Design of Two Alternative Routes for the Synthesis of Naftifine and Analogues as Potential Antifungal Agents
Abstract
:1. Introduction
2. Results and Discussion
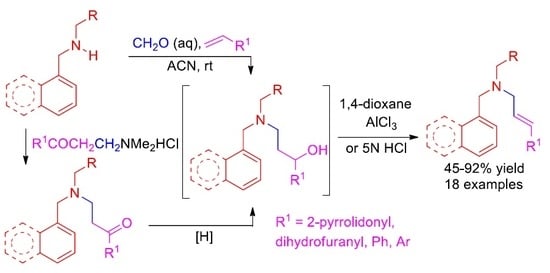
2.1. Chemistry
2.2. Antifungal Activity Studies
- (i)
- In general, the series of precursors 15 and products 16 were significantly less active than the series of precursors 18–20 and products 21–22.
- (ii)
- Almost all compounds 15–22 showed either very low to moderate activities or were inactive against A. flavus, A. niger and M. gypseum (data not shown).
- (iii)
- Compounds 15–22 identified with the letters a and d–k showed moderate to low (31.2–250 µg/mL) activities against the rest of the fungal panel (data not shown).
- (iv)
- Among compounds 18–22, the structures 18b, 18c and 21c displayed outstanding activities against one or more dermatophytes (0.5–7.8 µg/mL), (Table 1), while 19b was moderately active (MICs = 31.2–62.5 µg/mL) against four of the fungi tested (i.e., C. neoformans, M. gypseum, T. rubrum and T. mentagrophytes), being the only compound of the series showing activity against M. gypseum. It is worth to take into account that the most active compounds within 18–22 possess a CH3 group as R substituent although with variations in the substituent R1 of the phenyl ring. This finding is in accordance with the required features found by Stütz et al. for the allylamines to display antifungal activity [10].
- (v)
- Compound 18b was the most active of the whole series, showing activity not only against dermatophytes but also against Candida spp., S. cerevisiae, C. neoformans and A. flavus (MICs between 7.8 to 15.6 µg/mL). From these results it is clear that within the compounds bearing 4-Br and 3,4-methylenedioxy R1 substituents, the β-aminoketones 18 displayed the best activities, suggesting that the ketone group play an important role in the antifungal activity of these structures. Instead, among the γ-aminoalcohols 19–20, only 19b showed moderate activity against dermatophytes and C. neoformans, while its corresponding allylamine 21b displayed very low activities (MICs = 125–250 µg/mL) against the whole fungal panel. It is remarkable that allylamine 21c displayed the most outstanding activities against T. rubrum and T. mentagrophytes (MICs = 0.5–1.0 µg/mL) constituting this datum a finding that deserves great attention for future research.
3. Materials and Methods
3.1. General Information
3.2. Synthesis
3.2.1. General Procedure for the Synthesis of Secondary Amines 13a–e
3.2.2. General Procedure for the Synthesis of γ-Aminoalcohols 15
3.2.3. General Procedure for the Synthesis of Allylamines 16
3.2.4. General Procedure for the Synthesis of the γ-Aminoalcohols 19 and 20
3.2.5. General Procedure for the Synthesis of Allylamines 21 and 22
3.3. Antifungal Evaluation
3.3.1. Microorganisms and Media
3.3.2. Antifungal Susceptibility Testing
3.3.3. Fungal Growth Inhibition Percentage Determination
3.3.4. MIC100, MIC80 and MIC50 Determinations
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Batra, S.; Nag, S. Applications of allylamines for the syntheses of aza-heterocycles. Tetrahedron 2011, 67, 8959–9061. [Google Scholar]
- Ghorai, M.K.; Kumar, A.; Das, K. Lewis acid-mediated unprecedented ring-opening rearrangement of 2-aryl-N-tosylazetidines to enantiopure (E)-allylamines. Org. Lett. 2007, 9, 5441–5444. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Wang, X.; Bao, H.; Cheng, C.; Liu, N.; Hu, Y. Total syntheses of (−)-hanishin, (−)-longmide B, and (−)-longmide B methyl ester via a novel preparation of N-substituted pyrrole 2-carboxylates. Org. Lett. 2012, 14, 1062–1065. [Google Scholar] [CrossRef] [PubMed]
- Cannillo, A.; Norsikian, S.; Retailleau, P.; Tran Huu Dau, M.-E.; Iorga, B.I.; Beau, J.-M. From enantiopure hydroxyaldehydes to complex heterocyclic scaffolds: Development of domino Petasis/Diels–Alder and cross-metathesis/Michael addition reactions. Chem. Eur. J. 2014, 20, 12133–12143. [Google Scholar] [CrossRef] [PubMed]
- Scholtz, A.-W.; Ilgner, J.; Loader, B.; Pritschow, B.W.; Weisshaar, G. Cinnarizine and dimenhydrinate in the treatment of vertigo in medical practice. Wien Klin. Wochenschr. 2016, 128, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Kitahata, N.; Han, S.Y.; Noji, N.; Saito, T.; Kobayashi, M.; Nakano, T.; Kuchitsu, K.; Shinozaki, K.; Yoshida, S.; Matsumoto, S.; et al. A 9-cis-epoxycarotenoid dioxygenase inhibitor for use in the elucidation of abscisic acid action mechanisms. Bioorg. Med. Chem. 2006, 14, 5555–5561. [Google Scholar] [CrossRef] [PubMed]
- Donghi, D.; Hauser, V.; Bosshard, P.P. Microsporum audouinii tinea capitis in a Swiss school: Assessment and management of patients and asymptomatic carriers. Med. Mycol. 2011, 49, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Cuenca-Estrella, M.; Gomez-Lopez, A.; Mellado, E.; Buitrago, M.J.; Monzon, A.; Rodriguez-Tudela, J.L. Head-to-head comparison of the activities of currently available antifungal agents against 3,378 Spanish clinical isolates of yeasts and filamentous fungi. Antimicrob. Agents Chemother. 2006, 3, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Borba-Santos, L.P.; Rodrigues, A.M.; Gagini, T.B.; Fernandes, G.F.; Castro, R.; de Camargo, Z.P.; Nucci, M.; Lopes-Bezerra, L.M.; Ishida, K.; Rozental, S. Susceptibility of Sporothrix brasiliensis isolates to amphotericin B, azoles, and terbinafine. Med. Mycol. 2015, 53, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Stütz, A.; Georgopoulos, A.; Granitzer, W.; Petranyi, G.; Berney, D. Synthesis and structure-activity relationships of naftifine-related allylamine antimycotics. J. Med. Chem. 1986, 29, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Petasis, N.A.; Akritopoulou, I. The boronic acid Mannich reaction: A new method for the synthesis of geometrically pure allylamines. Tetrahedron Lett. 1993, 34, 583–586. [Google Scholar] [CrossRef]
- Prediger, P.; Barbosa, L.F.; Génisson, Y.; Correia, C. Substrate-directable Heck reactions with arenediazonium salts. The regio- and stereoselective arylation of allylamine derivatives and applications in the synthesis of naftifine and abamines. J. Org. Chem. 2011, 76, 7737–7749. [Google Scholar] [CrossRef] [PubMed]
- Nigam, P.K. Antifungal drugs and resistance: Current concepts. Our Dermatol. Online 2015, 6, 212–221. [Google Scholar] [CrossRef]
- Chamilos, G.; Kontoyiannis, D.P. Update on antifungal drug resistance mechanisms of Aspergillus fumigatus. Drug Resist. Update 2005, 8, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Abonia, R.; Castillo, J.; Insuasty, B.; Quiroga, J.; Nogueras, M.; Cobo, J. An efficient synthesis of 7-(arylmethyl)-3-tert-butyl-1-phenyl-6,7-dihydro-1H,4H-pyrazolo[3,4-d][1,3]oxazines. Eur. J. Org. Chem. 2010, 6454–6463. [Google Scholar] [CrossRef]
- Castillo, J.; Abonia, R.; Cobo, J.; Glidewell, C. Seven 5-benzylamino-3-tert-butyl-1-phenyl-1H-pyrazoles: Unexpected isomorphisms, and hydrogen-bonded supramolecular structures in zero, one and two dimensions. Acta Cryst. 2009, C65, o303–o310. [Google Scholar] [CrossRef] [PubMed]
- Abonia, R.; Castillo, J.; Insuasty, B.; Quiroga, J.; Nogueras, M.; Cobo, J. Efficient catalyst-free four-component synthesis of novel γ-aminoethers mediated by a Mannich type reaction. ACS Comb. Sci. 2013, 15, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Abonia, R.; Arteaga, D.; Castillo, J.; Insuasty, B.; Quiroga, J.; Ortíz, A. A straightforward and efficient method for the synthesis of diversely substituted β-aminoketones and γ-aminoalcohols from 3-(N,N-dimethylamino)propiophenones as starting materials. J. Braz. Chem. Soc. 2013, 24, 1396–1402. [Google Scholar] [CrossRef]
- Abonia, R.; Castillo, J.C.; Garay, A.; Insuasty, B.; Quiroga, J.; Nogueras, M.; Cobo, J.; D´Vries, R. A facile synthesis of stable β-amino-N-/O-hemiacetals through a catalyst-free three-component Mannich-type reaction. Tetrahedron Lett. 2017, 58, 1490–1494. [Google Scholar] [CrossRef]
- Veeraiah, M.K. Antimicrobial copolymers of N-vinylpyrrolidone. Indian J. Adv. Chem. Sci. S1 2016, 2–55. [Google Scholar]
- Sun, X.; Cao, Z.; Yeh, C.K.; Sun, Y. Antifungal activity, biofilm-controlling effect, and biocompatibility of poly(N-vinyl-2-pyrrolidinone)-grafted denture materials. Colloids Surf. B Biointerf. 2013, 110, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Veloso-Freire, C.P.; Baptista-Ferreira, S.; Melo de Oliveira, N.S.; Jackisch-Matsuura, A.B.; Gama, I.L.; da Silva, F.C.; de Souza, M.C.B.V.; Silva-Lima, E.; Ferreira, V.F. Synthesis and biological evaluation of substituted α- and β-2,3-dihydrofuran naphthoquinones as potent anticandidal agents. Med. Chem. Commun. 2010, 1, 229–232. [Google Scholar] [CrossRef]
- Zanatta, N.; Alves, S.H.; Coelho, H.S.; Borchhardt, D.M.; Machado, P.; Flores, K.M.; da Silva, F.M.; Spader, T.B.; Santurio, J.M.; Bonacorso, H.G.; et al. Synthesis, antimicrobial activity, and QSAR studies of furan-3-carboxamides. Bioorg. Med. Chem. 2007, 15, 1947–1958. [Google Scholar] [CrossRef] [PubMed]
- Senogles, E.; Roderick, R.A. The kinetics and mechanism of the acid-catalysed hydrolysis of N-vinylpyrrolidin-2-one. J. Chem. Soc. Perkin Trans. 2 1980, 825–828. [Google Scholar] [CrossRef]
- Jeffery, G.H.; Bassett, J.; Mendham, J.; Denney, R.C. Vogel’s Textbook of Practical Organic Chemistry, 4th ed.; Longman Inc.: New York, NY, USA, 1978; p. 815. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Document M27A3. In Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; CLSI: Wayne, PA, USA, 2008; pp. 1–25. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Document M38A2. In Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; CLSI: Wayne, PA, USA, 2008; pp. 1–35. [Google Scholar]
- Gerpe, A.; Boiani, L.; Hernández, P.; Sortino, M.; Zacchino, S.; González, M.; Cerecetto, H. Naftifine-analogues as anti-Trypanosoma cruzi agents. Eur. J. Med. Chem. 2010, 45, 2154–2164. [Google Scholar] [CrossRef] [PubMed]
- Trpković, A.; Pekmezović, M.; Barać, A.; Crnčević-Radović, L.; Arsić-Arsenijević, V. In vitro antifungal activities of amphotericin B, 5-fluorocytosine, fluconazole and itraconazole against Cryptococcus neoformans isolated from cerebrospinal fluid and blood from patients in Serbia. J. Mycol. Med. 2012, 22, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Messer, S.A.; Boyken, L.; Rice, C.; Tendolkar, S.; Hollis, R.J.; Doern, G.V.; Diekema, D.J. Global trends in the antifungal susceptibility of Cryptococcus neoformans (1990 to 2004). J. Clin. Microbiol. 2005, 43, 2163–2167. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute, formerly NCCLS (CLSI). Document M27-A2. In Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; CLSI: Wayne, PA, USA, 2002. [Google Scholar]
- Mutulis, F.; Mutule, L.; Lapins, M.; Wikberg, J.E.S. Reductive amination products containing naphthalene and indole moieties bind to melanocortin receptors. Bioorg. Med. Chem. Lett. 2002, 12, 1035–1038. [Google Scholar] [CrossRef]
- Nişancı, B.; Ganjehyan, K.; Metin, O.; Daştan, A.; Török, B. Graphene-supported NiPd alloy nanoparticles: A novel and highly efficient heterogeneous catalyst system for the reductive amination of aldehydes. J. Mol. Catal. A Chem. 2015, 409, 191–197. [Google Scholar] [CrossRef]
- Ye, Z.; Brust, T.F.; Watts, V.J.; Dai, M. Palladium-catalyzed regio- and stereoselective γ-arylation of tertiary allylic amines: Identification of potent adenylyl cyclase inhibitors. Org. Lett. 2015, 17, 892–895. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Lee, S. Additive-free decarboxylative coupling of cinnamic acid derivatives in water: Synthesis of allyl amines. Org. Lett. 2015, 17, 1300–1303. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |











 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | R | R1 | Ca | Sc | Cn | Afu | Afl | An | Mg | Tr | Tm | |
| 18 | b | CH3 | 4-Br | 15.6/62.5 | 15.6/31.3 | 7.8/15.6 | 7.8/15.6 | 250/250 | 250/>250 | >250/>250 | 3.9/3.9 | 2.0/2.0 |
| c | CH3 | 3,4-OCH2O | 125/125 | 125/125 | 31.25/62.5 | 31.3/62.5 | 250/250 | 250/>250 | >250/>250 | 7.8/15.6 | 7.8/15.6 | |
| 19 | b | CH3 | 4-Br | 125/250 | 125/250 | 62.5/125 | 250/>250 | 250/>250 | 250/>250 | 62.5/125 | 31.2/62.5 | 31.2/62.5 |
| c | CH3 | 3,4-OCH2O | >250 | >250 | >250 | >250 | >250 | >250 | 250/250 | 250/250 | 250/250 | |
| 21 | b | CH3 | 4-Br | 250 | >250 | >250 | >250 | >250 | >250 | 125/>250 | 125/>250 | 125/>250 |
| c | CH3 | 3,4-OCH2O | 125/>250 | 250/250 | 125/250 | 250/>250 | 250/>250 | 250/>250 | 125/>250 | 1.0/1.8 | 0.5/1.0 | |
| Amphotericin B | 1.0/1.0 | 1.0/1.0 | 1.0/2.0 | 2.0/2.0 | 2.0/2.0 | 2.0/2.0 | 0.5/0.5 | 0.5/0.5 | 0.5/0.5 | |||
| Terbinafine | - | - | - | - | - | - | 0.008/0.015 | 0.004/0.008 | 0.004/0.015 | |||
| Compound | Fungus | Concentrations of the Compounds (µg/mL) | MIC (µg/mL) | MFC (µg/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 250 | 125 | 62.5 | 31.2 | 15.6 | 7.8 | 3.9 | MIC100 | MIC80 | MIC50 | MFC | |||
| 18b |  | Ca | 100 | 100 | 100 | 100 | 100 | 87.4 ± 1.7 | 28.8 ± 2.6 | 15.6 | 7.8 | 7.8 | 62.5 |
| Cn | 100 | 100 | 100 | 100 | 100 | 98.9 ± 3.6 | 39.3 ± 3.7 | 7.8 | 7.8 | 7.8 | 15.6 | ||
| 19b | 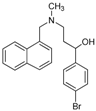 | Ca | 100 | 100 | 86.8 ± 1.3 | 54.3 ± 2.2 | 20.7 ± 3.2 | 7.7 ± 1.6 | 4.5 ± 0.7 | 125 | 62.5 | 31.2 | 250 |
| Cn | 100 | 100 | 100 | 87.3 ± 1.6 | 20.2 ± 1.3 | 8.37 ± 1.3 | 0 | 62.5 | 31.2 | 31.2 | 125 | ||
| 21b | 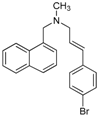 | Ca | 95.4 ± 8.3 | 83.2 ± 10.2 | 74.9 ± 5.7 | 52.4 ± 2.1 | 14.9 ± 1.6 | 5.6 ± 1.2 | 1.9 ± 1.5 | 250 | 62.5 | 31.2 | >250 |
| Cn | 58.1 ± 2.7 | 52.5 ± 4.2 | 49.4 ± 2.3 | 48.8 ± 1.8 | 35.1 ± 0.0 | 19.3 ± 1.2 | 0 | >250 | >250 | 31.2 | >250 | ||
| Amphotericin B | Ca | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 1.0 | 0.5 | 0.2 | 1.0 | |
| Cn | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 1.2 | 0.5 | 0.2 | 1.2 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abonia, R.; Garay, A.; Castillo, J.C.; Insuasty, B.; Quiroga, J.; Nogueras, M.; Cobo, J.; Butassi, E.; Zacchino, S. Design of Two Alternative Routes for the Synthesis of Naftifine and Analogues as Potential Antifungal Agents. Molecules 2018, 23, 520. https://doi.org/10.3390/molecules23030520
Abonia R, Garay A, Castillo JC, Insuasty B, Quiroga J, Nogueras M, Cobo J, Butassi E, Zacchino S. Design of Two Alternative Routes for the Synthesis of Naftifine and Analogues as Potential Antifungal Agents. Molecules. 2018; 23(3):520. https://doi.org/10.3390/molecules23030520
Chicago/Turabian StyleAbonia, Rodrigo, Alexander Garay, Juan C. Castillo, Braulio Insuasty, Jairo Quiroga, Manuel Nogueras, Justo Cobo, Estefanía Butassi, and Susana Zacchino. 2018. "Design of Two Alternative Routes for the Synthesis of Naftifine and Analogues as Potential Antifungal Agents" Molecules 23, no. 3: 520. https://doi.org/10.3390/molecules23030520
APA StyleAbonia, R., Garay, A., Castillo, J. C., Insuasty, B., Quiroga, J., Nogueras, M., Cobo, J., Butassi, E., & Zacchino, S. (2018). Design of Two Alternative Routes for the Synthesis of Naftifine and Analogues as Potential Antifungal Agents. Molecules, 23(3), 520. https://doi.org/10.3390/molecules23030520