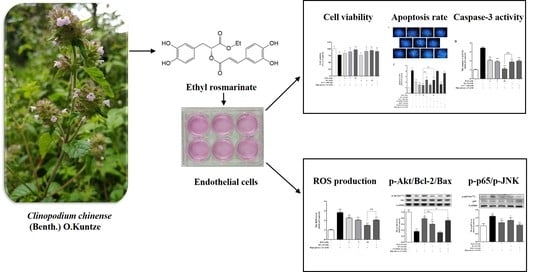
Ethyl Rosmarinate Protects High Glucose-Induced Injury in Human Endothelial Cells
Abstract
:1. Introduction
2. Results
2.1. Effect of RAE on Cell Viability Induced by High Glucose
2.2. Effect of RAE on ROS Generation in Human Endothelial Cells Induced by High Glucose
2.3. Effects of RAE on Cell Apoptosis Induced by High Glucose
2.4. Effect of RAE on PI3K/Akt Signal Pathway
2.5. Effect of RAE on the Expression of Bcl-2 and Bax
2.6. Effect of RAE on the Expression of p-p65 and p-JNK
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Reagents
4.3. Cell Culture
4.4. Cell Viability Assay
4.5. Detection of Intracellular ROS Production
4.6. Cell Morphology Assays by Hoechst Staining
4.7. Analysis of Apoptosis by Annexin V-FITC/PI Staining
4.8. Caspase-3 Activity Assay
4.9. Analysis of Western Blot
4.10. Molecular Docking Analysis
4.11. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seino, Y.; Nanjo, K.; Tajima, N.; Kadowaki, T.; Kashiwagi, A.; Araki, E.; Ito, C.; Inagaki, N.; Iwamoto, Y.; Kasuga, M.; et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J. Diabetes Investig. 2010, 1, 212–228. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Zimmet, P.; Shaw, J. International diabetes federation: a consensus on type 2 diabetes prevention. Diabet. Med. 2007, 24, 451–463. [Google Scholar] [CrossRef]
- Allen, D.A.; Yaqoob, M.M.; Harwood, S.M. Mechanisms of high glucose-induced apoptosis and its relationship to diabetic complications. J. Nutr. Biochem. 2005, 16, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, R.; Han, S.F.; Bu, L.; Wang, S.W.; Ma, H.; Jia, G.L. Alpha-linolenic acid attenuates high glucose-induced apoptosis in cultured human umbilical vein endothelial cells via PI3K/Akt/eNOS pathway. Nutrition 2007, 23, 762–770. [Google Scholar] [CrossRef]
- Shen, H.; Rong, H. Pterostilbene impact on retinal endothelial cells under high glucose environment. Int. J. Clin. Exp. Pathol. 2015, 8, 12589–12594. [Google Scholar] [PubMed]
- Liu, R.; Shen, H.; Wang, T.; Ma, J.; Yuan, M.; Huang, J.; Wei, M.; Liu, F. TRAF6 mediates high glucose-induced endothelial dysfunction. Exp. Cell Res. 2018, 370, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.Y.; Wang, C.C.; Lai, T.Y.; Tsu, H.N.; Wang, C.H.; Liang, H.Y.; Kuo, W.W. Antioxidant effects of diallyl trisulfide on high glucose-induced apoptosis are mediated by the PI3K/Akt-dependent activation of Nrf2 in cardiomyocytes. Int. J. Cardiol. 2013, 168, 1286–1297. [Google Scholar] [CrossRef] [PubMed]
- Ho, F.M.; Lin, W.W.; Chen, B.C.; Chao, C.M.; Yang, C.R.; Lin, L.Y.; Lai, C.C.; Liu, S.H.; Liau, C.S. High glucose-induced apoptosis in human vascular endothelial cells is mediated through NF-kappaB and c-jun NH2-terminal kinase pathway and prevented by PI3K/Akt/eNOS pathway. Cell. Signal. 2006, 18, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Yang, F.; Cao, X.; Chen, C.; Zhang, X.; Zhang, X.; Lin, W.L.; Wang, X.F.; Liang, C.W. Gab1 regulates SDF-1-induced progression via inhibition of apoptosis pathway induced by PI3K/Akt/Bcl-2/Bax pathway in human chondrosarcoma. Tumor Biol. 2016, 37, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Nizamutdinova, I.T.; Guleria, R.S.; Singh, A.B.; Jr, J.A.K.; Baker, K.M.; Pan, J. Retinoic acid protects cardiomyocytes from high glucose-induced apoptosis through inhibition of NF-κB signaling pathway. J. Cell. Physiol. 2013, 228, 380–392. [Google Scholar] [CrossRef]
- Kuo, W.W.; Wang, W.J.; Tsai, C.Y.; Way, C.L.; Hsu, H.H.; Chen, L.M. Diallyl trisufide (dats) suppresses high glucose-induced cardiomyocyte apoptosis by inhibiting JNK/NF-κB signaling via attenuating ROS generation. Int. J. Cardiol. 2013, 168, 270–280. [Google Scholar] [CrossRef]
- Nishikawa, T.; Araki, E. Impact of mitochondrial ROS production in the pathogenesis of diabetes mellitus and its complications. Antioxid. Redox Signal. 2007, 9, 343–353. [Google Scholar] [CrossRef] [PubMed]
- National Pharmacopoeia Committee. Pharmacopoeia of People’s Republic of China: Part. 1, 1st ed.; Chinese medical science press: Beijing, China, 2015; pp. 326–327. [Google Scholar]
- Li, J.; Wu, F.H.; Chen, K.; Liang, J.Y.; Ma, S.P. Extract of Clinopodium chinense inhibits high glucose induced apoptosis in human umbilical vein endothelial cells. J. Cardiovasc. Pharmacol. 2013, 61, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Zeng, B.; Chen, K.; Du, P.; Wang, S.S.; Ren, B.; Ren, Y.L.; Yan, H.S.; Liang, Y.; Wu, F.H. Phenolic compounds from Clinopodium chinense (Benth.) O. Kuntze and their inhibitory effects on α-glucosidase and vascular endothelial cells injury. Chem. Biodivers 2016, 13, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Thammason, H.; Khetkam, P.; Pabuprapap, W.; Suksamrarn, A.; Kunthalert, D. Ethyl rosmarinate inhibits lipopolysaccharide-induced nitric oxide and prostaglandin E2 production in alveolar macrophages. Eur. J. Pharmacol. 2018, 824, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Wicha, P.; Tocharus, J.; Nakaew, A.; Pantan, R.; Suksamrarn, A.; Tocharus, C. Ethyl rosmarinate relaxes rat aorta by an endothelium-independent pathway. Eur. J. Pharmacol. 2015, 766, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Mesaik, M.A.; Jabeen, A.; Halim, S.A.; Begum, A.; Khalid, A.S.; Asif, M.; Fatima, B.; Ul-Haq, Z.; Choudhary, M.I. In silico and in vitro immunomodulatory studies on compounds of Lindelofia stylosa. Chem. Biol. Drug Des. 2012, 79, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Sotnikova, R.; Okruhlicova, L.; Vlkovicova, J.; Navarova, J.; Gajdacova, B.; Pivackova, L.; Fialova, S.; Krenek, P. Rosmarinic acid administration attenuates diabetes-induced vascular dysfunction of the rat aorta. J. Pharm. Pharmacol. 2013, 65, 713–723. [Google Scholar] [CrossRef]
- Al-Musayeib, N.; Perveen, S.; Fatima, I.; Nasir, M.; Hussain, A. Antioxidant, anti-glycation and anti-inflammatory activities of phenolic constituents from Cordia sinensis. Molecules 2011, 16, 10214–10226. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Hauck, C.; Yum, M.Y.; Rizshsky, L.; Widrlechner, M.P.; McCoy, J.A.; Murphy, P.A.; Dixon, P.M.; Nikolau, B.J.; Birt, D.F. Rosmarinic acid in Prunella vulgaris ethanol extract inhibits lipopolysaccharide-induced prostaglandin E2 and nitric oxide in RAW 264.7 mouse macrophages. J. Agric. Food Chem. 2009, 57, 10579–10589. [Google Scholar] [CrossRef] [PubMed]
- Luan, H.Y.; Kan, Z.C.; Xu, Y.; Lv, C.J.; Jiang, W.L. Rosmarinic acid protects against experimental diabetes with cerebral ischemia: relation to inflammation response. J. Neuroinflammation 2013, 10, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altıntop, M.D.; Sever, B.; Akalın, G.; Özdemir, A. Design, synthesis, and evaluation of a new series of thiazole-based anticancer agents as potent Akt inhibitors. Molecules 2018, 23, 1318. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Mishra, M.; Sudhakar, D.R.; Siddiqui, M.H. In-silico designing of a potent analogue against HIV-1 Nef protein and protease by predicting its interaction network with host cell proteins. J. Pharm. Bioallied Sci. 2013, 5, 66–73. [Google Scholar] [CrossRef]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998, 352, 837–853. [Google Scholar] [CrossRef]
- Zinman, B.; Genuth, S.; Nathan, D.M. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study: 30th anniversary presentations. Diabetes Care 2014, 37, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Turunen, S.P.; Kummu, O.; Veneskoski, M.; Lehtimäki, J.; Nissinen, A.E. Natural antibodies of newborns recognize oxidative stress-related malondialdehyde acetaldehyde adducts on apoptotic cells and atherosclerotic plaques. Int. Immunol. 2013, 25, 575–587. [Google Scholar] [CrossRef] [Green Version]
- Du, X.L.; Edelstein, D.; Rossetti, L.; Fantus, I.G.; Goldberg, H.; Ziyadeh, F.; Wu, J.; Brownlee, M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc. Natl. Acad. Sci. USA. 2000, 97, 12222–12226. [Google Scholar] [CrossRef] [Green Version]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Hammes, H.P.; et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000, 404, 787–790. [Google Scholar] [CrossRef]
- Li, K.; Li, Y.; Mi, J.; Mao, L.; Han, X.; Zhao, J. Resveratrol protects against sodium nitroprusside induced nucleus pulposus cell apoptosis by scavenging ROS. Int. J. Mol. Med. 2018, 41, 2485–2492. [Google Scholar] [CrossRef]
- Zhu, M.; Chen, J.; Hui, J.; Miao, C. Propofol protects against high glucose-induced endothelial adhesion molecules expression in human umbilical vein endothelial cells. Cardiovasc. Diabetol. 2013, 12, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K. Molecular mechanisms of hepatic apoptosis. Cell Death Dis. 2014, 5, e996. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, L.; Wang, Y.; Wang, L.; Liu, X.; Liu, X.; Chen, Z.; Liu, L. Exendin-4 protects HUVECs from t-BHP-induced apoptosis via PI3K/Akt-Bcl-2-caspase-3 signaling. Endocr. Res. 2016, 41, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Ma, S.; Wang, C.; Ke, J.; Yang, J.; Li, W.; Liu, Y.; Hou, W.; Feng, X.; Wang, G.; et al. Exenatide exerts direct protective effects on endothelial cells through the AMPK/Akt/eNOS pathway in a GLP-1 receptor-dependent manner. Am. J. Physiol. Endocrinol. MeTable 2016, 310, E947–957. [Google Scholar] [CrossRef]
- Franke, T.F.; Hornik, C.P.; Segev, L.; Shostak, G.A.; Sugimoto, C. PI3K/Akt and apoptosis: Size matters. Oncogene 2003, 22, 8983–8998. [Google Scholar] [CrossRef] [PubMed]
- Kucharczak, J.; Simmons, M.J.; Fan, Y.; Gélinas, C. To be, or not to be: NF-κB is the answer-role of rel/NF-κB in the regulation of apoptosis. Oncogene 2003, 22, 8961–8982. [Google Scholar] [CrossRef]
- Dutta, J.; Fan, Y.N.; Fan, G.; Gélinas, C. Current insights into the regulation of programmed cell death by NF-kappaB. Oncogene 2006, 25, 6800–6816. [Google Scholar] [CrossRef]
- Chen, G.; Chen, Y.; Chen, H.; Li, L.; Yao, J.; Jiang, Q.; Lin, X.; Wen, J.; Lin, L. The effect of NF-κB pathway on proliferation and apoptosis of human umbilical vein endothelial cells induced by intermittent high glucose. Mol. Cell. Biochem. 2011, 347, 127–133. [Google Scholar] [CrossRef]
- Ho, D.T.; Bardwell, A.J.; Abdollahi, M.; Bardwell, L. A docking site in MKK4 mediates high affinity binding to JNK MAPKs and competes with similar docking sites in JNK substrates. J. Biol. Chem. 2003, 278, 32662–32672. [Google Scholar] [CrossRef]
- Bennett, B.L.; Sasaki, D.T.; Murray, B.W.; O’Leary, E.C.; Sakata, S.T.; Xu, W.; Leisten, J.C.; Motiwala, A.; Pierce, S.; Satoh, Y.; et al. Sp600125, an anthrapyrazolone inhibitor of jun N-terminal kinase. Proc. Natl. Acad. Sci. USA 2001, 98, 13681–13686. [Google Scholar] [CrossRef]
- Xia, Z.; Dickens, M.; Raingeaud, J.; Davis, R.J.; Greenberg, M.E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 1995, 270, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, G.Q.; Yu, G.P.; Liu, C.J. Pyrroloquinoline quinone protects mouse brain endothelial cells from high glucose-induced damage in vitro. Acta. Pharmacol. Sin. 2014, 35, 1402–1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, G.; Han, H.; Yang, Y.; Jin, Y.; Wang, X.; Liu, X. Neferine prevented hyperglycemia-induced endothelial cell apoptosis through suppressing ROS/Akt/NF-κB signal. Endocrine 2014, 47, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, F. Reactive oxygen species (ROS), troublemakers between nuclear factor-kappaB (NF-kappaB) and c-jun NH(2)-terminal kinase (JNK). Cancer Res. 2004, 64, 1902–1905. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.W.; Deng, L.X.; Chen, H.Y.; Su, Z.Q.; Ye, S.L.; Xu, W.Y. MiR-124 affects the apoptosis of brain vascular endothelial cells and ROS production through regulating PI3K/Akt signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhang, C.Z.; Ran, R.X.; Cao, Y.F.; Du, Z.; Fu, Z.W.; Huang, C.T.; Zhao, Z.Y.; Zhang, W.H.; Fang, Z.Z. In vitro comparative study of the inhibitory effects of Mangiferin and its aglycone norathyriol towards UDP-glucuronosyl transferase (UGT) isoforms. Molecules 2017, 22, 1008. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are all available from the authors. |










| Compound | Docking Score (kcal/mol) | Ki Value (μM) |
|---|---|---|
| RAE | −8.50 | 0.59 |
| RA | −8.03 | 1.30 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.-H.; Wang, L.-Y.; Zhang, B.-B.; Hu, Q.-M.; Wang, P.; He, B.-Q.; Bao, G.-H.; Liang, J.-Y.; Wu, F.-H. Ethyl Rosmarinate Protects High Glucose-Induced Injury in Human Endothelial Cells. Molecules 2018, 23, 3372. https://doi.org/10.3390/molecules23123372
Shen Y-H, Wang L-Y, Zhang B-B, Hu Q-M, Wang P, He B-Q, Bao G-H, Liang J-Y, Wu F-H. Ethyl Rosmarinate Protects High Glucose-Induced Injury in Human Endothelial Cells. Molecules. 2018; 23(12):3372. https://doi.org/10.3390/molecules23123372
Chicago/Turabian StyleShen, Yan-Hui, Li-Ying Wang, Bao-Bao Zhang, Qi-Ming Hu, Pu Wang, Bai-Qiu He, Guan-Hu Bao, Jing-Yu Liang, and Fei-Hua Wu. 2018. "Ethyl Rosmarinate Protects High Glucose-Induced Injury in Human Endothelial Cells" Molecules 23, no. 12: 3372. https://doi.org/10.3390/molecules23123372