. Food Sci. Technol. 2002, 34, 269–273. [Google Scholar]
- Ha, J.H.; Hawer, W.S.; Kim, Y.J.; Nam, Y.J. Changes of free sugars in kimchi during fermentation. Korean J. Food Sci. Technol. 1989, 21, 633–638. [Google Scholar]
- Oh, Y.A.; Kim, S.D. Changes in enzyme activities of salted Chinese cabbage and kimchi during salting and fermentation. J. Korean Soc. Food Sci. Nutr. 1997, 26, 404–410. [Google Scholar]
- Dol, M.; Chraibi, W.; Remaud-Simeon, M.; Lindley, N.D.; Monsan, P.F. Growth and energetics of Leuconostoc mesenteroides NRRL B-1299 during metabolism of various sugars and their consequences for dextransucrase production. Appl. Environ. Microb. 1997, 63, 2159–2165. [Google Scholar]
- Aarnikunnas, J.; Rönnholm, K.; Palva, A. The mannitol dehydrogenase gene (mdh) from Leuconostoc mesenteroides is distinct from other known bacterial mdh genes. Appl. Environ. Microb. 2002, 59, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Grobben, G.J.; Peters, S.W.P.G.; Wisselink, H.W.; Weusthuis, R.A.; Hoefnagel, M.H.N.; Hugenholtz, J.; Eggink, G. Spontaneous formation of a mannitol-producing variant of Leuconostoc pseudomesenteroies grown in the presence of fructose. Appl. Environ. Microb. 2001, 67, 2867–2870. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, G.E.; Vermeylen, R.; Geeroms, J.; Delcour, J.A. Rice starches. III. Structural aspects provide insight in amylopectin retrogradation properties and gel texture. J. Cereal Sci. 2003, 38, 61–68. [Google Scholar] [CrossRef]
- You, S.Y.; Oh, S.K.; Kim, H.S.; Chung, H.J. Influence of molecular structure on physicochemical properties and digestibility of normal rice starches. Int. J. Biol. Macromol. 2015, 77, 375–382. [Google Scholar] [CrossRef]
- Hanashiro, I.; Abe, J.I.; Hizukuri, S. A periodic distribution of the chain length of amylopectin as revealed by high-performance anion-exchange chromatography. Carbohydr. Res. 1996, 283, 151–159. [Google Scholar] [CrossRef]
- Hu, Y.; Ketabi, A.; Buchko, A.; Gänzle, M.G. Metabolism of isomalto-oligosaccharides by Lactobacillus reuteri and bifidobacterial. Lett. Appl. Microbiol. 2013, 57, 108–114. [Google Scholar] [CrossRef]
- Bijttebier, A.; Goesaert, H.; Delcour, J.A. Hydrolysis of amylopectin by amylolytic enzymes: Structural analysis of the residual amylopectin population. Carbohydr. Res. 2010, 345, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Bae, E.K.; Ha, S.D.; Park, Y.S.; Mok, C.K.; Hong, K.P.; Kim, S.P.; Park, J. Evaluation of dry rehydratable film method for enumeration of microorganisms in Korean traditional foods. J. Food Hyg. Saf. 2004, 19, 209–216. [Google Scholar]
- AACC International. AACC Methods 76-13.01. In Approved Methods of the American Association of Cereal Chemists, 11th ed.; AACC International: St. Paul, MN, USA, 2000. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |




| Distribution (%) | Fermentation Time (Days) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.5 | 1 | 2 | 5 | 7 | 10 | 20 | |
| DP 6–12 | 31.3 ± 0.4 g | 36.6 ± 0.1 f | 41.1 ± 0.2 e | 43.1 ± 0.2 d | 49.6 ± 0.3 a | 41.8 ± 0.1 d | 48.9 ± 0.0 b | 45.3 ± 0.1 c |
| DP 13–24 | 46.0 ± 0.4 a | 42.4 ± 0.1 c | 39.3 ± 0.4 d | 38.7 ± 0.3 d | 39.8 ± 0.2 d | 43.4 ± 0.0 b | 42.2 ± 0.1 c | 45.8 ± 0.1 a |
| DP 25–36 | 11.6 ± 0.1 b | 11.8 ± 0.1 ab | 11.8 ± 0.0 a | 11.7 ± 0.0 b | 8.5 ± 0.0 d | 11.3 ± 0.1 c | 6.9 ± 0.0 e | 7.0 ± 0.1 e |
| DP ≥ 37 | 11.1 ± 0.1 a | 9.2 ± 0.0 b | 7.7 ± 0.1 c | 6.6 ± 0.1 d | 2.2 ± 0.1 f | 3.6 ± 0.1 e | 2.0 ± 0.0 f | 1.8 ± 0.1 f |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, D.; Lee, J.-H.; Chung, H.-J. Analysis of Targeted Metabolites and Molecular Structure of Starch to Understand the Effect of Glutinous Rice Paste on Kimchi Fermentation. Molecules 2018, 23, 3324. https://doi.org/10.3390/molecules23123324
Jeong D, Lee J-H, Chung H-J. Analysis of Targeted Metabolites and Molecular Structure of Starch to Understand the Effect of Glutinous Rice Paste on Kimchi Fermentation. Molecules. 2018; 23(12):3324. https://doi.org/10.3390/molecules23123324
Chicago/Turabian StyleJeong, Duyun, Jong-Hee Lee, and Hyun-Jung Chung. 2018. "Analysis of Targeted Metabolites and Molecular Structure of Starch to Understand the Effect of Glutinous Rice Paste on Kimchi Fermentation" Molecules 23, no. 12: 3324. https://doi.org/10.3390/molecules23123324
APA StyleJeong, D., Lee, J.-H., & Chung, H.-J. (2018). Analysis of Targeted Metabolites and Molecular Structure of Starch to Understand the Effect of Glutinous Rice Paste on Kimchi Fermentation. Molecules, 23(12), 3324. https://doi.org/10.3390/molecules23123324