Metal-Incorporated Mesoporous Silicates: Tunable Catalytic Properties and Applications
Abstract
:1. Introduction
Need for Mesoporous Silica
2. Experimental
2.1. Scalable and Reproducible One-Pot Synthesis of M-KIT-6
2.2. Unique Characteristics of M-KIT-6
3. Results and Discussion
3.1. Applications of M-KIT-6 Materials in Gas Phase Reactions
3.1.1. Selective Dehydration
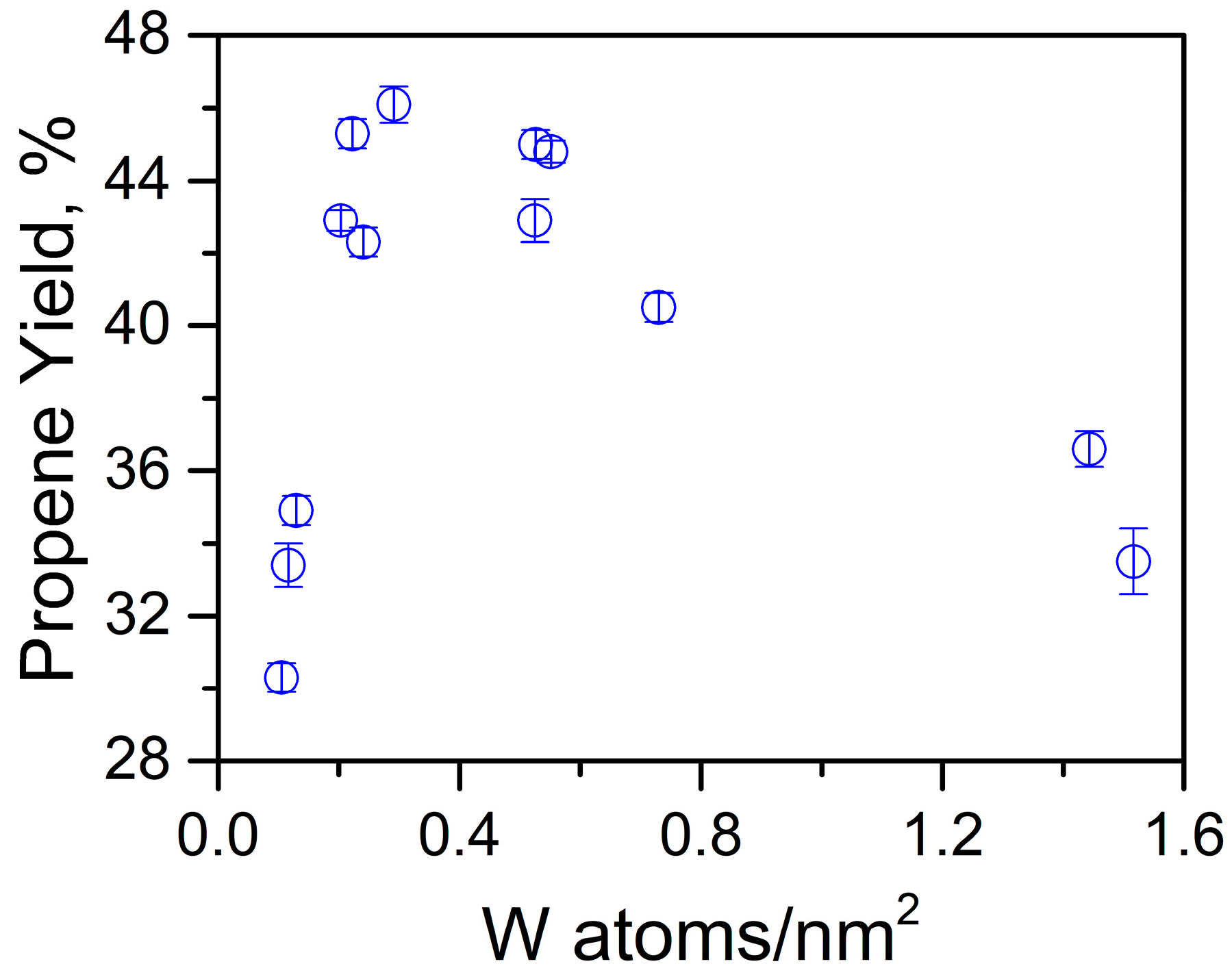
3.1.2. Olefin Metathesis
3.2. Applications of M-KIT-6 Materials in Liquid Phase Reactions
3.2.1. Liquid Phase Epoxidations
3.2.2. Liquid Phase Lignin Depolymerization
4. Catalyst Deactivation
5. Summary and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; et al. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Zhao, D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-Y.; Chen, L.-H.; Li, Y.; Rooke, J.C.; Sanchez, C.; Su, B.-L. Hierarchically porous materials: Synthesis strategies and structure design. Chem. Soc. Rev. 2017, 46, 481–558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Xu, L.-L.; Jiang, J.-G.; Calin, N.; Lam, K.-F.; Zhang, S.-J.; Wu, H.-H.; Wu, G.-D.; Albela, B.; Bonneviot, L.; et al. Facile Large-Scale Synthesis of Monodisperse Mesoporous Silica Nanospheres with Tunable Pore Structure. J. Am. Chem. Soc. 2013, 135, 2427–2430. [Google Scholar] [CrossRef] [PubMed]
- Möller, K.; Bein, T. Talented Mesoporous Silica Nanoparticles. Chem. Mater. 2017, 29, 371–388. [Google Scholar] [CrossRef]
- Kleitz, F.; Hei Choi, S.; Ryoo, R. Cubic Ia3d large mesoporous silica: Synthesis and replication to platinum nanowires, carbon nanorods and carbon nanotubes. Chem. Commun. 2003, 2136–2137. [Google Scholar] [CrossRef]
- Kim, T.-W.; Kleitz, F.; Paul, B.; Ryoo, R. MCM-48-like Large Mesoporous Silicas with Tailored Pore Structure: Facile Synthesis Domain in a Ternary Triblock Copolymer−Butanol−Water System. J. Am. Chem. Soc. 2005, 127, 7601–7610. [Google Scholar] [CrossRef] [PubMed]
- Nava, R.; Pawelec, B.; Castaño, P.; Álvarez-Galván, M.C.; Loricera, C.V.; Fierro, J.L.G. Upgrading of bio-liquids on different mesoporous silica-supported CoMo catalysts. Appl. Catal. B Environ. 2009, 92, 154–167. [Google Scholar] [CrossRef]
- Aho, A.; Salmi, T.; Murzin, D.Y. Catalytic Pyrolysis of Lignocellulosic Biomass. In The Role of Catalysis for the Sustainable Production of Bio-Fuels and Bio-Chemicals; Elsevier: Amsterdam, The Netherlands, 2013; pp. 137–159. ISBN 978-0-444-56330-9. [Google Scholar]
- Gürbüz, E.; Bond, J.Q.; Dumesic, J.A.; Román-Leshkov, Y. Role of Acid Catalysis in the Conversion of Lignocellulosic Biomass to Fuels and Chemicals. In The Role of Catalysis for the Sustainable Production of Bio-Fuels and Bio-Chemicals; Elsevier: Amsterdam, The Netherlands, 2013; pp. 261–288. ISBN 978-0-444-56330-9. [Google Scholar]
- Arun, N.; Sharma, R.V.; Dalai, A.K. Green diesel synthesis by hydrodeoxygenation of bio-based feedstocks: Strategies for catalyst design and development. Renew. Sustain. Energy Rev. 2015, 48, 240–255. [Google Scholar] [CrossRef]
- Nandiwale, K.Y.; Danby, A.M.; Ramanathan, A.; Chaudhari, R.V.; Subramaniam, B. Zirconium-Incorporated Mesoporous Silicates Show Remarkable Lignin Depolymerization Activity. ACS Sustain. Chem. Eng. 2017, 5, 7155–7164. [Google Scholar] [CrossRef]
- Soni, K.; Mouli, K.C.; Dalai, A.K.; Adjaye, J. Influence of Frame Connectivity of SBA-15 and KIT-6 Supported NiMo Catalysts for Hydrotreating of Gas Oil. Catal. Lett. 2010, 136, 116–125. [Google Scholar] [CrossRef]
- Vinu, A.; Srinivasu, P.; Balasubramanian, V.V.; Ariga, K.; Mori, T.; Nemoto, Y. Three-dimensional Mesoporous TiKIT-6 with Ia3d Symmetry Synthesized at Low Acid Concentration and Its Catalytic Performances. Chem. Lett. 2008, 37, 1016–1017. [Google Scholar] [CrossRef]
- Hussain, M.; Akhter, P.; Russo, N.; Saracco, G. Novel Ti-KIT-6 material for the photocatalytic reduction of carbon dioxide to methane. Catal. Commun. 2013, 36, 58–62. [Google Scholar] [CrossRef]
- Parlett, C.M.A.; Bruce, D.W.; Hondow, N.S.; Lee, A.F.; Wilson, K. Support-Enhanced Selective Aerobic Alcohol Oxidation over Pd/Mesoporous Silicas. ACS Catal. 2011, 1, 636–640. [Google Scholar] [CrossRef]
- Pirez, C.; Caderon, J.-M.; Dacquin, J.-P.; Lee, A.F.; Wilson, K. Tunable KIT-6 Mesoporous Sulfonic Acid Catalysts for Fatty Acid Esterification. ACS Catal. 2012, 2, 1607–1614. [Google Scholar] [CrossRef]
- Li, W.; Zhao, D. An overview of the synthesis of ordered mesoporous materials. Chem. Commun. 2013, 49, 943–946. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Luo, X.; Huang, J.; Wang, X.; Liang, Z. One-pot synthesis of ordered mesoporous Cu-KIT-6 and its improved catalytic behavior for the epoxidation of styrene: Effects of the pH value of the initial gel. Chin. J. Catal. 2017, 38, 518–528. [Google Scholar] [CrossRef]
- Zhan, W.; Guo, Y.; Wang, Y.; Guo, Y.; Lu, G. Synthesis of lathanum or La-B doped KIT-6 mesoporous materials and their application in the catalytic oxidation of styrene. J. Rare Earths 2010, 28, 369–375. [Google Scholar] [CrossRef]
- Prabhu, A.; Shoaibi, A.A.; Srinivasakannan, C. Development of gallium incorporated mesoporous silica catalysts for the selective removal of BTX. Appl. Catal. A Gen. 2013, 466, 137–141. [Google Scholar] [CrossRef]
- Merkache, R.; Fechete, I.; Maamache, M.; Bernard, M.; Turek, P.; Al-Dalama, K.; Garin, F. 3D ordered mesoporous Fe-KIT-6 catalysts for methylcyclopentane (MCP) conversion and carbon dioxide (CO2) hydrogenation for energy and environmental applications. Appl. Catal. A Gen. 2015, 504, 672–681. [Google Scholar] [CrossRef]
- Ramanathan, A.; Subramaniam, B.; Badloe, D.; Hanefeld, U.; Maheswari, R. Direct incorporation of tungsten into ultra-large-pore three-dimensional mesoporous silicate framework: W-KIT-6. J. Porous Mater. 2012, 19, 961–968. [Google Scholar] [CrossRef]
- Ramanathan, A.; Subramaniam, B.; Maheswari, R.; Hanefeld, U. Synthesis and characterization of Zirconium incorporated ultra large pore mesoporous silicate, Zr–KIT-6. Microporous Mesoporous Mater. 2013, 167, 207–212. [Google Scholar] [CrossRef]
- Ramanathan, A.; Maheswari, R.; Barich, D.H.; Subramaniam, B. Niobium incorporated mesoporous silicate, Nb-KIT-6: Synthesis and characterization. Microporous Mesoporous Mater. 2014, 190, 240–247. [Google Scholar] [CrossRef]
- Pan, Q.; Ramanathan, A.; Snavely, W.K.; Chaudhari, R.V.; Subramaniam, B. Synthesis and Dehydration Activity of Novel Lewis Acidic Ordered Mesoporous Silicate: Zr-KIT-6. Ind. Eng. Chem. Res. 2013, 52, 15481–15487. [Google Scholar] [CrossRef]
- Wu, J.-F.; Ramanathan, A.; Snavely, W.K.; Zhu, H.; Rokicki, A.; Subramaniam, B. Enhanced metathesis of ethylene and 2-butene on tungsten incorporated ordered mesoporous silicates. Appl. Catal. A Gen. 2016, 528, 142–149. [Google Scholar] [CrossRef]
- Zhu, H.; Chaudhari, R.V.; Subramaniam, B.; Ramanathan, A.; Wu, J.-F. Effects of tunable acidity and basicity of Nb-KIT-6 catalysts on ethanol conversion: Experiments and kinetic modeling. AIChE J. 2017, 63, 2888–2899. [Google Scholar] [CrossRef]
- Zhang, M.; Yu, Y. Dehydration of Ethanol to Ethylene. Ind. Eng. Chem. Res. 2013, 52, 9505–9514. [Google Scholar] [CrossRef]
- Nash, C.P.; Ramanathan, A.; Ruddy, D.A.; Behl, M.; Gjersing, E.; Griffin, M.; Zhu, H.; Subramaniam, B.; Schaidle, J.A.; Hensley, J.E. Mixed alcohol dehydration over Brønsted and Lewis acidic catalysts. Appl. Catal. A Gen. 2016, 510, 110–124. [Google Scholar] [CrossRef]
- Pan, Q.; Ramanathan, A.; Kirk Snavely, W.; Chaudhari, R.V.; Subramaniam, B. Intrinsic Kinetics of Ethanol Dehydration Over Lewis Acidic Ordered Mesoporous Silicate, Zr-KIT-6. Top. Catal. 2014, 57, 1407–1411. [Google Scholar] [CrossRef]
- Turek, W.; Haber, J.; Krowiak, A. Dehydration of isopropyl alcohol used as an indicator of the type and strength of catalyst acid centres. Appl. Surf. Sci. 2005, 252, 823–827. [Google Scholar] [CrossRef]
- Huo, H.; Peng, L.; Gan, Z.; Grey, C.P. Solid-State MAS NMR Studies of Brønsted Acid Sites in Zeolite H-Mordenite. J. Am. Chem. Soc. 2012, 134, 9708–9720. [Google Scholar] [CrossRef] [PubMed]
- Pazè, C.; Zecchina, A.; Spera, S.; Cosma, A.; Merlo, E.; Spanò, G.; Girotti, G. Comparative IR and 1H-MAS NMR study of adsorption of CD3CN on zeolite H-β: Evidence of the presence of two families of bridged Brnsted sites. Phys. Chem. Chem. Phys. 1999, 1, 2627–2629. [Google Scholar] [CrossRef]
- Phung, T.K.; Proietti Hernández, L.; Lagazzo, A.; Busca, G. Dehydration of ethanol over zeolites, silica alumina and alumina: Lewis acidity, Brønsted acidity and confinement effects. Appl. Catal. A Gen. 2015, 493, 77–89. [Google Scholar] [CrossRef]
- Phung, T.K.; Proietti Hernández, L.; Busca, G. Conversion of ethanol over transition metal oxide catalysts: Effect of tungsta addition on catalytic behaviour of titania and zirconia. Appl. Catal. A Gen. 2015, 489, 180–187. [Google Scholar] [CrossRef]
- Katryniok, B.; Paul, S.; Dumeignil, F. Recent Developments in the Field of Catalytic Dehydration of Glycerol to Acrolein. ACS Catal. 2013, 3, 1819–1834. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Wu, S.-B.; Liu, Y. Advances in the Catalytic Production and Utilization of Sorbitol. Ind. Eng. Chem. Res. 2013, 52, 11799–11815. [Google Scholar] [CrossRef]
- Lwin, S.; Wachs, I.E. Olefin Metathesis by Supported Metal Oxide Catalysts. ACS Catal. 2014, 4, 2505–2520. [Google Scholar] [CrossRef]
- Mol, J. Industrial applications of olefin metathesis. J. Mol. Catal. Chem. 2004, 213, 39–45. [Google Scholar] [CrossRef]
- Maksasithorn, S.; Praserthdam, P.; Suriye, K.; Debecker, D.P. Preparation of super-microporous WO3–SiO2 olefin metathesis catalysts by the aerosol-assisted sol–gel process. Microporous Mesoporous Mater. 2015, 213, 125–133. [Google Scholar] [CrossRef]
- Debecker, D.P.; Stoyanova, M.; Rodemerck, U.; Colbeau-Justin, F.; Boissère, C.; Chaumonnot, A.; Bonduelle, A.; Sanchez, C. Aerosol route to nanostructured WO3-SiO2-Al2O3 metathesis catalysts: Toward higher propene yield. Appl. Catal. A Gen. 2014, 470, 458–466. [Google Scholar] [CrossRef]
- Liu, H.; Tao, K.; Zhang, P.; Xu, W.; Zhou, S. Enhanced catalytic performance for metathesis reactions over ordered tungsten and aluminum co-doped mesoporous KIT-6 catalysts. New J. Chem. 2015, 39, 7971–7978. [Google Scholar] [CrossRef]
- Howell, J.G.; Li, Y.-P.; Bell, A.T. Propene Metathesis over Supported Tungsten Oxide Catalysts: A Study of Active Site Formation. ACS Catal. 2016, 6, 7728–7738. [Google Scholar] [CrossRef]
- Lwin, S.; Li, Y.; Frenkel, A.I.; Wachs, I.E. Nature of WOx Sites on SiO2 and Their Molecular Structure–Reactivity/Selectivity Relationships for Propylene Metathesis. ACS Catal. 2016, 6, 3061–3071. [Google Scholar] [CrossRef]
- Maksasithorn, S.; Debecker, D.P.; Praserthdam, P.; Panpranot, J.; Suriye, K.; Ayudhya, S.K.N. NaOH modified WO3/SiO2 catalysts for propylene production from 2-butene and ethylene metathesis. Chin. J. Catal. 2014, 35, 232–241. [Google Scholar] [CrossRef]
- Ouyang, X.; Hwang, S.-J.; Xie, D.; Rea, T.; Zones, S.I.; Katz, A. Heteroatom-Substituted Delaminated Zeolites as Solid Lewis Acid Catalysts. ACS Catal. 2015, 5, 3108–3119. [Google Scholar] [CrossRef]
- Gallo, A.; Tiozzo, C.; Psaro, R.; Carniato, F.; Guidotti, M. Niobium metallocenes deposited onto mesoporous silica via dry impregnation as catalysts for selective epoxidation of alkenes. J. Catal. 2013, 298, 77–83. [Google Scholar] [CrossRef]
- Tiozzo, C.; Palumbo, C.; Psaro, R.; Bisio, C.; Carniato, F.; Gervasini, A.; Carniti, P.; Guidotti, M. The stability of niobium-silica catalysts in repeated liquid-phase epoxidation tests: A comparative evaluation of in-framework and grafted mixed oxides. Inorg. Chim. Acta 2015, 431, 190–196. [Google Scholar] [CrossRef]
- Ivanchikova, I.D.; Maksimchuk, N.V.; Skobelev, I.Y.; Kaichev, V.V.; Kholdeeva, O.A. Mesoporous niobium-silicates prepared by evaporation-induced self-assembly as catalysts for selective oxidations with aqueous H2O2. J. Catal. 2015, 332, 138–148. [Google Scholar] [CrossRef]
- Tiozzo, C.; Bisio, C.; Carniato, F.; Guidotti, M. Grafted non-ordered niobium-silica materials: Versatile catalysts for the selective epoxidation of various unsaturated fine chemicals. Catal. Today 2014, 235, 49–57. [Google Scholar] [CrossRef]
- Buffum, J.E.; Kowaleski, R.M.; Gerdes, W.H. Ethylene Oxide Catalyst. U.S. Patent 5,145,824, 8 September 1992. [Google Scholar]
- Yan, W.; Ramanathan, A.; Ghanta, M.; Subramaniam, B. Towards highly selective ethylene epoxidation catalysts using hydrogen peroxide and tungsten- or niobium-incorporated mesoporous silicate (KIT-6). Catal. Sci. Technol. 2014, 4, 4433–4439. [Google Scholar] [CrossRef]
- Morey, M.S.; Bryan, J.D.; Schwarz, S.; Stucky, G.D. Pore Surface Functionalization of MCM-48 Mesoporous Silica with Tungsten and Molybdenum Metal Centers: Perspectives on Catalytic Peroxide Activation. Chem. Mater. 2000, 12, 3435–3444. [Google Scholar] [CrossRef]
- Coelho, J.V.; Oliveira, L.C.A.; Moura, F.C.C.; de Souza, P.P.; Silva, C.A.; Batista, K.B.; da Silva, M.J. β-pinene oxidation by hydrogen peroxide catalyzed by modified niobium-MCM. Appl. Catal. A Gen. 2012, 419–420, 215–220. [Google Scholar] [CrossRef]
- Lee, H.-J.; Ghanta, M.; Busch, D.H.; Subramaniam, B. Toward a CO2-free ethylene oxide process: Homogeneous ethylene oxide in gas-expanded liquids. Chem. Eng. Sci. 2010, 65, 128–134. [Google Scholar] [CrossRef]
- Oyama, S.T. Rates, Kinetics, and Mechanisms of Epoxidation. In Mechanisms in Homogeneous and Heterogeneous Epoxidation Catalysis; Elsevier: Amsterdam, The Netherlands, 2008; pp. 3–99. ISBN 978-0-444-53188-9. [Google Scholar]
- Yan, W.; Ramanathan, A.; Patel, P.D.; Maiti, S.K.; Laird, B.B.; Thompson, W.H.; Subramaniam, B. Mechanistic insights for enhancing activity and stability of Nb-incorporated silicates for selective ethylene epoxidation. J. Catal. 2016, 336, 75–84. [Google Scholar] [CrossRef]
- Maiti, S.K.; Ramanathan, A.; Thompson, W.H.; Subramaniam, B. Strategies to Passivate Brønsted Acidity in Nb-TUD-1 Enhance Hydrogen Peroxide Utilization and Reduce Metal Leaching during Ethylene Epoxidation. Ind. Eng. Chem. Res. 2017, 56, 1999–2007. [Google Scholar] [CrossRef]
- Bruijnincx, P.C.A.; Rinaldi, R.; Weckhuysen, B.M. Unlocking the potential of a sleeping giant: Lignins as sustainable raw materials for renewable fuels, chemicals and materials. Green Chem. 2015, 17, 4860–4861. [Google Scholar] [CrossRef]
- Final Renewable Fuel Standards for 2014, 2015 and 2016, and the Biomass-Based Diesel Volume for 2017. Available online: https://www.epa.gov/renewable-fuel-standard-program/final-renewable-fuel-standards-2014-2015-and-2016-and-biomass-based (accessed on 30 November 2017).
- Xu, C.; Arancon, R.A.D.; Labidi, J.; Luque, R. Lignin depolymerisation strategies: Towards valuable chemicals and fuels. Chem. Soc. Rev. 2014, 43, 7485–7500. [Google Scholar] [CrossRef] [PubMed]
- Deepa, A.K.; Dhepe, P.L. Lignin Depolymerization into Aromatic Monomers over Solid Acid Catalysts. ACS Catal. 2015, 5, 365–379. [Google Scholar] [CrossRef]
- Wu, J.-F.; Ramanathan, A.; Subramaniam, B. Novel tungsten-incorporated mesoporous silicates synthesized via evaporation-induced self-assembly: Enhanced metathesis performance. J. Catal. 2017, 350, 182–188. [Google Scholar] [CrossRef]
- Ramanathan, A.; Zhu, H.; Maheswari, R.; Subramaniam, B. Remarkable epoxidation activity of neat and carbonized niobium silicates prepared by evaporation-induced self-assembly. Microporous Mesoporous Mater. 2018, 261, 158–163. [Google Scholar] [CrossRef]
Sample Availability: Not available. |



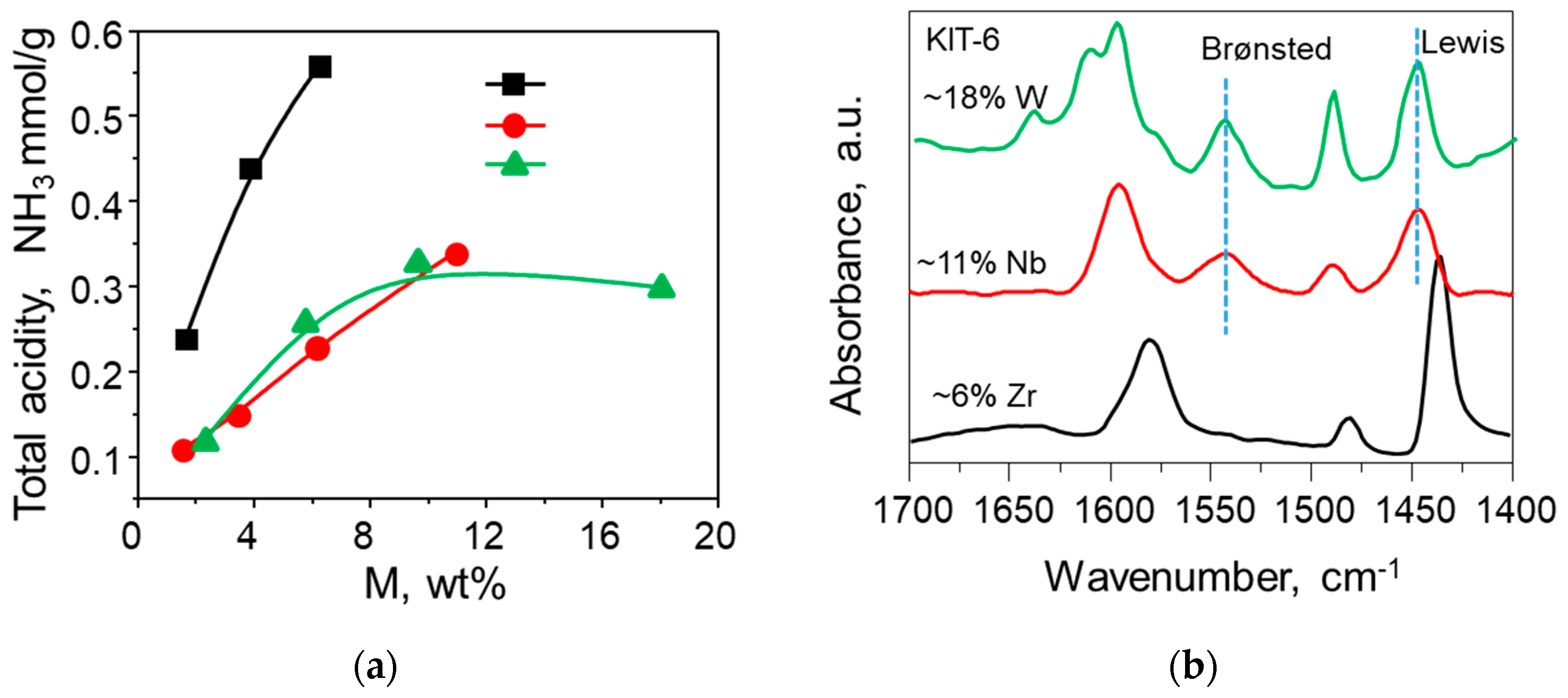

| KIT-6 (Si/M) a | Si/M b | M wt % b | SBET c m²/g | Vtp d cm3/g | dP, BJH e nm | Total Acidity g mmol NH3/g |
|---|---|---|---|---|---|---|
| Si-KIT-6 | - | - | 1013 | 1.38 | 9.3 | 0.04 |
| Zr-KIT-6(100) | 92 | 1.6 | 980 | 1.65 | 9.3 | 0.19 |
| Zr-KIT-6(40) | 39 | 3.8 | 881 | 1.42 | 9.3 | 0.40 |
| Zr-KIT-6(20) | 23 | 6.2 | 810 | 1.07 | 9.3 | 0.49 |
| Nb-KIT-6(100) | 98 | 1.5 | 997 | 1.46 | 9.3 | 0.11 |
| Nb-KIT-6(40) | 41 | 3.4 | 991 | 1.29 | 9.3 | 01.5 |
| Nb-KIT-6(20) | 21 | 6.1 | 926 | 1.28 | 9.3 | 0.23 |
| Nb-KIT-6(10) | 9.8 | 10.9 | 804 | 1.12 | 9.3 | 0.34 |
| W-KIT-6(100) | 104 | 2.9 | 880 | 1.03 | 9.3 | 0.13 |
| W-KIT-6(40) | 51 | 5.6 | 764 | 0.81 | 9.3 | 0.26 |
| W-KIT-6(20) | 29 | 9.5 | 661 | 0.69 | 9.3 | 0.33 |
| W-KIT-6(10) | 14 | 18.0 | 536 | 0.60 | 8.1 | 0.33 |
| Catalyst | W-KIT-6 (8.7) | W-KIT-6 (2 h, 9.2) |
|---|---|---|
| W (at %) 1 | 2.74 | 3.86 |
| Si (at %) 2 | 97.3 | 96.1 |
| W/Si Atomic Ratio 3 | 0.0282 | 0.0402 |
| W/Si Atomic Ratio from ICP | 0.03204 | 0.03421 |
| W/Si increment from XPS, % | - | 42.6 |
| W/Si increment from ICP, % | - | 6.8 |
| Apparent TOF (mmolpropene mol w−1 s−1) | 3.58 | 4.69 |
| Propylene yield, % | 42.9 | 59.2 |
| Catalyst | M wt% | PEO a (±3%) | SEO % b (±3%) | XH2O2 % c (±3%) | UH2O2 % d (±3%) | Leaching (±5%) |
|---|---|---|---|---|---|---|
| W-KIT-6 | 17.9 | 34.4 | 81.4 | 10.2 | 3.6 | 74.1–100 |
| 9.4 | 43.4 | 80.0 | 6.4 | 3.9 | ||
| 5.7 | 66.5 | 84.0 | 6.0 | 3.5 | ||
| 2.2 | 152.6 | 80.0 | 4.2 | 5.0 | ||
| Nb-KIT-6 | 13.4 | 234 | 46.8 | 17.1 | 18.8 | 33.7 |
| 7.2 | 340 | 52.7 | 17.1 | 13.1 | 32.4 | |
| 3.7 | 513 | 62.6 | 17.5 | 8.4 | 61.6 | |
| 1.5 | 794 | 73.4 | 11.2 | 7.1 | 72.4 | |
| Nb-TUD-1 | 4.0 | 1186 | 89.1 | 15.7 | 8.6 | 52.5 |
| 1.4 | 2539 | 87.8 | 12.8 | 9.1 | 60.8 | |
| 0.88 | 4304 | 91.7 | 5.8 | 20.6 | 62.2 | |
| Benzylated Nb-TUD-1 | 597 | 98.7 | 0.65 | 59.7 | 3.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramanathan, A.; Subramaniam, B. Metal-Incorporated Mesoporous Silicates: Tunable Catalytic Properties and Applications. Molecules 2018, 23, 263. https://doi.org/10.3390/molecules23020263
Ramanathan A, Subramaniam B. Metal-Incorporated Mesoporous Silicates: Tunable Catalytic Properties and Applications. Molecules. 2018; 23(2):263. https://doi.org/10.3390/molecules23020263
Chicago/Turabian StyleRamanathan, Anand, and Bala Subramaniam. 2018. "Metal-Incorporated Mesoporous Silicates: Tunable Catalytic Properties and Applications" Molecules 23, no. 2: 263. https://doi.org/10.3390/molecules23020263
APA StyleRamanathan, A., & Subramaniam, B. (2018). Metal-Incorporated Mesoporous Silicates: Tunable Catalytic Properties and Applications. Molecules, 23(2), 263. https://doi.org/10.3390/molecules23020263





