Antiangiogenic Potential of Microbial Metabolite Elaiophylin for Targeting Tumor Angiogenesis
Abstract
:1. Introduction
2. Results
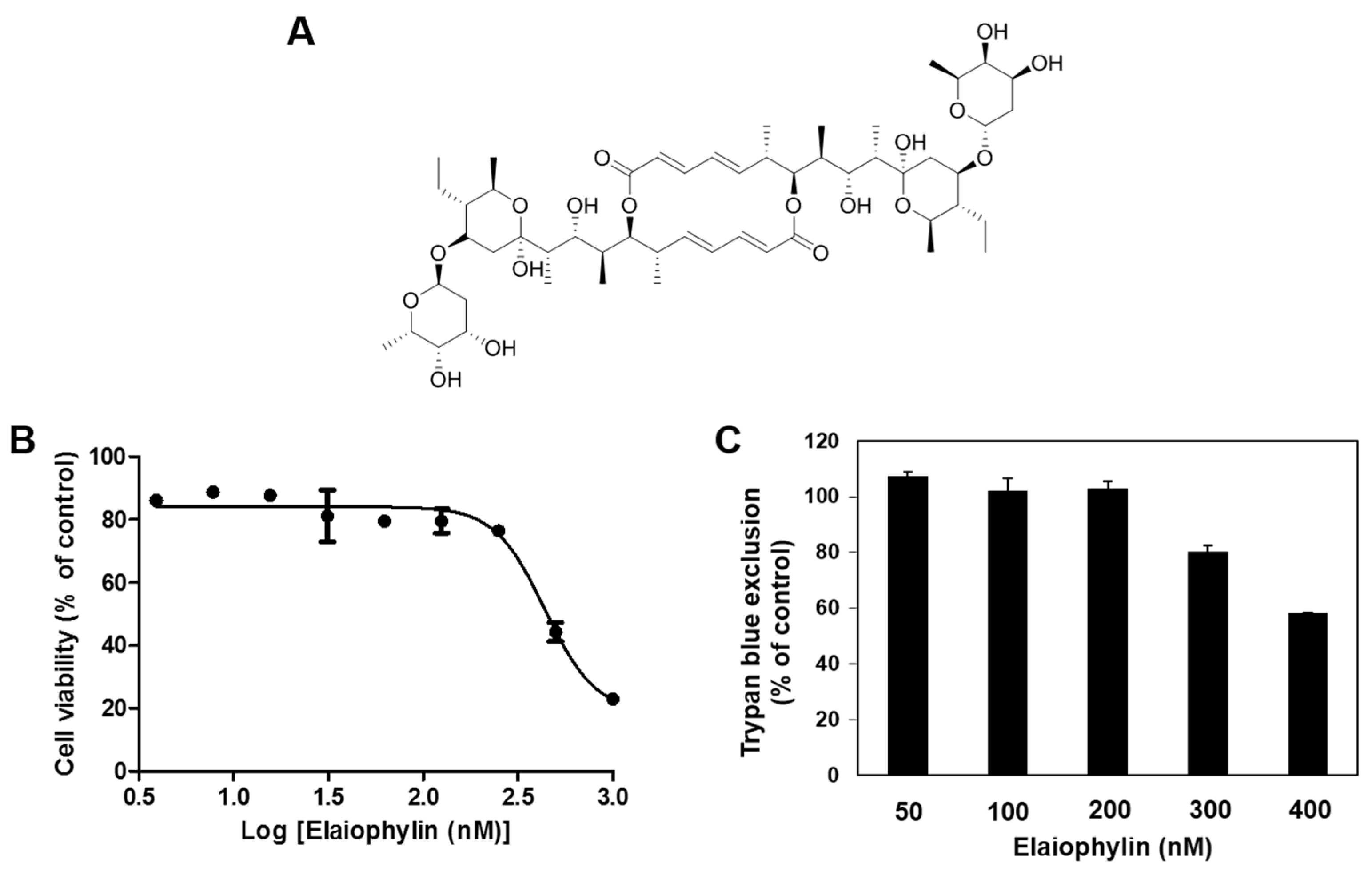
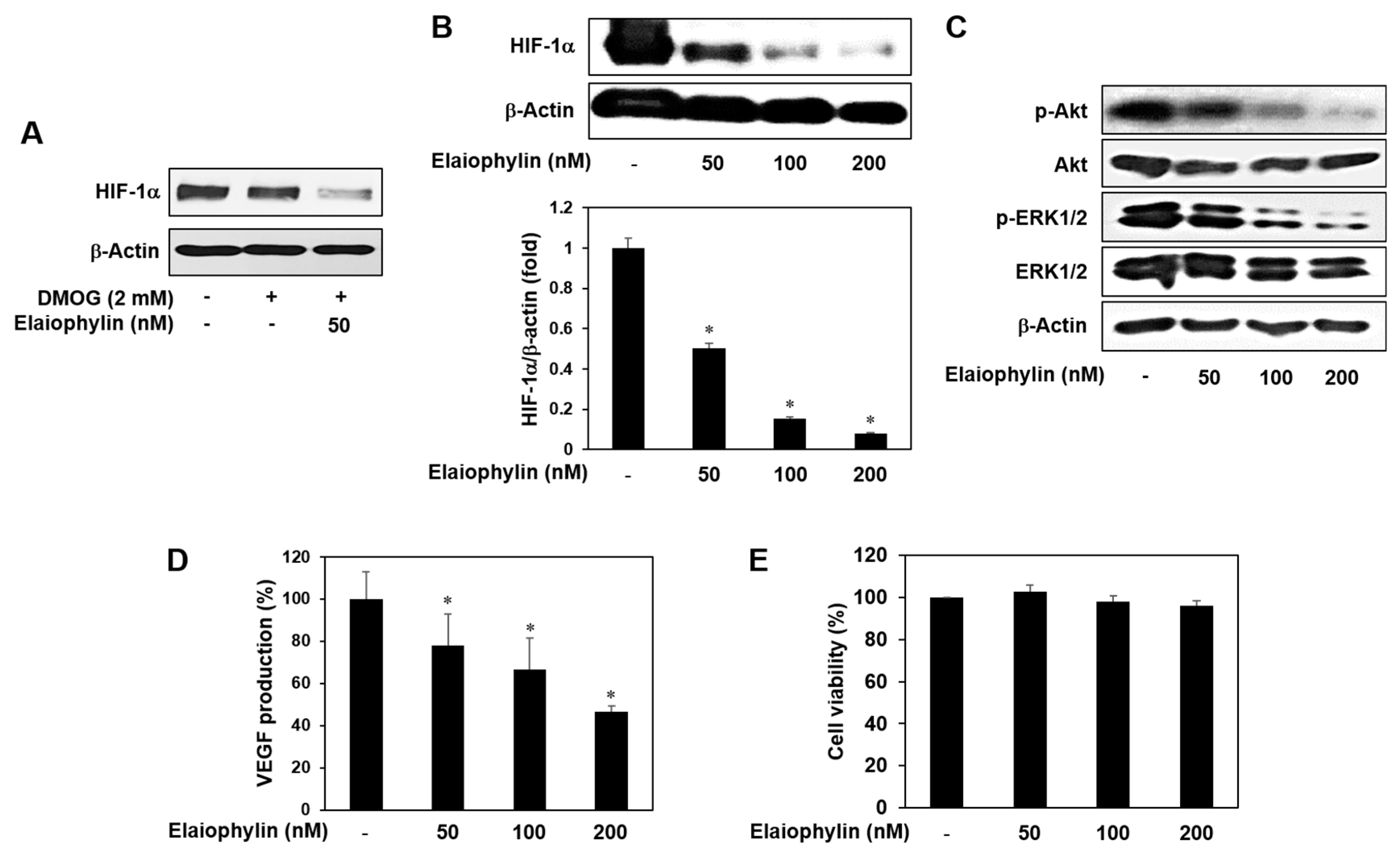
2.1. The Effect of Elaiophylin on the Viability of HUVECs
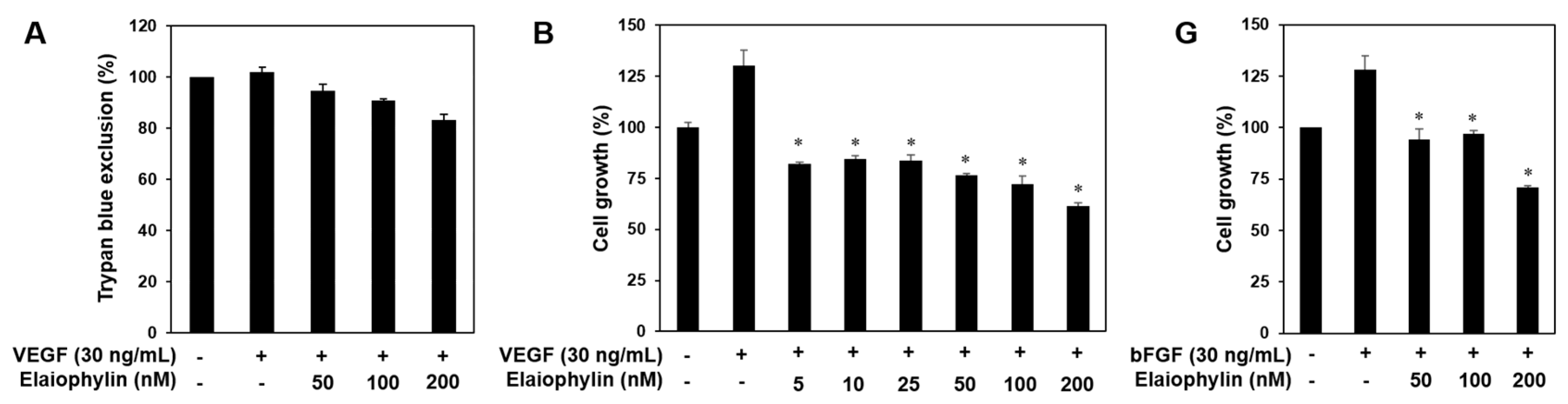
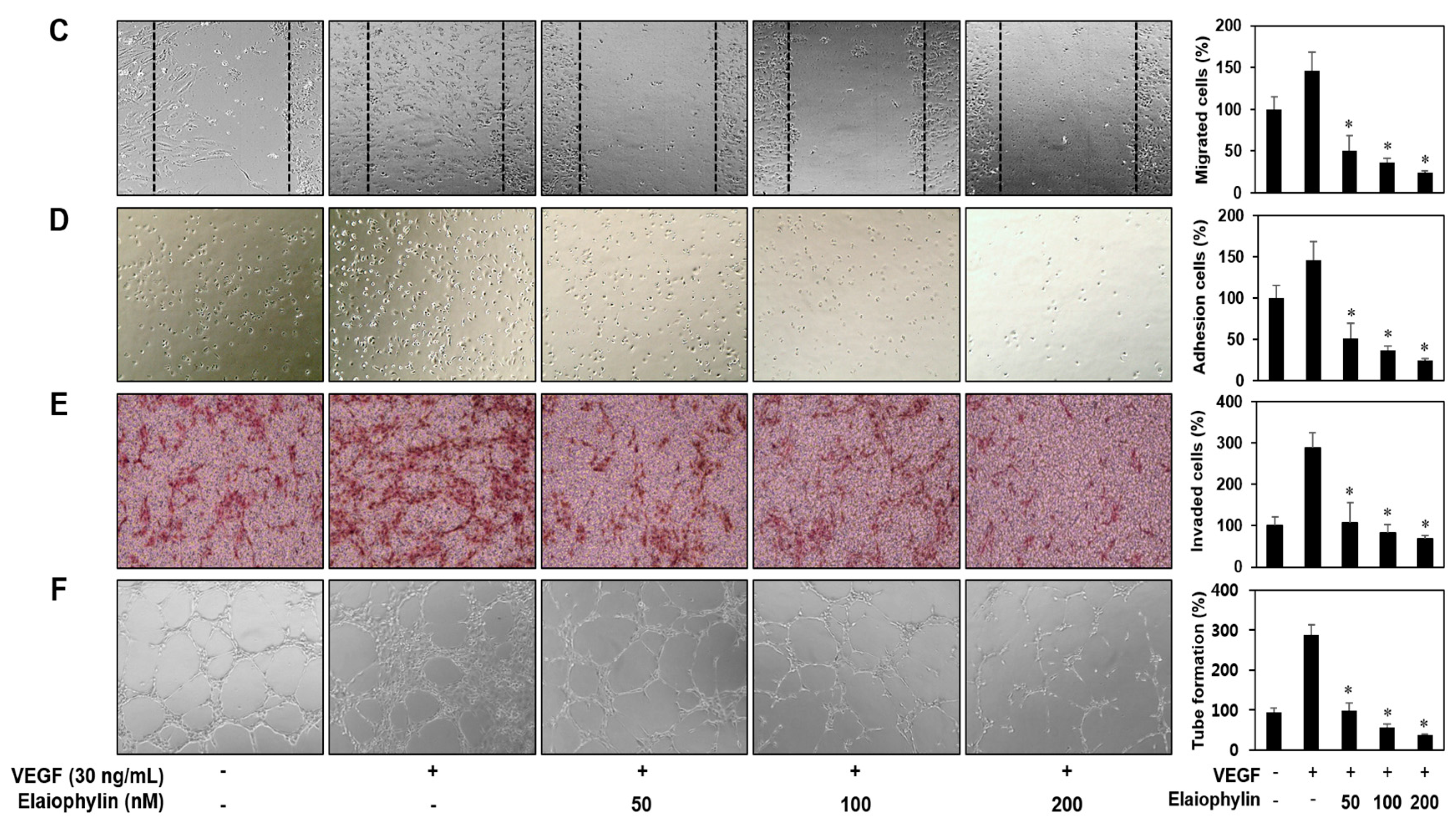
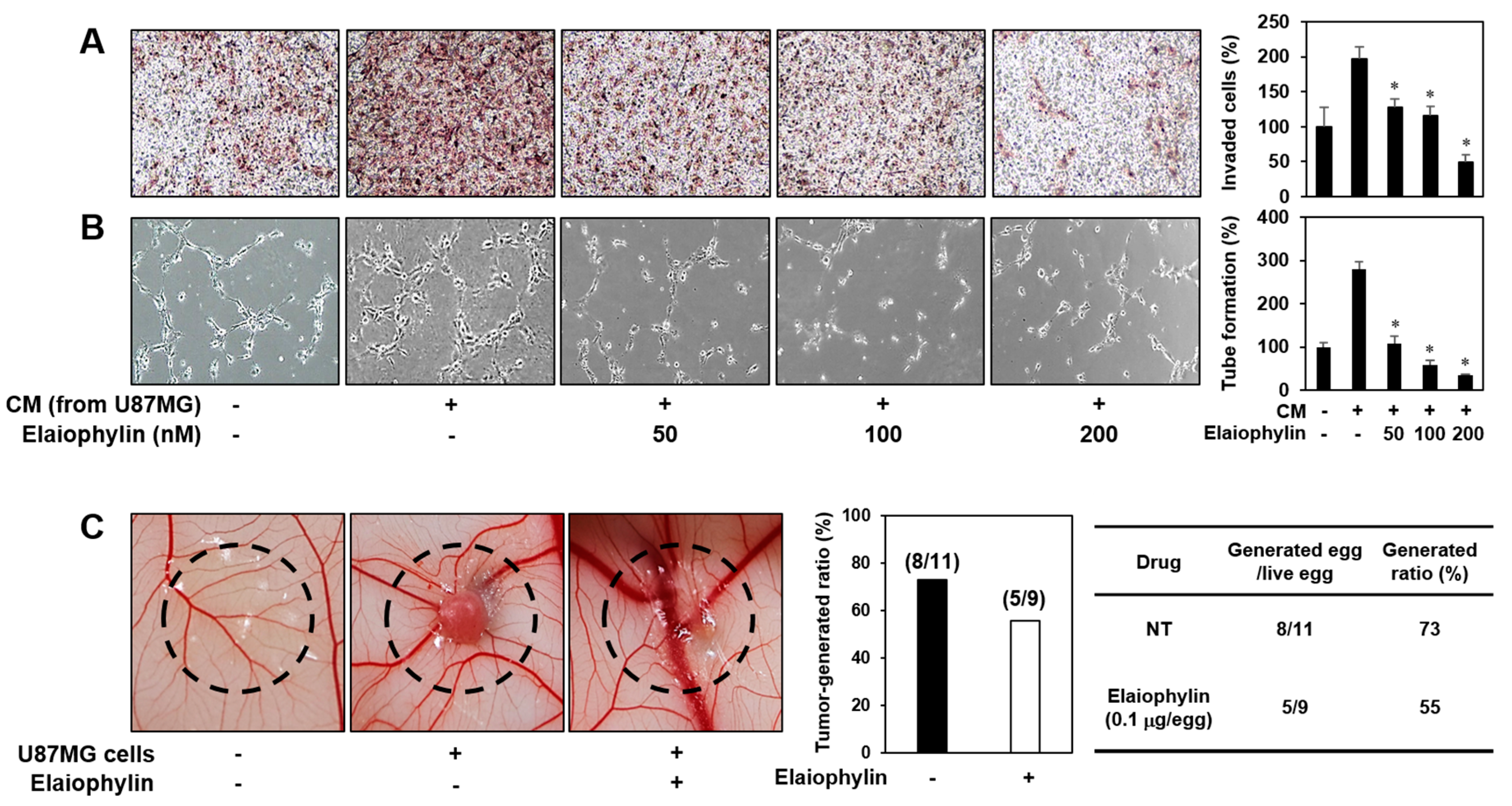
2.2. The Effect of Elaiophylin on the Angiogenesis In Vitro
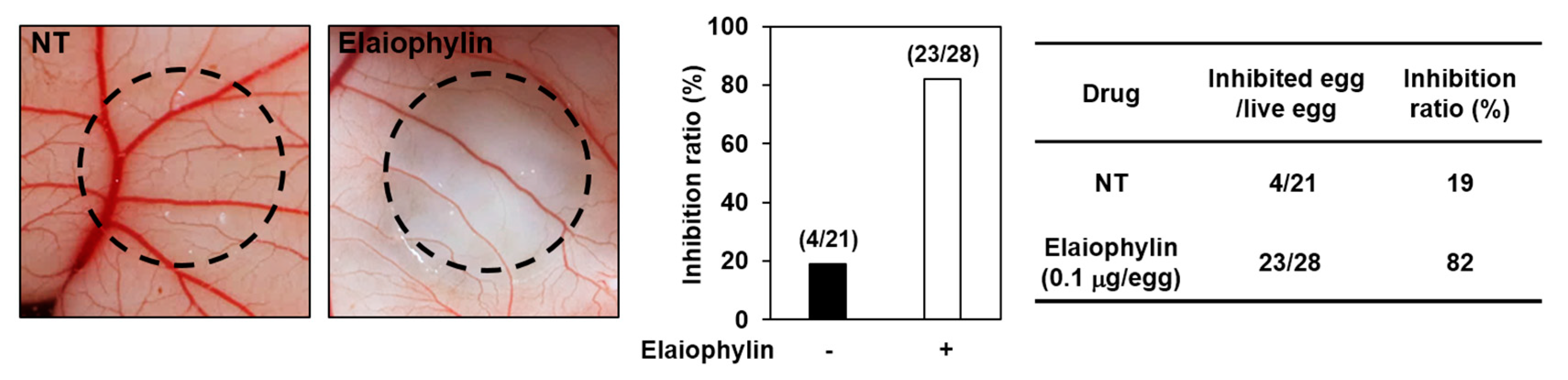
2.3. The Effect of Elaiophylin on the Angiogenesis In Vivo
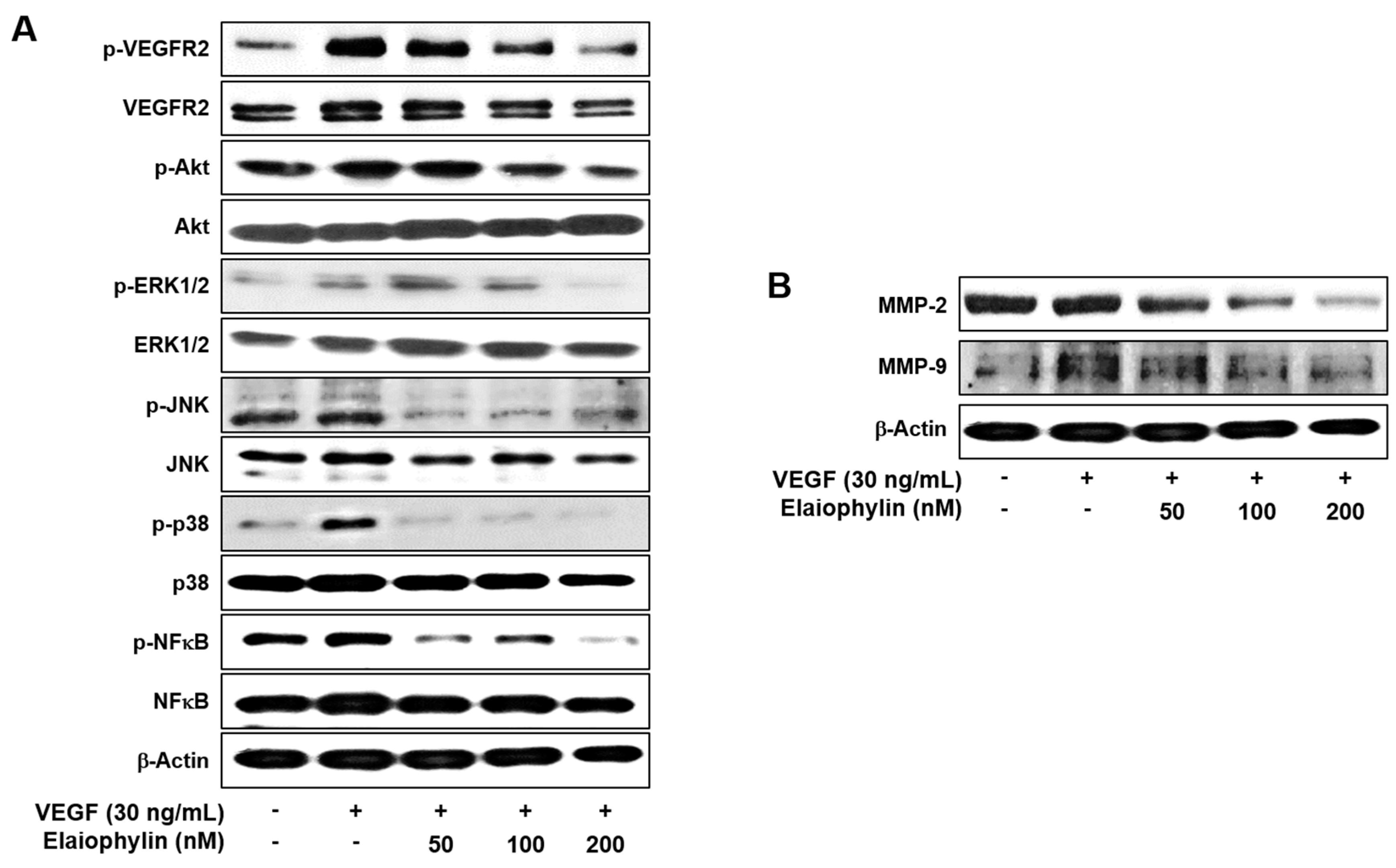
2.4. The Effect of Elaiophylin on the VEGFR2-Dependent Signaling
2.5. The Effect of Elaiophylin on the Tumor Cell-Induced Angiogenesis
2.6. The Effect of Elaiophylin on the Accumulation of HIF-1α Protein
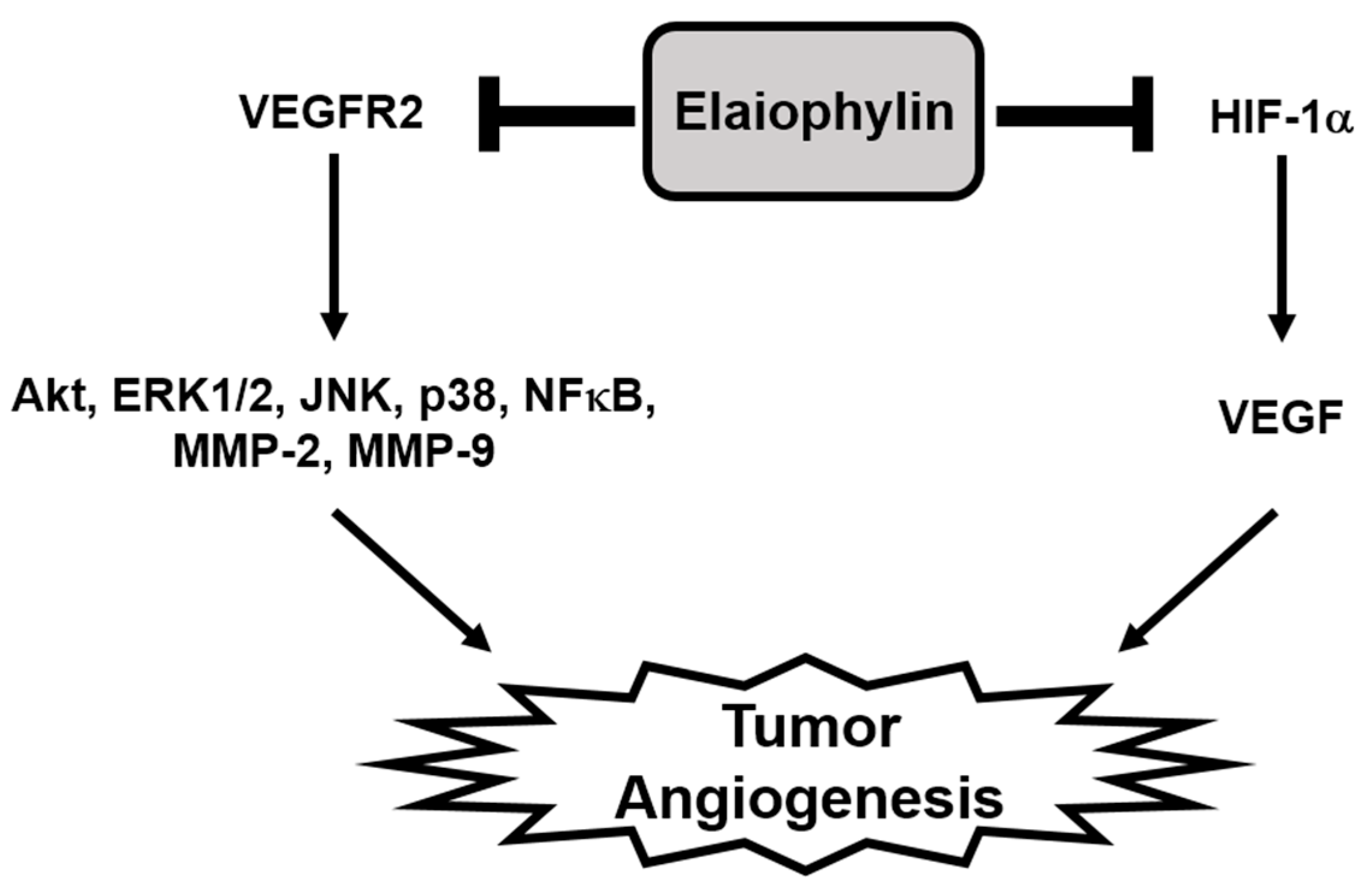
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Fermentation, Extraction and Purification of Elaiophylin
4.3. Cell Culture
4.4. Cell Viability Assay
4.5. Trypan Blue Exclusion Assay
4.6. Wound Healing Assay
4.7. Adhesion Assay
4.8. Chemoinvasion Assay
4.9. Capillary Tube Formation Assay
4.10. Chorioallantoic Membrane (CAM) Assay
4.11. Western Blot Analysis
4.12. Tumor Cell-Induced Angiogenesis Assay
4.13. Tumor Angiogenesis Assay with CAM Model
4.14. Measurement of VEGF by Enzyme-Linked Immunosorbent Assay (ELISA)
4.15. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Folkman, J. Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N. Engl. J. Med. 1995, 333, 1757–1763. [Google Scholar] [CrossRef] [PubMed]
- Risau, W. Mechanisms of angiogenesis. Nature 1997, 386, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Karamysheva, A.F. Mechanisms of angiogenesis. Biochemistry (Mosc.) 2008, 73, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Holash, J.; Wiegand, S.J.; Yancopoulos, G.D. New model of tumor angiogenesis: Dynamic balance between vessel regression and growth mediated by angiopoietins and VEGF. Oncogene 1999, 18, 5356–5362. [Google Scholar] [CrossRef] [PubMed]
- Holmes, K.; Roberts, O.L.; Thomas, A.M.; Cross, M.J. Vascular endothelial growth factor receptor-2: Structure, function, intracellular signalling and therapeutic inhibition. Cell Signal. 2007, 19, 2003–2012. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu. Rev. Cell Dev. Biol. 1999, 15, 551–578. [Google Scholar] [CrossRef] [PubMed]
- Greer, S.N.; Metcalf, J.L.; Wang, Y.; Ohh, M. The updated biology of hypoxia inducible factor. EMBO J. 2012, 31, 2448–2460. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, J.A.; Jiang, B.H.; Iyer, N.V.; Agani, F.; Leung, S.W.; Koos, R.D.; Semenza, G.L. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 1996, 16, 4604–4613. [Google Scholar] [CrossRef] [PubMed]
- Höckel, M.; Vaupel, P. Tumor hypoxia: Definitions and current clinical, biologic, and molecular aspects. J. Natl. Cancer Inst. 2001, 93, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Arai, M. Azalomycins B and F, two new antibiotics. I. Production and isolation. J. Antibiot. 1960, 13, 46–50. [Google Scholar] [PubMed]
- Ritzau, M.; Heinze, S.; Fleck, W.F.; Dahse, H.M.; Gräfe, U. New macrodiolide antibiotics, 11-O-monomethyl-and 11,11′-O-dimethylelaiophylins, from Streptomyces sp. HKI-0113 and HKI-0114. J. Nat. Prod. 1998, 61, 1337–1339. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Tan, Y.; Gan, M.; Wang, Y.; Guan, Y.; Hu, X.; Zhou, H.; Shang, X.; You, X.; Yang, Z.; et al. Identification of elaiophylin derivatives from the marine-derived actinomycete Streptomyces sp. 7-145 using PCR-based screening. J. Nat. Prod. 2013, 76, 2153–2157. [Google Scholar] [CrossRef] [PubMed]
- Gerlitz, M.; Hammann, P.; Thiericke, R.; Rohr, J. The biogenetic origin of the carbon skeleton and the oxygen atoms of elaiophylin, a symmetric macrodiolide antibiotic. J. Org. Chem. 1992, 57, 4030–4033. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, M.S.; Kim, H.S.; Kim, Y.H.; Hong, S.D.; Lee, J.J. Structure determination and biological activities of elaiophylin produced by Streptomyces sp. MCY-846. J. Microbiol. Biotechnol. 1996, 6, 245–249. [Google Scholar]
- Han, Y.; Tian, E.; Xu, D.; Ma, M.; Deng, Z.; Hong, K. Halichoblelide D, a new elaiophylin derivative with potent cytotoxic activity from mangrove-derived Streptomyces sp. 219807. Molecules 2016, 21, 970. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Woo, J.K.; Kim, D.; Kim, M.; Cho, S.K.; Kim, J.H.; Park, S.P.; Lee, H.Y.; Riu, K.Z.; Lee, D.S. Antiviral activity of methylelaiophylin, an alpha-glucosidase inhibitor. J. Microbiol. Biotechnol. 2011, 21, 263–266. [Google Scholar] [PubMed]
- Lee, S.Y.; Kim, H.S.; Kim, Y.H.; Han, S.B.; Kim, H.M.; Hong, S.D.; Lee, J.J. Immunosupressive activity of elaiophylins. J. Microbiol. Biotechnol. 1997, 7, 272–277. [Google Scholar]
- Zhao, X.; Fang, Y.; Yang, Y.; Qin, Y.; Wu, P.; Wang, T.; Lai, H.; Meng, L.; Wang, D.; Zheng, Z.; et al. Elaiophylin, a novel autophagy inhibitor, exerts antitumor activity as a single agent in ovarian cancer cells. Autophagy 2015, 11, 1849–1863. [Google Scholar] [CrossRef] [PubMed]
- Stetler-Stevenson, W.G. Matrix metalloproteinases in angiogenesis: A moving target for therapeutic intervention. J. Clin. Investig. 1999, 103, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Méndez, O.; Zavadil, J.; Esencay, M.; Lukyanov, Y.; Santovasi, D.; Wang, S.C.; Newcomb, E.W.; Zagzag, D. Knock down of HIF-1alpha in glioma cells reduces migration in vitro and invasion in vivo and impairs their ability to form tumor spheres. Mol. Cancer 2010, 9, 133. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Jung, H.J.; Seo, I.; Jha, B.K.; Suh, S.I.; Suh, M.H.; Baek, W.K. Translational suppression of HIF-1α by miconazole through the mTOR signaling pathway. Cell. Oncol. 2014, 37, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Seo, I.; Jha, B.K.; Suh, S.I.; Suh, M.H.; Baek, W.K. Minocycline inhibits angiogenesis in vitro through the translational suppression of HIF-1α. Arch. Biochem. Biophys. 2014, 545, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y. Future options of anti-angiogenic cancer therapy. Chin. J. Cancer 2016, 35, 21. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Kwon, H.J. Exploring the role of mitochondrial UQCRB in angiogenesis using small molecules. Mol. Biosyst. 2013, 9, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.P.; Jung, H.J.; Han, J.M.; Jung, N.; Kim, Y.; Kwon, H.J.; Ko, S.K.; Soung, N.K.; Jang, J.H.; Ahn, J.S. Two cyclic hexapeptides from Penicillium sp. FN070315 with antiangiogenic activities. PLoS ONE 2017, 12, e0184339. [Google Scholar] [CrossRef] [PubMed]
- Han, J.M.; Kwon, H.J.; Jung, H.J. Tricin, 4′,5,7-trihydroxy-3′,5′-dimethoxyflavone, exhibits potent antiangiogenic activity in vitro. Int. J. Oncol. 2016, 49, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.X.; Cleck, J.N. Adverse effects of anticancer agents that target the VEGF pathway. Nat. Rev. Clin. Oncol. 2009, 6, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Verheul, H.M.; Pinedo, H.M. Possible molecular mechanisms involved in the toxicity of angiogenesis inhibition. Nat. Rev. Cancer 2007, 7, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, H.P.; Wörner, W.; Zähner, H.; Kaiser, H.P.; Keller-Schierlein, W.; Müller, A. Metabolic products of microorganisms. 200 Isolation and characterization of niphithricins A, B, and elaiophylin, antibiotics produced by Streptomyces violaceoniger. J. Antibiot. 1981, 34, 1107–1118. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.K.; Dhakal, D.; Sohng, J.K. An insight into the “-omics” based engineering of streptomycetes for secondary metabolite overproduction. Biomed. Res. Int. 2013, 2013, 968518. [Google Scholar] [CrossRef] [PubMed]
- Otoguro, K.; Iwatsuki, M.; Ishiyama, A.; Namatame, M.; Nishihara-Tsukashima, A.; Sato, S.; Hatsu, M.; Iinuma, H.; Shibahara, S.; Nimura, S.; et al. In vitro and in vivo antiprotozoal activities of bispolides and their derivatives. J. Antibiot. 2010, 63, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Fang, A.; Wong, G.K.; Demain, A.L. Enhancement of the antifungal activity of rapamycin by the coproduced elaiophylin and nigericin. J. Antibiot. 2000, 53, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Grigoriev, P.A.; Schlegel, R.; Gräfe, U. Cation selective ion channels formed by macrodiolide antibiotic elaiophylin in lipid bilayer membranes. Bioelectrochemistry 2001, 54, 11–15. [Google Scholar] [CrossRef]
- Cikosová, M.; Blazsek, M.; Kubis, M.; Gajdosíková, J.; Borosová, G. Biotechnological preparation of the elaiophylin. Folia Microbiol. 2004, 49, 731–736. [Google Scholar] [CrossRef]
- Nakakoshi, M.; Kimura, K.; Nakajima, N.; Yoshihama, M.; Uramoto, M. SNA-4606-1, a new member of elaiophylins with enzyme inhibition activity against testosterone 5 alpha-reductase. J. Antibiot. 1999, 52, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, H.; Keller-Schierlein, W. Structure elucidation of elaiophylin: spectroscopy and chemical degradation. Helv. Chim. Acta 1981, 64, 407–424. [Google Scholar] [CrossRef]
- Nair, M.G.; Chandra, A.; Thorogood, D.L.; Ammermann, E.; Walker, N.; Kiehs, K.J. Gopalamicin, an antifungal macrodiolide produced by soil Actinomycetes. Agric. Food Chem. 1994, 42, 2308–2310. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, H.N.; Jang, J.-P.; Han, J.M.; Jang, J.-H.; Ahn, J.S.; Jung, H.J. Antiangiogenic Potential of Microbial Metabolite Elaiophylin for Targeting Tumor Angiogenesis. Molecules 2018, 23, 563. https://doi.org/10.3390/molecules23030563
Lim HN, Jang J-P, Han JM, Jang J-H, Ahn JS, Jung HJ. Antiangiogenic Potential of Microbial Metabolite Elaiophylin for Targeting Tumor Angiogenesis. Molecules. 2018; 23(3):563. https://doi.org/10.3390/molecules23030563
Chicago/Turabian StyleLim, Haet Nim, Jun-Pil Jang, Jang Mi Han, Jae-Hyuk Jang, Jong Seog Ahn, and Hye Jin Jung. 2018. "Antiangiogenic Potential of Microbial Metabolite Elaiophylin for Targeting Tumor Angiogenesis" Molecules 23, no. 3: 563. https://doi.org/10.3390/molecules23030563
APA StyleLim, H. N., Jang, J. -P., Han, J. M., Jang, J. -H., Ahn, J. S., & Jung, H. J. (2018). Antiangiogenic Potential of Microbial Metabolite Elaiophylin for Targeting Tumor Angiogenesis. Molecules, 23(3), 563. https://doi.org/10.3390/molecules23030563



