Mitochondria: Central Organelles for Melatonin?s Antioxidant and Anti-Aging Actions
Abstract
:1. Introduction
2. Melatonin Origin and Distribution
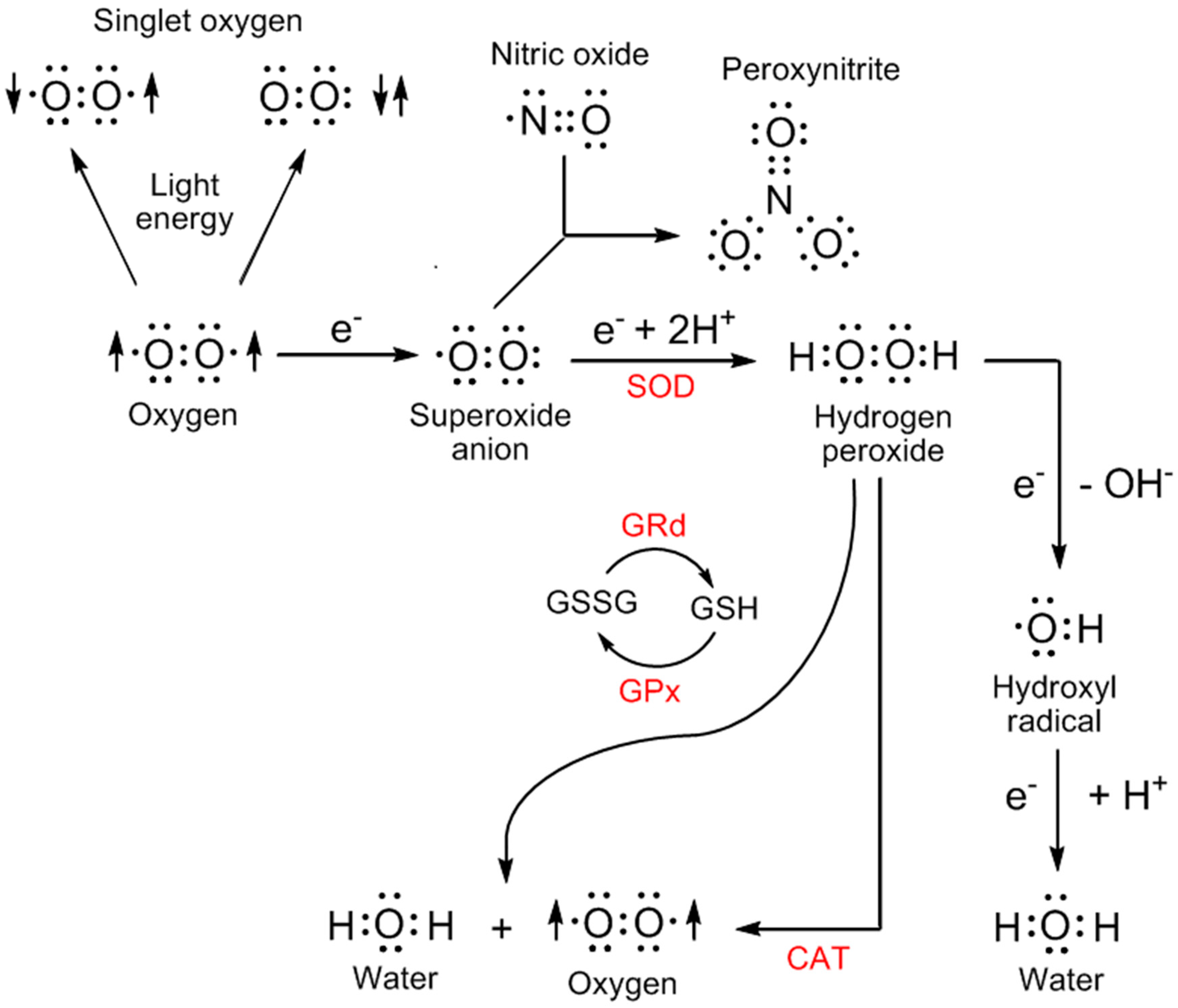
3. Sites of Reactive Oxygen Species Generation
4. Melatonin and Its Metabolites: Determinants of Oxidative Stress
5. Melatonin in Mitochondria: A Fortuitous Association
6. Melatonin, Oxidative Stress, and Aging
7. Concluding Remarks
Acknowledgments
Conflicts of Interest
References
- Halliwell, B. Biochemistry of Oxidative Stress. Biochem. Soc. Trans. 2007, 35, 1147–1150. [Google Scholar] [CrossRef] [PubMed]
- Dasuri, K.; Zhang, L.; Keller, J.N. Oxidative Stress, Neurodegeneration and the Balance of Protein Degradation of Protein Synthesis. Free Radic. Biol. Med. 2013, 62, 170–185. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Reactive Oxygen Species in Living Systems: Source, Biochemistry and Role in Human Disease. Am. J. Med. 1991, 91, 145–225. [Google Scholar] [CrossRef]
- Yang, M.L.; Doyle, H.; Clarke, S.G.; Herold, K.; Mamula, M. Oxidative Modification in Tissue Pathology and Autoimmune Diseases. Antioxid. Redox. Signal. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Agmon, E.; Stockwell, B.R. Lipid Homeostasis and Regulated Cell Death. Curr. Opin. Chem. Biol. 2017, 39, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.D.; Huang, B.W.; Tsaji, Y. Reactive Oxygen Species (ROS) Homeostasis and Redox Regulation in Cellular Signalling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Weidings, A.; Kozlov, A.V. Biological Activities of Reactive Oxygen and Reactive Nitrogen Species: Oxidative Stress versus Signal Transduction. Biomolecules 2015, 5, 472–484. [Google Scholar] [CrossRef] [PubMed]
- D’Autreaux, M.B.; Toledano, M.B. ROS as Signalling Molecules: Mechanisms that Generate Specificity in ROS Homeostasis. Nat. Rev. Mol. Cell. Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, J.; Alexandre, J.; Huang, P. Targeting Cancer Cells by ROS-Mediated Mechanisms: A Radical Therapeutic Approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Hydrogen Peroxide as a Central Redox Signaling Molecule in Physiological Oxidative Stress: Oxidative Eustress. Redox Biol. 2017, 11, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Manchester, L.C.; Poeggeler, B.; Alvares, F.L.; Ogden, G.B.; Reiter, R.J. Melatonin Immunoreactivity in the Photosynthetic Prokaryote Rhodospirillum rubrum: Implications for an Ancient Antioxidant System. Cell. Mol. Biol. Res. 1995, 41, 391–395. [Google Scholar] [PubMed]
- Ma, Y.; Jiao, J.; Fan, X.; Sun, H.; Zhang, Y.; Jiang, J.; Liu, C. Endophytic Bacterium (Pseudomonas fluorescens RG11) May Transform Tryptophan to Melatonin and Promote Endogenous Melatonin Levels in the Roots of Four Grape Cultivars. Front. Plant Sci. 2016, 7, 2068. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Hardeland, R.; Manchester, L.C.; Paredes, S.D.; Korkmaz, A.; Sainz, R.M.; Mayo, J.C.; Fuentes-Broto, L.; Reiter, R.L. The Changing Biological Roles of Melatonin during Evolution: From an Antioxidant to Signals of Darkness, Sexual Selection and Fitness. Biol. Rev. Camb. Philos. Soc. 2010, 85, 607–623. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Galano, A. Melatonin: Exceeding Expectations. Physiology 2014, 29, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Manchester, L.C.; Coto-Montes, A.; Boga, J.A.; Andersen, L.P.; Zhou, Z.; Galano, A.; Vriend, J.; Tan, D.X.; Reiter, R.J. Melatonin: An Ancient Molecule that Makes Oxygen Metabolically Tolerable. J. Pineal Res. 2015, 59, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Fraschini, F. Endocrine Aspects of the Mammalian Pineal Gland. Neuroendocrinology 1969, 5, 219–255. [Google Scholar] [CrossRef] [PubMed]
- Su, S.C.; Hsieh, M.J.; Yang, M.E.; Chung, W.H.; Reiter, R.J.; Yang, S.F. Cancer Metastases: Mechanisms of Inhibition by Melatonin. J. Pineal Res. 2017, 62, e12370. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Fang, Y.; Lu, Y.; Tan, D.; Du, C.; Li, Y.; Ma, Q.; Yu, J.; Chen, M.; Zhou, C.; et al. Melatonin Alleviates Cadmium-induced Liver Injury by Inhibiting the TXNIP-NLRP3 Inflammasome. J. Pineal Res. 2017, 62, e12389. [Google Scholar] [CrossRef] [PubMed]
- Dubbels, R.; Reiter, R.J.; Klemke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.W.; Schloat, W. Melatonin in Edible Plants Identified by Radioimmunoassay and High Performance Liquid Chromatography-Mass Spectrometry. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Hattori, A.; Migitaka, H.; Iigo, M.; Ito, M.; Yamamoto, K.; Ohtani-Kandro, G.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of Melatonin in Plants and Its Effects on Plasma Melatonin Levels and Binding to Melatonin Receptor in Vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar] [PubMed]
- Arnao, M.B.; Hernandez-Ruiz, J. Functions of Melatonin in Plants. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Erland, L.A.; Murch, S.J.; Reiter, R.J.; Saxena, D.K. A New Balancing Act: The Many Roles of Melatonin and Serotonin in Plant Growth and Development. Plant Signal. Behav. 2015, 10, e1096469. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Burkhardt, S.; Manchester, L.C. Melatonin in Plants. Nutr. Rev. 2001, 59, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Back, K. Melatonin is Required for H2O2 and NO-mediated Defense Signaling through MAPKKK3 and OXI1 in Arabidopis thaliana. J. Pineal Res. 2017, 62, e12379. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Hu, W.; Wang, Q.; Zeng, H.; Li, X.; Yan, Y.; Reiter, R.J.; He, C.; Shi, H. Identification, Transcriptional and Functional Analysis of Heat-Shock Protein 90s in Banana (Musa acuminata L.) Highlight Their Novel Role in Melatonin-Mediated Plant Response to Fusarium Wilt. J. Pineal Res. 2017, 62, e12367. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Zhou, Z.; Cruz, M.H.; Fuentes-Broto, L.; Galano, A. Phytomelatonin: Assisting Plants to Survive and Thrive. Molecules 2015, 20, 7396–7437. [Google Scholar] [CrossRef] [PubMed]
- Fridovich, I. Oxygen Toxicity: A Radical Explanation. J. Exp. Biol. 1998, 201, 1203–1209. [Google Scholar] [PubMed]
- Venegas, C.; Garcia, J.A.; Escames, G.; Ortiz, F.; Lopez, A.; Doerrier, C.; Garcia-Corzo, L.; Lopez, L.C.; Reiter, R.J.; Acuna-Castroviejo, D. Extrapineal Melatonin: Analysis of Its Subcellular Distribution and Daily Fluctuations. J. Pineal Res. 2012, 52, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Van der Bliek, A.M.; Sedensky, M.M.; Morgan, P.G. Cell Biology of the Mitochondrion. Genetics 2017, 207, 843–871. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an Antioxidant: Under Promises but Over Delivers. J. Pineal Res. 2016, 61, 259–278. [Google Scholar] [CrossRef] [PubMed]
- Skinner, D.C.; Malpaux, B. High Melatonin Concentrations in the Third Ventricular Cerebrospinal Fluid are not Due to Galen Vein Blood Recirculating through the Choroid Plexus. Endocrinology 1999, 140, 4399–4405. [Google Scholar] [CrossRef] [PubMed]
- Brzezinski, A.; Seibel, M.M.; Lynch, H.J.; Deng, M.H.; Wurtman, R.J. Melatonin in Human Preovulatory Follicular Fluid. J. Clin. Endocrinol. Metab. 1987, 64, 865–867. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Manchester, L.C.; Reiter, R.J.; Qi, W.; Hanes, M.A.; Farley, N.J. High Physiological Levels of Melatonin in the Bile of Mammals. Life Sci. 1999, 65, 2523–2529. [Google Scholar] [CrossRef]
- Stehle, J.H.; von Gall, C.; Korf, H.W. Melatonin: A Clock-output, A Clock-input. J. Neuroendocrinol. 2003, 15, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, G.M.; Pelham, R.W.; Pang, S.F.; Loughlin, L.L.; Wilson, K.M.; Sandock, K.L.; Vaughan, M.K.; Koslow, S.H.; Reiter, R.J. Nocturnal Elevation of Plasma Melatonin and Urinary 5-Hydroxyindoleacetic Acid in Young Men: Attempts at Modification by Brief Changes in Environmental Lighting and Sleep and by Autonomic Drugs. J. Clin. Endocrinol. Metab. 1976, 42, 752–764. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, B.; Ravault, J.P.; Ferrandi, B.; Reiter, R.J. Melatonin Concentration in the Cerebral Vascular Sinuses of Sheep and Evidence for Its Episodic Release. J. Pineal Res. 1988, 5, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, A.; Petren, S.; Plannthin, J.; Eklundh, T.; Nordin, C. Serum and Cerebrospinal Fluid Concentrations of Melatonin: A Pilot Study in Healthy Male Volunteers. J. Neurol Transm. 1999, 106, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Nakamura, Y.; Korkmaz, A.; Manchester, L.C.; Tan, D.X.; Sugino, N.; Reiter, R.J. Melatonin and the Ovary: Physiological and Pathophysiological Implications. Fertil. Steril. 2009, 92, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Manchester, L.C.; Tan, D.X. Melatonin in Walnuts: Influence on Levels of Melatonin and Total Antioxidant Capacity of Blood. Nutrition 2005, 21, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Troiani, M.E.; Reiter, R.J.; Tannenbaum, M.G.; Puig-Domingo, M.; Guerrero, J.M.; Menendez-Pelaez, A. Neither the Pituitary Gland nor the Sympathetic Nervous System is Responsible for Eliciting the Large Drop in Elevated Rat Pineal Melatonin Levels due to Swimming. J. Neural Transm. 1980, 74, 149–160. [Google Scholar] [CrossRef]
- Dauchy, R.T.; Wren-Dail, M.A.; Hoffman, A.E.; Hanifin, J.P.; Warfield, B.; Brainard, G.C.; Hill, S.M.; Belancio, V.P.; Dauchy, E.M.; Blask, D.E. Effects of Daytime Exposure to Light from Blue-Enriched Light-Emitting Diodes on the Nighttime Melatonin Amplitude and Circadian Regulation of Rodent Metabolism and Physiology. Comp. Med. 2016, 66, 373–383. [Google Scholar] [PubMed]
- Arendt, J. Melatonin. Clin. Endocrinol. 1988, 29, 205–229. [Google Scholar] [CrossRef]
- Tamura, H.; Takasaki, A.; Taketani, M.; Tanabe, M.; Lee, L.; Tamura, I.; Maekawa, R.; Aasada, H.; Yamagata, Y.; Sugino, N. Melatonin and Female Reproduction. J. Obstet. Gynaecol. Res. 2014, 40, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Milan-Tomas, A.; Shapiro, C.M. Circadian Rhythm Disturbances in Alzheimer Disease: Current Concepts, Diagnosis, and Management. Alzheimer Dis. Assoc. Disord. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Brainard, G.C.; Barker, P.M.; Hoffman, R.J.; Stetson, M.H.; Hanifin, J.P.; Podolin, P.L.; Rollag, M.D. Ultraviolet Regulation of Neuroendocrine and Circadian Physiology of Rodents. Vis. Res. 1994, 34, 1521–1533. [Google Scholar] [CrossRef]
- Tan, D.X.; Chen, L.D.; Poeggeler, B.; Manchester, L.C.; Reiter, R.J. Melatonin: A Potent Endogenous Hydroxyl Radical Scavenger. Endocrine 1993, 1, 57–60. [Google Scholar]
- Galano, A.; Tan, D.X.; Reiter, R.J. Melatonin as a Natural Ally against Oxidative Stress: A Physiochemical Examination. J. Pineal Res. 2011, 51, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Boutin, J.A. Quinone Reductase 2 as a Promising Target of Melatonin Therapeutic Actions. Expert Opin. Ther. Targets 2016, 20, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Koppisepi, S. Medical Implications of Melatonin: Receptor-Mediated and Receptor-Independent Actions. Adv. Med. Sci. 2007, 52, 11–28. [Google Scholar] [PubMed]
- Barberino, R.S.; Menezes, V.G.; Ribeiro, A.E.A.S.; Palheta, R.C., Jr.; Jiang, X.; Smitz, J.E.J.; Matos, M.H.T. Melatonin Protects against Cisplatin-induced Ovarian Damage in Mice Via the MT1 Receptor and Antioxidant Activity. Biol. Reprod. 2017, 96, 1244–1255. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.L.; Sun, T.C.; Yu, K.; Wang, Z.P.; Zhang, B.L.; Zhang, Y.; Wang, X.X.; Lian, Z.X.; Liu, Y.X. Melatonin Reduces Oxidative Damage and Upregulates Heat Shock Protein 80 Expression in Cryopreserved Human Semen. Free Radic. Biol. Med. 2017, 113, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Stankov, B.; Reiter, R.J. Melatonin Receptors: Current Status, Facts and Hypotheses. Life Sci. 1990, 46, 971–982. [Google Scholar] [CrossRef]
- Dubocovich, M.L.; Delagrange, P.; Krause, D.N.; Sudgen, D.; Cardinali, D.P.; Olcese, J. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, Classification, and Pharmacology of G Protein-coupled Melatonin Receptors. Pharmacol. Rev. 2010, 62, 343–380. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sirianni, A.; Pei, Z.; Cormier, K.; Smith, K.; Jiang, J.; Zhou, S.; Wang, H.; Zhao, R.; Yano, H.; et al. The Melatonin MT1 Receptor Axis Modulates Mutant Huntingtin-mediated Toxicity. J. Neurosci. 2011, 31, 14496–15507. [Google Scholar] [CrossRef] [PubMed]
- Urata, Y.; Honma, S.; Goto, S.; Todoroki, S.; Iida, T.; Cho, S.; Honma, K.; Kondo, T. Melatonin Induces Gamma-glutamylcysteine Synthetase Mediated by activator Protein-1 in Human Vascular Endothelial Cells. Free Radic. Bio. Med. 1999, 27, 838–847. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Naaz, S.; Bhattacharjee, B.; Ghosal, N.; Chattopadhyay, A.; Roy, S.; Reiter, R.J.; Bandyopadhyay, D. Mechanism of Melatonin Protection against Copper-ascorbate-induced Oxidative Damage in Vitro through Isothermal Titration Calorimetry. Life Sci. 2017, 180, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Qin, L.; Reiter, R.J. Melatonin: A Mitochondrial Targeting Molecule Involving Mitochondrial Protection and Dynamics. Int. J. Mol. Sci. 2016, 17, 2124. [Google Scholar] [CrossRef] [PubMed]
- Back, K.; Tan, D.X.; Reiter, R.J. Melatonin Biosynthesis in Plants: Multiple Pathways Catalyze Tryptophan to Melatonin in Cytoplasm or Chloroplasts. J. Pineal Res. 2016, 61, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Tan, D.X.; Allan, A.C.; Zuo, B.; Zhao, Y.; Reiter, R.J.; Wang, L.; Wang, Z.; Guo, Y.; Zhou, J.; et al. Chloroplastic Biosynthesis of Melatonin and Its Involvement in Protection of Plants from Salt Stress. Sci. Rep. 2017, 7, 41236. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Hardeland, R.; Manchester, L.C.; Korkmaz, A.; Ma, S.; Rosales-Corral, S.; Reiter, R.J. Functional Roles of Melatonin in Plants, and Perspectives in Nutritional and Agricultural Science. J. Exp. Bot. 2012, 63, 577–597. [Google Scholar] [CrossRef] [PubMed]
- Champney, T.H.; Holtorf, A.P.; Steger, R.W.; Reiter, R.J. Concurrent Determination of Enzymatic Activities and Substrate Concentrations in the Melatonin Synthetic Pathway within the Same Rat Pineal Gland. J. Neurosci. Res. 1984, 11, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Stehle, J.H.; Saade, A.; Rawashdeh, O.; Ackermann, K.; Jilg, A.; Sebesteny, T.; Maronde, E. A Survey of the Molecular Details in the Human Pineal Gland in the Light of Phylogeny, Structure, Function and Chronobiological Diseases. J. Pineal Res. 2011, 51, 17–43. [Google Scholar] [CrossRef] [PubMed]
- Pablos, M.I.; Reiter, R.J.; Ortiz, G.G.; Guerrero, J.M.; Agapito, M.T.; Chuang, J.I.; Sewerynek, E. Rhythms of Glutathione Peroxidase and Glutathione, Reductase in Brain of Chick and Their Inhibition by Light. Neurochem. Int. 1998, 32, 69–75. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Manchester, L.C. The Universal Nature, Unequal Distribution and Antioxidant Functions of Melatonin and Its Derivatives. Mini-Rev. Med. Chem. 2013, 13, 373–384. [Google Scholar] [PubMed]
- Hardeland, R.; Fuhrberg, B.; Uria, H.; Behrmann, G.; Meyer, T.J.; Burkhardt, S.; Poeggeler, B. Chronobiology of Indoleamines in the Dinoflagellate Gonyaulax polyedra: Metabolism and Effects Related to Circadian Rhythmicity and Photoperiodism. Braz. J. Med. Biol. Res. 1996, 29, 119–123. [Google Scholar] [PubMed]
- Reczek, CR.; Chandel, N.S. ROS-dependent Signal Transduction. Curr. Opin. Cell Biol. 2014, 33C, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Crow, J.P.; Spruel, C.; Chen, J.; Gunn, C.; Ischiropaulos, H.; Tsai, M.; Smith, C.D.; Koppenol, W.H.; Bechman, J.S. On the pH-dependent Yield of Hydroxyl Radical Products from Peroxynitrite. Free Radic. Biol. Med. 1994, 10, 331–338. [Google Scholar] [CrossRef]
- Hardeland, R. Neuroprotection by Radical Avoidance. Molecules 2009, 14, 5054–5102. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, A.; Reiter, R.J.; Topal, T.; Manchester, L.C.; Oter, S.; Tan, D.X. Melatonin: An Established Antioxidant Worthy of Its Use in Clinical Trials. Mol. Med. 2009, 15, 43–50. [Google Scholar] [PubMed]
- Brazao, V.; Santello, F.H.; Colato, R.P.; Mazotti, T.T.; Tazinafo, L.F.; Toldo, M.P.A.; do Vale, G.T.; Tirapelli, C.R.; do Prado, J.C., Jr. Melatonin: Antioxidant and Modulatory Properties in Age-related Changes during Trypanosoma cruzi Infection. J. Pineal Res. 2017, 63, e12409. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Rosales-Corral, S.; Tan, D.X.; Jou, M.J.; Galano, A.; Xu, B. Melatonin as a Mitochondria-targeted Antioxidant: One of Evolution’s Best Ideas. Cell. Mol. Life Sci. 2017, 74, 3863–3881. [Google Scholar] [CrossRef] [PubMed]
- Barlow-Walden, L.R.; Reiter, R.J.; Abe, M.; Pablos, M.I.; Menendez-Pelaez, A.; Chen, L.R.; Poeggeler, B. Melatonin Stimulates Brain Glutathione Peroxidase Activity. Neurochem. Int. 1995, 26, 497–502. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Osuna, C.; Gitto, E. Actions of Melatonin in the Reduction of Oxidative Stress. J. Biomed. Sci. 2000, 7, 444–458. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Zhang, T.; Zhang, P.; Wang, Z.Y. Melatonin Attenuates Postharvest Physiological Deterioration of Cassava Storage Roots. J. Pineal Res. 2016, 60, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.H.; Cho, E.H.; Kim, M.O.; Koh, P.O. Identification of Protein Differentially Expressed by Melatonin Treatment in Cerebral Ischemic Injury—A Proteomics Approach. J. Pineal Res. 2009, 46, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Mendez, I.; Vazquez-Martinez, O.; Hernandez-Munoz, R.; Valente-Godinez, H.; Diaz-Munoz, M. Redox Regulation and Pro-oxidant Reactions in the Physiology of Circadian Systems. Biochimie 2015, 124, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Vriend, J.; Reiter, R.J. Melatonin, Bone Regulation and the Ubiquitin-Proteasome Connection: A Review. Life Sci. 2016, 145, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Janjetovic, Z.; Jarrett, S.G.; Lee, E.F.; Duprey, C.; Reiter, R.J.; Slominski, A. Melatonin and Its Metabolites Protect Human Melanocytes against UVB-induced Damage: Involvement of Nrf2-mediated Pathways. Sci. Rep. 2017, 7, 1274. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D. The Sites and Topology of Mitochondrial Superoxide Production. Exp. Gerontol. 2010, 45, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.S.; D’Imprima, E.; Vonck, J. Mitochondrial Respiratory Chain Complexes. Subcell. Biochem. 2018, 87, 167–227. [Google Scholar] [PubMed]
- Muller, F.L.; Liu, Y.; Van Rammen, H. Complex III Releases Superoxide to Both Sides of the Inner Mitochondrial Membrane. J. Biol. Chem. 2004, 279, 49064–49073. [Google Scholar] [CrossRef] [PubMed]
- Seva, L.A.; Chandel, N.S. Physiological Roles of Mitochondrial Reactive Oxygen Species. Mol. Cell. 2012, 48, 158–167. [Google Scholar]
- Kalous, M.; Drahota, Z. The Role of Mitochondria in Aging. Physiol. Res. 1996, 45, 351–359. [Google Scholar] [PubMed]
- Kauppila, T.E.S.; Kauppila, J.H.K.; Larsson, N.G. Mammalian Mitochondria and Aging: An Update. Cell. Metab. 2017, 25, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Corral, S.A.; Acuna-Castroviejo, D.; Coto-Montes, A.; Boga, J.A.; Manchester, L.C.; Fuentes-Broto, L.; Korkmaz, A.; Ma, S.; Tan, D.X.; Reiter, R.J. Alzheimer’s Disease: Pathophysiological Mechanisms and the Beneficial Role of Melatonin. J. Pineal Res. 2012, 52, 167–202. [Google Scholar] [CrossRef] [PubMed]
- Coto-Montes, A.; Boga, J.A.; Tan, D.X.; Reiter, R.J. Melatonin as a Potential Agent in the Treatment of Sacropenia. In, J. Mol. Sci. 2016, 17, E1771. [Google Scholar] [CrossRef] [PubMed]
- Acuna-Castroviejo, D.; Rahim, I.; Acuna-Fernandez, C.; Fernandez-Ortiz, M.; Solera-Marin, J.; Sayed, R.K.A.; Diaz-Casado, M.E.; Dusanova, I.; Lopez, L.C.; Escames, G. Melatonin, Clock Genes and Mitochondria in Sepsis. Cell. Mol. Life Sci. 2017, 74, 3965–3988. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, D.P.; Vigo, D.E. Melatonin, Mitochondria and Metabolic Syndrome. Cell. Mol. Life Sci. 2017, 74, 3941–3954. [Google Scholar] [CrossRef] [PubMed]
- Kanaan, G.N.; Harper, M.E. Cellular Redox Dysfunction in the Development of Cardiovascular Diseases. Biochem. Biophys. Acta 2017, 1861, 2822–2829. [Google Scholar] [CrossRef] [PubMed]
- Drummond, G.R.; Selemidis, S.; Griendling, K.K.; Sobey, C.G. Combatting Oxidative Stress in Vascular Disease: NADPH Oxidases as Therapeutic Targets. Nat. Rev. Drug Discov. 2011, 10, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Krause, K.H. Aging: A Revisited Theory Based on Free Radicals Generated by NOX Family NADPH Oxidases. Exp. Gerontol. 2007, 42, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Vida, C.; Corpas, I.; De la Fuente, M.; Gonzalez, E.M. Age-related Changes in Xanthine Oxidase Activity and Lipid Peroxidation as well as in the Correlation between Both Parameters, in Plasma and Several Organs from Female Mice. J. Physiol. Biochem. 2011, 67, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Alper, G.; Girgin, F.K.; Ozgonul, M.; Mentes, G.; Erroz, B. MAO Inhibitors and Oxidative Stress in Aging Brain Tissue. Eur. Neuropsychopharmacol. 1999, 9, 247–252. [Google Scholar] [CrossRef]
- Naskar, A.; Prabhakar, V.; Singh, R.; Dutta, D.; Mohanakumar, K.P. Melatonin Enhances L-DOPA Therapeutic Effects, Helps to Reduce Its Dose, and Protects Dopaminergic Neurons in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinsonism in Mice. J. Pineal Res. 2015, 58, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Casado, M.E.; Lima, E.; Garcia, J.A.; Doerrier, C.; Aranda, P.; Sayed, R.K.A.; Guerra-Librero, A.; Escames, G.; Lopez, L.C.; Acuna-Castroviejo, D. Melatonin Rescues Zebrafish Embryos from the Parkinsonian Phenotype Restoring the Parkin/PINK1/DJ-1/MUL1 Network. J. Pineal Res. 2016, 61, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Titorenko, V.I.; Terlecky, S.R. Peroxisome Metabolism and Cellular Aging. Traffic 2011, 12, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Legakis, J.E.; Koepke, J.I.; Jedeszko, C.; Barlaskar, F.; Terlecky, L.J.; Edwards, H.J.; Walton, P.A.; Terlecky, S.R. Peroxisome Senescence in Human Fibroblasts. Mol. Biol. Cell 2002, 13, 4243–4255. [Google Scholar] [CrossRef] [PubMed]
- Terman, A.; Kurz, T.; Navratil, M.; Arriaga, E.A.; Brunk, U.T. Mitochondrial Turnover and Aging of Long-lived Postmitotic Cells: The Mitochondrial-lysosomal Axis Theory of Aging. Antioxid. Redox Signal. 2010, 12, 503–535. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Wang, C.; Yu, Z.; Peng, Y.; Wang, S.; Feng, S.; Zhang, S.; Tian, X.; Sun, C.; Liu, K.; et al. Human Transporters, PEPT 1/2, Facilitate Melatonin Transportation into Mitochondria of Cancer Cells: An Implication of the Therapeutic Potential. J. Pineal Res. 2017, 62, e12390. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Liu, X.; Rosales-Corral, S.A.; Acuna-Castroviejo, D.; Reiter, R.J. Mitochondria and Chloroplasts as the Original Sites of Melatonin Synthesis: A Hypothesis Related to Melatonin’s Primary Function and Evolution in Eukaryotes. J. Pineal Res. 2013, 54, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Feng, C.; Zhang, X.; Guo, Y.; Zhou, F.; Shan, D.; Liu, X.; Kong, J. Plant Mitochondria Synthesize Melatonin and Enhance the Tolerance of Plants to Drought Stress. J. Pineal Res. 2017, 63, e12429. [Google Scholar] [CrossRef] [PubMed]
- Suofu, Y.; Li, W.; Jean-Alphonse, F.G.; Jia, J.; Khattar, N.K.; Li, J.; Baranov, S.V.; Leronni, D.; Mihalik, A.C.; He, Y.; et al. Dual Role of Mitochondria in Producing Melatonin and Driving GPCR Signaling to Block Cytochrome c Release. Proc. Natl. Acad. Sci. USA 2017, 114, E7997–E8006. [Google Scholar] [CrossRef] [PubMed]
- Menendez-Pelaez, A.; Reiter, R.J. Distribution of Melatonin in Mammalian Tissues: Relative Importance of Nuclear Versus Cytosolic Localization. J. Pineal Res. 1993, 15, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Mitochondrial Bioenergetics Decay in Aging: Beneficial Effects of Melatonin. Cell. Mol. Life Sci. 2017, 74, 3897–3911. [Google Scholar] [CrossRef] [PubMed]
- Quintana, L.; Cobrera, J.; Perdomo, J.; Estevez, F.; Loro, J.F.; Reiter, R.J.; Quintana, J. Melatonin Enhances Hypothermia-induced Apoptotic Cell Death in Human Leukemia Cells. J. Pineal Res. 2016, 61, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Ning, J.; Feng, N.; Li, Z.; Liu, Z.; Wang, Y.; Li, X.; Hue, C.; Jia, X.; Xu, R.; et al. Dynamin-related Protein-1-mediated Mitochondrial Fission Contributes to Post-traumatic Cardiac Dysfunction in Rats and the Protective Effect of Melatonin. J. Pineal Res. 2017, 63, e12447. [Google Scholar] [CrossRef] [PubMed]
- Kleszczyriski, K.; Zillikens, D.; Fischer, T.W. Melatonin Enhances Mitochondrial ATP Synthesis, Reduces Reactive Oxygen Species Formation, and Mediates Translocation of the Nuclear Erythroid 2-related Factor 2 in Activation of Phase-2 Antioxidant Enzymes (γ-GCS, HO-1, NQO1) in Ultraviolet Radiation-treated Normal Human Epidermal Keratinocytes (NHEK). J. Pineal Res. 2016, 61, 187–197. [Google Scholar]
- Areti, A.; Komirishetty, P.; Akuthota, M.; Malik, R.A.; Kurman, A. Melatonin Prevents Mitochondrial Dysfunction and Promotes Neuroprotection by Inducing Autophagy during Oxaliplatin-evoked Peripheral Neuropathy. J. Pineal Res. 2017, 62, e12393. [Google Scholar] [CrossRef] [PubMed]
- Dehdashtian, E.; Mehrzadi, S.; Yousefi, B.; Hosseinzadeh, A.; Reiter, R.J.; Ghaznavi, H.; Naseripour, M. Diabetic Retinopathy Pathogenesis and the Ameliorating Effects of Melatonin: Involvement of Autophagy, Inflammation and Oxidative Stress. Life Sci. 2018, 193, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Mandala, A.; Naaz, S.; Giri, S.; Jain, M.; Bandyopadhyay, D.; Reiter, R.J.; Roy, S.S. Melatonin Protects against Lipid-induced Mitochondrial Dysfunction in Hepatocytes and Inhibits Stellate Cell Activation during Hepatic Fibrosis in Mice. J. Pineal Res. 2017, 62, e12404. [Google Scholar] [CrossRef] [PubMed]
- Dragicevic, N.; Copes, N.; O’Neal-Moffitt, G.; Jin, J.; Buzzeo, R.; Mamcarz, M.; Tan, J.; Cao, C.; Olcese, J.J.; Arendash, G.W.; et al. Melatonin Treatment Restores Mitochondrial Function in Alzheimer’s Mice: A Mitochondrial Protective Role of Melatonin Membrane Receptor Signaling. J. Pineal Res. 2011, 51, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Pi, H.; Zhang, L.; Zhang, N.; Li, Y.; Zhang, H.; Tang, J.; Li, H.; Feng, M.; Deng, P.; et al. Melatonin Prevents Abnormal Mitochondrial Dynamics Resulting from Neurotoxicity of Cadmium by Blocking Cadmium-dependent Translocation of Drp1 to the Mitochondria. J. Pineal Res. 2016, 60, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Melchiorri, D.; Reiter, R.J.; Attica, A.M.; Hara, M.; Burgas, A.; Nistico, G. Potent Protective Effect of Melatonin on in Vivo Paraquat-induced Oxidative Damage in Rats. Life Sci. 1995, 56, 83–89. [Google Scholar] [CrossRef]
- Maity, P.; Bindu, S.; Dey, S.; Goyal, M.; Alam, A.; Pal, C.; Reiter, R.J.; Bandyopadhaya, D. Melatonin Reduces Indomethacin-induced Gastric Mucosal Cell Apoptosis in Preventing Mitochondrial Oxidative Stress and the Activation of Mitochondrial Pathway of Apoptosis. J. Pineal Res. 2009, 46, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Chuang, J.I.; Pan, I.L.; Hsieh, C.Y.; Huang, C.Y.; Chen, P.C.; Shin, J.W. Melatonin Prevents the Dynamin-related Protein 1-dependent Mitochondrial Fission and Oxidative Insult in the Cortical Neurons after 1-Methyl-4-phenylpyridinium Treatment. J. Pineal Res. 2016, 61, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.F.; Hou, J.N.; Wei, F.P.; Xue, Q.; Zhang, F.; Peng, C.F.; Yang, Y.; Tian, Y.; Feng, J.; Du, J.; et al. Melatonin Attenuates Postmyocardial Infarction Injury Via Increasing Tom70 Expression. J. Pineal Res. 2017, 62, e12371. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, C.; Chatzimichail, G.; Xenofontos, R.; Pavlou, J.J.; Panagiotou, E.; Christou, A.; Fotopoulos, V. Melatonin Systemically Ameliorates Drought Stress-induced Damage in Medicago sativa Plants by Modulating Nitro-oxidative Homeostasis and Proline Metabolism. J. Pineal Res. 2017, 62, e12401. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Tan, D.X.; Reiter, R.J. Melatonin and Related Compounds: Chemical Insights into Their Protective Effects against Oxidative Stress. Curr. Org. Chem. 2017, 21, 2077–2095. [Google Scholar] [CrossRef]
- Galano, A. On the Direct Scavenging Activity of Melatonin towards Hydroxyl and a Series of Peroxyl Radicals. Phys. Chem. Chem. Phys. 2011, 13, 7178–7188. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Reiter, R.J.; Qi, W.; Karbownik, M.; Calvo, J.R. Significance of Melatonin in Antioxidative Defense System: Reactions and Products. Biol. Signals Recept. 2000, 9, 137–159. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Antioxidative Protection by Melatonin: Multiplicity of Mechanisms from Radical Detoxification to Radical Avoidance. Endocrine 2005, 27, 119–130. [Google Scholar] [CrossRef]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolin, I.; Herrera, F.; Martin, V.; Reiter, R.J. Regulation of Antioxidant Enzymes: A Significant Role for Melatonin. J. Pineal Res. 2004, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Paredes, S.D.; Bejarano, I.; Terron, M.P.; Berriga, C.; Reiter, R.J.; Rodriguez, A.B. Melatonin and Tryptophan Counteract Lipid Peroxidation and Modulate Superoxide Dismutase Activity in Heterophils In Vivo: Effect of Antigen-induced Activation and Age. Age 2009, 31, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Fan, C.; Li, Z.; Zhang, J.; Xue, X.; Zhao, G.; Yang, Y.; Wang, H. Melatonin Rescues Cardiac Thioredoxin during Ischemia-reperfusion Injury in Acute Hyperglycemic State by Restoring Notch1/Hes1/Akt Signaling in a Membrane Receptor-dependent Manner. J. Pineal Res. 2017, 62, e12375. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wang, H.; Li, L.; Li, X.; Ge, J.; Reiter, R.J.; Wang, Q. Melatonin Protects Against Maternal Obesity-associated Oxidative Stress and Meiotic Defects in Oocytes Via SIRT3-SOD2-dependent Pathways. J. Pineal Res. 2017, 63, e12431. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Rosales-Corral, S.; Zhou, X.; Tan, D.X. Role of SIRT3/SOD2 Signaling in Mediating the Antioxidant Actions of Melatonin in Mitochondria. Curr. Trends Endocrinol. 2017, 9, 45–49. [Google Scholar]
- Zhai, M.; Li, B.; Duan, W.; Jing, L.; Zhang, B.; Zhang, M.; Yu, L.; Liu, Z.; Yu, B.; Ren, K.; et al. Melatonin Ameliorates Myocardial Ischemia Reperfusion Injury Through SIRT3-dependent Regulation of Oxidative Stress and Apoptosis. J. Pineal Res. 2017, 63, e12433. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Aranda, A.; Fernandez-Vasquez, G.; Mohammad A-Serrano, M.; Reiter, R.J.; Agil, A. Melatonin Improves Mitochondrial Function in Inguinal White Adipose Tissue of Zucker Diabetic Rats. J. Pineal Res. 2014, 57, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Yin, T.C.; Sung, P.H.; Chiang, J.Y.; Sun, C.K.; Yip, H.K. Melatonin Enhances Survival and Reserves Functional Integrity of Stem Cells: A Survey. J. Pineal Res. 2017, 62, e12372. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.Y.; Zhang, Y.; Xu, Y.P.; Qi, Z.Y.; Li, M.Q.; Ahammed, G.J.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Reiter, R.J.; et al. HsfA1a Upregulates Melatonin Biosynthesis to Confer Cadmium Tolerance in Tomato Plants. J. Pineal Res. 2017, 62, e12387. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Xin, Z.; Di, W.; Yan, X.; Li, X.; Reiter, R.J.; Yang, Y. Melatonin and Mitochondrial Function during Ischemia/Reperfusion Injury. Cell. Mol. Life Sci. 2017, 72, 3989–3998. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Tan, D.X.; Reiter, R.J. Kynuramines Metabolites of Melatonin and Other Indoles: The Resurrection of an Almost Forgotten Class of Biogenic Amines. J. Pineal Res. 2009, 47, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Tan, D.X.; Reiter, R.J. On the Free Radical Scavenging Activities of Melatonin’s Metabolites, AFMK and AMK. J. Pineal Res. 2013, 54, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One Molecule, Many Derivatives: A Never Ending Interaction of Melatonin with Reactive Oxygen and Nitrogen Species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Hardeland, R.; Manchester, L.C.; Galano, A.; Reiter, R.J. Cyclic 3-hydroxy-melatonin (C3OHM), a Potent Antioxidant, Scavenges Free Radicals and Suppresses Oxidative Reactions. Curr. Med. Chem. 2014, 21, 1557–1585. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Medina, M.E.; Tan, D.X.; Reiter, R.J. Melatonin and Its Metabolites as Cooper Chelating Agents and their Role in Inhibiting Oxidative Stress: a Physiochemical Analysis. J. Pineal Res. 2015, 58, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Diduk, R.; Galano, A.; Tan, D.X.; Reiter, R.J. N-acetylserotonin and 6-hydroxy-melatonin Against Oxidative Stress: Implications for the Overall Protection Exerted by Melatonin. J. Phys. Chem. B 2015, 119, 8535–8543. [Google Scholar] [CrossRef] [PubMed]
- Majidinia, M.; Sadeghpour, A.; Mehrzadi, S.; Reiter, R.J.; Khatami, N.; Yousefi, B. Melatonin: A Pleiotropic Molecule that Modulates DNA Damage Response and Repair Pathways. J. Pineal Res. 2017, 63, e12416. [Google Scholar] [CrossRef] [PubMed]
- Jou, M.J.; Peng, T.I.; Reiter, R.J.; Jou, S.B.; Wu, H.Y.; Wen, S.T. Visualization of the Antioxidant Effects of Melatonin at the Mitochondrial Level during Oxidative Stress-induced Apoptosis of Rat Brain Astrocytes. J. Pineal Res. 2004, 37, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Jou, M.J.; Peng, T.I.; Hsu, L.F.; Jou, S.B.; Reiter, R.J.; Yang, C.M.; Chiao, C.C.; Lin, Y.F.; Chen, C.C. Visualization of Melatonin’s Multiple Mitochondrial Levels of Protection Against Mitochondrial Ca2+-mediated Permeability Transition and Beyond in Rat Brain Astrocytes. J. Pineal Res. 2010, 48, 20–38. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.I.; Hsiao, C.W.; Reiter, R.J.; Tanaka, M.; Lai, Y.K.; Jou, M.J. mtDNA T8993G Mutation-induced Mitochondrial Complex V Inhibition Augments Cardiolipin-dependent Alterations in Mitochondrial Dynamics during Oxidative, Ca2+, and Lipid Insults in NARP Cybrids: A Potential Therapeutic Target for Melatonin. J. Pineal Res. 2012, 52, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Paredes, S.D.; Korkmaz, A.; Jou, M.J.; Tan, D.X. Melatonin Combats Molecular Terrorism at the Mitochondrial Level. Interdiscip. Toxicol. 2008, 1, 127–149. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Macias, M.; Escames, G.; Reiter, R.J.; Agapita, M.T.; Ortiz, G.G.; Acuna-Castroviejo, D. Melatonin-induced Increased Activity of the Respiratory Chain Complexes I and IV can Prevent Mitochondrial Damage Induced by Ruthenium Red In Vivo. J. Pineal Res. 2000, 28, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Macias, M.; Escames, G.; Leon, J.; Acuna-Castroviejo, D. Melatonin but not Vitamins C and E Maintains Glutathione Homeostasis in Butyl-hydroperoxide-induced Mitochondrial Oxidative Stress. FASEB J. 2000, 14, 1677–1679. [Google Scholar] [CrossRef] [PubMed]
- Acuna-Castroviejo, D.; Lopez, L.C.; Escames, G.; Lopez, A.; Garcia, J.A.; Reiter, R.J. Melatonin-mitochondria Interplay in Health and Disease. Curr. Top. Med. Chem. 2011, 11, 221–240. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.H.; Lee, H.Y.; Back, K. Chloroplast Overexpression of Rice Caffeic Acid O-methyltransferase Increases Melatonin Production in Chloroplasts Via 5-methoxytryptamine Pathway in Transgenic Rice Plants. J. Pineal Res. 2017, 63, 12412. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin and the Electron Transport Chain. Cell. Mol. Life Sci. 2017, 71, 3883–3896. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, L.; Lischko, M.M.; Rollag, M.D.; Niswender, G.D. Melatonin: Daily Cycle in Plasma and Cerebrospinal Fluid in Calves. Science 1977, 195, 686–687. [Google Scholar] [CrossRef] [PubMed]
- Tricoire, H.; Locatelli, A.; Chemineau, P.; Malpaux, B. Melatonin Enters the Cerebrospinal Fluid through the Pineal Recess. Endocrinology 2002, 143, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Kim, S.J.; Cruz, M.H.C. Delivery of Pineal Melatonin to the brain and SCN: Role of Canaliculi, Cerebrospinal Fluid, Tanycytes and Virchow-Robin Perivascular Spaces. Brain Struct. Funct. 2014, 219, 1873–1887. [Google Scholar] [CrossRef] [PubMed]
- Mayo, J.C.; Sainz, R.M.; Gonzalez-Menendez, P.; Hevia, D.; Cermuda-Cermuda, R. Melatonin Transplant into Mitochondria. Cell. Mol. Life Sci. 2017, 74, 3927–3940. [Google Scholar] [CrossRef] [PubMed]
- Hevia, D.; Gonzalez-Menendez, P.; Quiros-Gonzales, I.; Miar, A.; Rodriguez-Garcia, A.; Tan, D.X.; Reiter, R.J.; Mayo, J.C.; Sainz, R.M. Melatonin Uptake through Glucose Transporters: A New Target for Melatonin Inhibition of Cancer. J. Pineal Res. 2015, 58, 234–250. [Google Scholar] [CrossRef] [PubMed]
- Lowes, D.A.; Webster, N.R.; Murphy, M.P.; Galley, H.F. Antioxidants that Protect Mitochondria Reduce Interleukin-6 and Oxidative Stress, Improve Mitochondrial Function, and Reduce Biochemical Markers of Organ Dysfunction in a Rat Model of sepsis. Br. J. Anaesth. 2013, 110, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Zubero, E.; Alatorre-Jimenez, M.A.; Lopez-Pingarron, L.; Reyes-Gonzalez, M.C.; Almeida-Sauza, P.; Cantin-Golet, A.; Ruiz-Ruiz, F.J.; Tan, D.X.; Garcia, J.J.; Reiter, R.J. Melatonin’s Role in Preventing Toxin-related and Sepsis-mediated Hepatic Damage: A Review. Pharmacol. Res. 2016, 105, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Deng, C.; Ma, Z.; Wang, D.; Fan, C.; Li, T.; Di, S.; Gong, B.; Reiter, R.J.; Yang, Y. Utilizing Melatonin to Combat Bacterial Infections, and Septic Injury. Br. J. Pharmacol. 2017, 174, 754–788. [Google Scholar] [CrossRef] [PubMed]
- Gitto, E.; Karbownik, M.; Reiter, R.J.; Tan, D.X.; Cuzzocrea, S.; Chiurazzi, P.; Cordaro, S.; Corona, G.; Trimarchi, G.; Barberi, I. Effects of Melatonin Treatment in Septic Newborns. Pediatr. Res. 2001, 50, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Mayo, J.C.; Sainz, R.M.; Gonzalez-Menendez, P.; Cepas, V.; Tan, D.X.; Reiter, R.J. Melatonin and Sirtuins: A Not-so Unexpected Relationship. J. Pineal Res. 2017, 62, e12391. [Google Scholar] [CrossRef] [PubMed]
- Byeon, Y.; Back, K. Melatonin Production in Escherichia coli by Dual Expression of Serotonin N-acetyltransferase and Caffeic Acid O-Methyltransferase. Appl. Microbiol. Biotechnol. 2016, 100, 6683–6691. [Google Scholar] [CrossRef] [PubMed]
- Zimorski, V.; Ku, C.; Martin, W.F.; Gould, S.B. Endosymbiotic Theory for Organelle Origins. Curr. Opin. Microbiol. 2014, 22, 38–48. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Wang, J.; Zhang, Z.; Yang, M.; Li, Y.; Tian, X.; Ma, T.; Tao, J.; Zhu, K.; Song, Y.; et al. Mitochondria Synthesize Melatonin to Ameliorate Its Function and Improve Oocyte Quality Under In Vitro Conditions. Int. J. Mol. Sci. 2016, 17, 939. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Back, B. Cadmium Disrupts Subcellular Organelles, Including Chloroplasts, Resulting in Melatonin Induction in Plants. Molecules 2017, 22, E1791. [Google Scholar] [CrossRef] [PubMed]
- Byeon, Y.; Lee, H.Y.; Back, K. Chloroplastic and Cytoplasmic Overexpression of Sheep Serotonin N-acetyltransferase in Transgenic Rice Plants is Associated with Low Melatonin Production Despite High Enzyme Activity. J. Pineal Res. 2015, 58, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Gastel, J.A.; Weller, J.L.; Schwartz, C.; Jaffe, H.; Namboodiri, M.A.; Coon, S.L.; Hoffman, A.B.; Rollag, M.; Obsil, T.; et al. Melatonin Synthesis: 14–3-3-dependent Activation and Inhibition of Arylalkylamine N-acetyltransferase Mediated by Phosphoserine-205. Proc. Natl. Acad. Sci. USA 2005, 102, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Chang, Y.; Zeng, H.; Liu, G.; He, C.; Shi, H. RAV Transcription Factors are Essential for Disease Resistance Against Cassava Bacterial Blight Via Activation of Melatonin Biosynthesis Genes. J. Pineal Res. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Q.; Hasan, M.K.; Li, C.X.; Ahammed, G.J.; Xia, X.J.; Shi, K.; Zhou, V.H.; Reiter, R.J.; Yu, J.Q.; Xu, M.X.; et al. Melatonin Mediates Selenium-induced Tolerance to Cadmium Stress in Tomato Plants. J. Pineal Res. 2016, 61, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Chan, Z.; Shi, H. Improved Abiotic Tolerance of Bermudagrass by Exogenous Small Molecules. Front. Signal. Behav. 2015, 10, e971577. [Google Scholar] [CrossRef] [PubMed]
- Fuhrberg, B.; Hardeland, R.; Poeggela, B.; Behrmann, G. Dramatic Rises of Melatonin and 5-methoxytryptamine in Gonyaulax Exposed to Decreased Temperature. Biol. Rhythm Res. 1997, 28, 144–150. [Google Scholar] [CrossRef]
- Cedikova, M.; Pitule, P.; Kripnerova, M.; Markov, M.; Kuncova, J. Multiple Roles of Mitochondria in Aging Processes. Physiol. Res. 2016, 65, S519–S531. [Google Scholar] [PubMed]
- Stefonates, R.; Sanz, A. The Role of Mitochondrial ROS in the Aging Brain. FEBS Lett. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Aging: A Theory Based on Free Radical and Radiation Chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Lv, Z.; Qiao, X.; Li, X.; Li, Y.; Zhang, Y.; Chen, C. The Decay of Redox-stress Response Capacity is a Substantive Characteristic of Aging: Revising the Redox Theory of Aging. Redox Biol. 2017, 11, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Mock, J.T.; Chaudhari, K.; Sidhu, A.; Sumien, N. The Influence of Vitamins E and C and Exercise in Brain Aging. Exp. Gerontol. 2017, 94, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.N.; Wu, M.; Bondy, S.C. Telomere Shortening during Aging: Attenuation by Antioxidants and Anti-inflammatory Agents. Mech. Ageing Dev. 2017, 164, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Ramis, M.R.; Estaban, S.; Miralles, A.; Tan, D.X.; Reiter, R.J. Protective Effects of Melatonin and Mitochondrial-targeted Antioxidants against Oxidative Stress: A Review. Curr. Med. Chem. 2015, 22, 2690–2711. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.S.; Simoes, R.F.; Canto, R.; Oliveira, P.J. Targeting Mitochondria in Cardiovascular Diseases. Curr. Pharm. Res. 2016, 22, 5698–5717. [Google Scholar] [CrossRef]
- Prauchner, C.A. Oxidative Stress in Sepsis: Pathophysiological Implications Justifying Antioxidant Co-therapy. Burns 2016, 43, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Poeggeler, B.; Menendez-Pelaez, A.; Chen, L.D.; Saarda, S. Melatonin as a Free Radical Scavenger: Implications for Aging and Age-related Diseases. Ann. N. Y. Acad. Sci. 1994, 719, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Poeggeler, B. Melatonin, Aging, and Age-related Diseases: Perspectives for Prevention, Intervention, and Therapy. Endocrine 2005, 27, 201–212. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Kim, S.J.; Manchester, L.C.; Qi, W.; Garcia, J.J.; Cabrera, J.C.; El-Sokkary, G.; Rouvier-Garay, V. Augmentation of Indices of Oxidative Damage in Life-long Melatonin-deficient Rats. Mech. Ageing Dev. 1999, 110, 157–173. [Google Scholar] [CrossRef]
- Vriend, J.; Reiter, R.J. Melatonin Feedback on Clock Genes: A Theory Involving the Proteasome. J. Pineal Res. 2015, 58, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, G.; Otsuka, K. Chronobiology of Ageing: A Mini-review. Gerontology 2017, 63, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Sohal, R.S.; Allen, R.G. Oxidative Stress as a Causal Factor in Differentiation and Aging: A Unifying Hypothesis. Exp. Gerontol. 1990, 25, 499–522. [Google Scholar] [CrossRef]
- Reiter, R.J.; Rosales-Corral, S.A.; Tan, D.X.; Alatorre-Jimenez, M.; Lopez, C. Circadian Dysregulation and Melatonin Suppression in the Context of Aging. In Circadian Rhythms and Their Impact on Aging; Jazwenski, M., Belancio, V.P., Hill, S.M., Eds.; Springer: New York, NY, USA, 2017; pp. 1–25. [Google Scholar]
- Manella, G.; Asher, G. The Circadian Nature of Mitochondrial Biology. Front. Endocrinol. 2016, 7, 162. [Google Scholar] [CrossRef] [PubMed]
- Bratic, A.; Larsson, N.G. The Role of Mitochondria in Aging. J. Clin. Investig. 2013, 123, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Koltover, V.K. Free Radical Timer of Aging: From Chemistry of Free Radicals to Systems Theory of Reliability. Curr. Aging Sci. 2017, 10, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Ortiz, F.; Doerrier, C.; Venegas, C.; Fernandez-Ortiz, M.; Aranda, P.; Diaz-Casado, M.E.; Fernandez-Gil, B.; Barriocanal-Casado, E.; Escames, G.; et al. Mitochondrial Impairment and Melatonin Protection in Parkinsonian Mice Do Not Depend on Inducible or Nitric Oxide Syntheses. PLoS ONE 2017, 12, e0183090. [Google Scholar] [CrossRef] [PubMed]
- Pi, H.; Xu, S.; Reiter, R.J.; Guo, P.; Zhang, L.; Li, M.; Cao, Z.; Tian, L.; Xie, J.; Zhang, R.; et al. SIRT3-SOD2-mROS-dependent Autophagy in Cadmium-induced Hepatotoxicity and Salvage by Melatonin. Autophagy 2015, 11, 1037–1051. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Du, W.; Li, Y.; Shi, C.; Hu, N.; Ma, S.; Wang, W.; Ren, J. Effects of Melatonin on Fatty Liver Disease: The Role of NR4A1/DNA-RKcs/p53 Pathway, Mitochondrial Fission, and Mitophagy. J. Pineal Res. 2017, 63, e12490. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Zmijewski, M.A.; Semak, I.; Kim, T.K.; Janjetovic, Z.; Slominski, R.M.; Zmijewski, J.W. Melatonin, Mitochondria, and the Skin. Cell. Mol. Life Sci. 2017, 74, 3913–3926. [Google Scholar] [CrossRef] [PubMed]
- Baltatu, O.C.; Amaral, F.G.; Campos, L.A.; Cipolla-Neto, J. Melatonin, Mitochondria and Hypertension. Cell. Mol. Life Sci. 2017, 74, 3955–3964. [Google Scholar] [CrossRef] [PubMed]
- Wongprayoon, P.; Govitrapong, P. Melatonin as a Mitochondrial Protector in Neurodegenerative Diseases. Cell. Mol. Life Sci. 2017, 74, 3999–4014. [Google Scholar] [CrossRef] [PubMed]
- Benot, S.; Goberna, R.; Reiter, R.J.; Garcia-Maurino, S.; Osuna, C.; Guerrero, J.M. Physiological levels of Melatonin Contribute to the Antioxidant Capacity of Human Serum. J. Pineal Res. 1999, 27, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Richardson, B.A.; Johnson, L.Y.; Ferguson, B.N.; Dink, D.T. Pineal Melatonin Rhythm in Aging Syrian Hamsters. Science 1980, 210, 1372–1373. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Craft, C.M.; Johnson, J.E., Jr.; King, T.S.; Richardson, B.A.; Vaughan, G.M.; Vaughan, M.K. Age-associated Reduction in Nocturnal Pineal Melatonin Levels in Female Rats. Endocrinology 1981, 109, 1295–1297. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Hidalgo, M.; de la Lastra, C.; Carrascosa-Salmoral, M.P.; Naranjo, M.C.; Gomez-Corvera, A.; Caballero, B.; Guerrero, J.M. Age-related Changes in Melatonin Synthesis in Rat Extrapineal Tissues. Exp. Gerontol. 2009, 44, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Pappolla, M.A.; Simovich, M.J.; Bryant-Thomas, T.; Chyan, Y.L.; Poeggeler, B.; Dubocovich, M.; Bick, R.; Perry, G.; Cruz-Sanchez, F.; Smith, M.A. The Neuroprotective Activities of Melatonin against the Alzheimer Beta-protein are Not Mediated by Melatonin Membrane Receptors. J. Pineal Res. 2002, 32, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.; Morel, A.; Sasco, L.; Saluk, J. Melatonin Redox Activity: Its Potential Clinical Applications in Neurodegenerative Disorders. Curr. Top. Med. Chem. 2015, 15, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Shukla, M.; Govitrapong, P.; Boontem, P.; Reiter, R.J.; Satayavivad, J. Mechanisms of Melatonin in Alleviating Alzheimer’s Disease. Curr. Neuropharmacol. 2017, 15, 1010–1031. [Google Scholar] [CrossRef] [PubMed]
- Milani, M.; Sparavigna, A. Antiaging Efficacy of Melatonin-based Day and Night Creams: A Randomized Split Face, Assessor-blinded Proof-of-concept Trial. Clin. Cosmet. Investig. Dermatol. 2018, 11, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Kawamoto, M.; Sato, S.; Tamura, I.; Maekawa, R.; Taketani, T.; Aasada, H.; Takaki, E.; Nakai, A.; Reiter, R.J.; et al. Long-term Melatonin Treatment Delays Ovarian Aging. J. Pineal Res. 2017, 62, e12381. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, D.P.; Vigo, D.E.; Olivar, N.; Vidal, M.F.; Furio, A.M.; Brusco, L.I. Therapeutic Application of Melatonin in Mild Cognitive Impairment. Am. J. Neurogener. Dis. 2012, 1, 280–291. [Google Scholar]
- Boyko, Y.; Jennum, P.; Toft, P. Sleep Quality and Circadian Rhythm Disruption in the Intensive Care Unit: A Review. Nat. Sci. Sleep 2017, 9, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Barcelo, E.J.; Rueda, N.; Mediavilla, M.D.; Martinez-Cue, C.; Reiter, R.J. Clinical Uses of Melatonin in Neurological Diseases and Mental Behavioral Disorders. Curr. Med. Chem. 2017, 24, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Sanchez, N.; Cruz-Chamorro, I.; Diaz-Sanchez, M.; Sarmiento-Soto, H.; Medrano-Campillo, P.; Martinez-Lopez, A.; Lardone, P.J.; Guerrero, J.M.; Carrillo-Vico, A. Melatonin Reduces Inflammatory Response in Peripheral T Helper Lymphocytes from Relapsing-remitting Multiple Sclerosis Patients. J. Pineal Res. 2017, 63, e12442. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Gonzalez, A.; Alvarez-Sanchez, N.; Lardone, R.J.; Cruz-Chamorro, L.; Martinez-Lopez, A.; Guerrero, J.M.; Reiter, R.J.; Carrillo-Vico, A. Melatonin Treatment Improves Primary Progressive Multiple Sclerosis: A Case Report. J. Pineal Res. 2015, 58, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Leon, J.; Acuna-Castroviejo, D.; Sainz, R.M.; Mayo, J.C.; Tan, D.X.; Reiter, R.J. Melatonin and Mitochondrial Function. Life Sci. 2004, 1, 765–790. [Google Scholar] [CrossRef] [PubMed]
- Paradies, G.; Petrosillo, G.; Paradies, V.; Reiter, R.J.; Ruggiero, F.M. Melatonin, Cardiolipin and Mitochondrial Bioenergetics in Health and Disease. J Pineal Res 2010, 48, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Protective Role of Melatonin in Mitochondrial Function and Related Disorders. Arch. Toxicol. 2015, 89, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Changes in the Mitochondrial Permeability Transition Pore in Aging and Age-associated Diseases. Mech. Ageing Dev. 2013, 134, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sun, X.; Xie, Y.; Li, M.; Chen, W.; Zhang, S.; Liang, D.; Ma, F. Melatonin Regulates Proteomic Changes during Leaf Senescence in Malus hupehensis. J. Pineal Res. 2014, 57, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Zheng, G.; Li, W.; Wang, Y.; Hu, B.; Wang, H.; Wu, H.; Qian, Y.; Zhu, X.G.; Tan, D.X.; Chen, S.Y.; Chu, C. Melatonin Delays Leaf Senescence and Enhances Salt Stress Tolerance in Rice. J. Pineal Res. 2015, 59, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.C.; Ristow, M.; Vina, J. Antioxidant Supplements in Exercise: Worse Than Useless? Am. J. Physiol. Endocrinol. Metab. 2012, 302, E476–E477. [Google Scholar] [CrossRef] [PubMed]
- Soysal, P.; Isik, A.T.; Carvalho, A.F.; Fernandes, B.S.; Solmi, M.; Schofield, P.; Veronese, N.; Stubbs, B. Oxidative Stress and Frailty: A Systemic Review and Synthesis of the Best Evidence. Maturitas 2017, 99, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Li, Y.; Zhou, Y.; Gan, R.Y.; Xu, D.P.; Li, H.B. Dietary Sources and Bioactivities of Melatonin. Nutrients 2017, 9, E367. [Google Scholar] [CrossRef] [PubMed]
- Liochev, S.I. Which is the Most Significant Cause of Aging? Antioxidants 2015, 17, 793–810. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, P.J.; Thompson, A.M.; Stambolic, V. Diabetes, Metformin and Breast Cancer. J. Clin. Oncol. 2012, 30, 2812–2814. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Di Gennaro, E.; Bruzzese, F.; Avallone, A.; Budillon, A. New Perspective for an Old Antidiabetic Drug: Metformin as Anticancer Agent. Cancer Treat. Res. 2014, 159, 355–376. [Google Scholar] [PubMed]
- Barzilai, N.; Crandall, J.P.; Kritcherisky, S.B.; Espeland, M.A. Metformin as a Tool to Target Ageing. Cell Metab. 2016, 119, 662–665. [Google Scholar]
- Kurhaluk, N.; Bojkova, B.; Radkowski, M.; Zaitseva, O.V.; Kyrüenko, S.; Demkow, U.; Winklewski, P.J. Melatonin and Metformin Diminish Oxidative Stress in Heart Tissue in a Rat Model of High Fat Diet and Mammary Carcinogenesis. Arch. Exp. Med. Biol. 2017, 128. in press. [Google Scholar]
- Asghari, A.; Akbari, G.; Meghdadi, A.; Mortazavi, P. Effects of Melatonin and Metformin Co-administration on Testicular Ischemia/Reperfusion Injury in Rats. J. Pediatr. Urol. 2016, 12, 410.e1–410.e7. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Zhou, X.J.; Xu, B. Mitochondria: Central Organelles for Melatonin?s Antioxidant and Anti-Aging Actions. Molecules 2018, 23, 509. https://doi.org/10.3390/molecules23020509
Reiter RJ, Tan DX, Rosales-Corral S, Galano A, Zhou XJ, Xu B. Mitochondria: Central Organelles for Melatonin?s Antioxidant and Anti-Aging Actions. Molecules. 2018; 23(2):509. https://doi.org/10.3390/molecules23020509
Chicago/Turabian StyleReiter, Russel J., Dun Xian Tan, Sergio Rosales-Corral, Annia Galano, Xin Jia Zhou, and Bing Xu. 2018. "Mitochondria: Central Organelles for Melatonin?s Antioxidant and Anti-Aging Actions" Molecules 23, no. 2: 509. https://doi.org/10.3390/molecules23020509
APA StyleReiter, R. J., Tan, D. X., Rosales-Corral, S., Galano, A., Zhou, X. J., & Xu, B. (2018). Mitochondria: Central Organelles for Melatonin?s Antioxidant and Anti-Aging Actions. Molecules, 23(2), 509. https://doi.org/10.3390/molecules23020509






