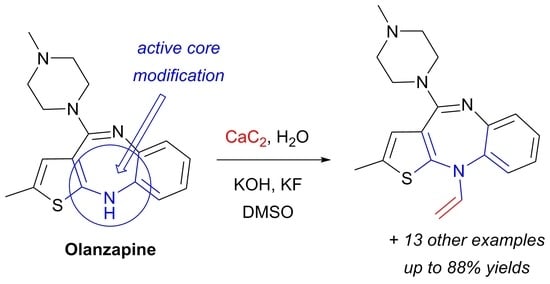
Vinylation of a Secondary Amine Core with Calcium Carbide for Efficient Post-Modification and Access to Polymeric Materials
Abstract
:1. Introduction
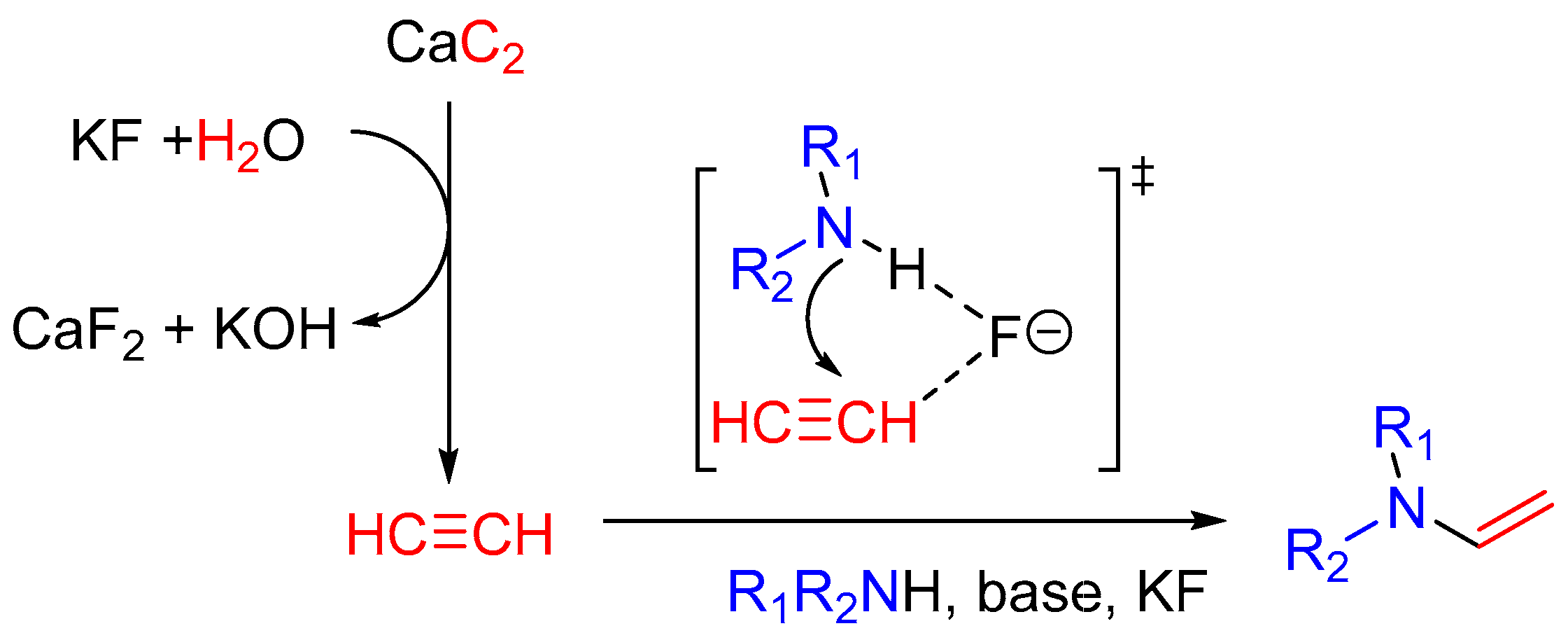
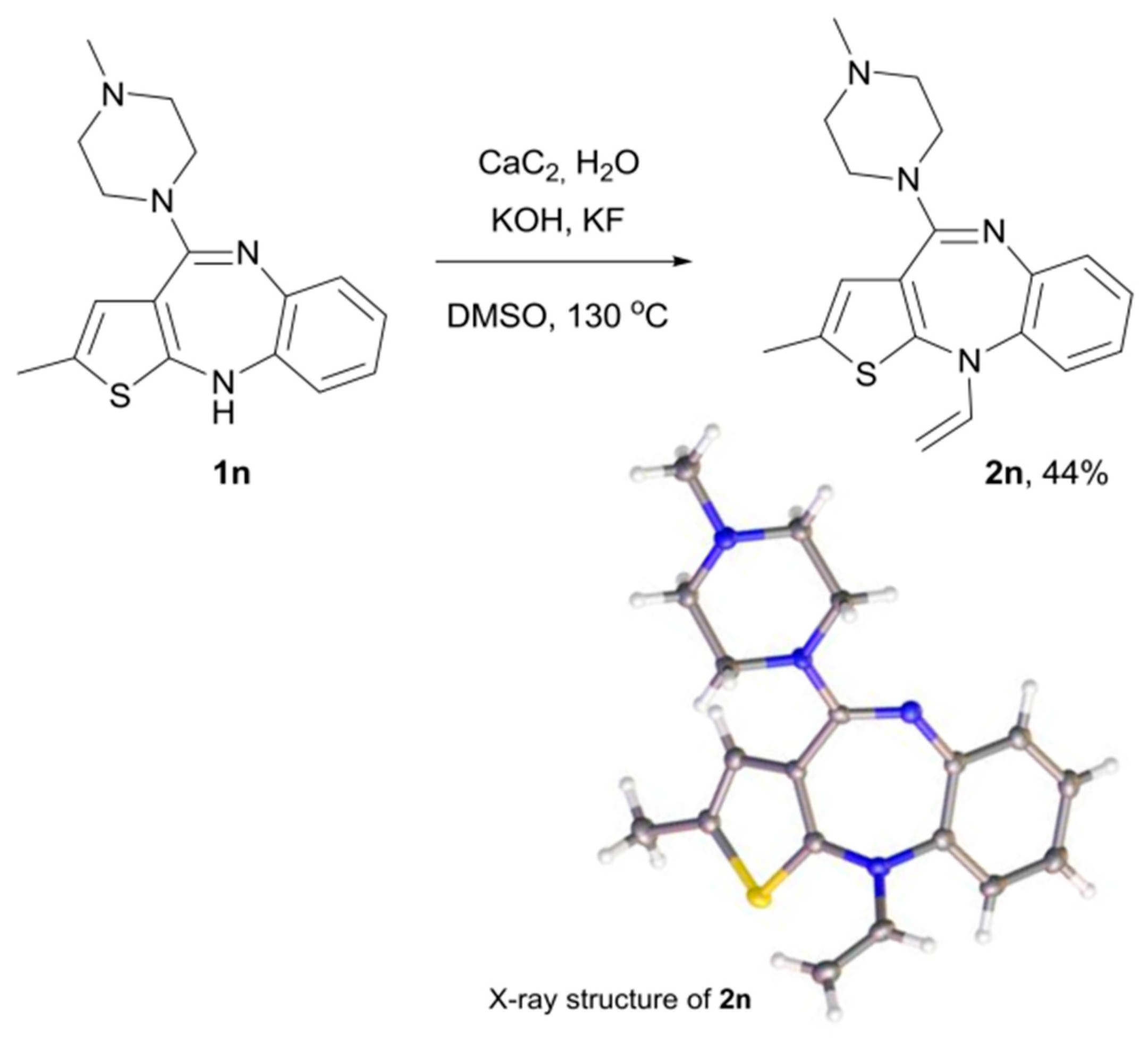
2. Results and Discussion
3. Materials and Methods
3.1. Synthetic Procedures
3.1.1. General Procedure of Vinylation
3.1.2. Experimental Procedure for the Radical Polymerization of N-vinyl-1,2,3,4-tetrahydrocarbazole (1l)
3.1.3. Experimental Procedure for the Cationic Polymerization of N-vinyl-1,2,3,4-tetrahydrocarbazole (1l)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- England, D.G.; Pawda, A. General access to the vinca and tacaman alkaloids using a Rh(II)-catalyzed cyclization/cycloaddition cascade. J. Org. Chem. 2008, 73, 2792–2802. [Google Scholar] [CrossRef] [PubMed]
- Oshiro, Y.; Shirota, Y.; Mikawa, H. Synthesis of polymers with polar side groups. III. Tricyanovinylation of poly(N-vinylindole) and N-Vinylindole—Fumaronitrile copolymer, and dielectric properties of these polymers. Polym. J. 1974, 6, 364–369. [Google Scholar] [CrossRef]
- Grazulevicius, J.V.; Strohriegl, P.; Pielichowski, J.; Pielichowski, K. Carbazole-containing polymers: Synthesis, properties and applications. Prog. Polym. Sci. 2003, 28, 1297–1353. [Google Scholar] [CrossRef]
- Hoegl, H. On photoelectric effects in polymers and their sensitization by dopants. J. Phys. Chem. 1965, 69, 755–766. [Google Scholar] [CrossRef]
- Watanabe, H.; Kanazawa, A.; Aoshima, S. Stereospecific living cationic polymerization of N-vinylcarbazole through the design of ZnCl2-derived counteranions. ACS Macro Lett. 2017, 6, 463–467. [Google Scholar] [CrossRef]
- Lyoo, W.S.; Choi, J.H.; Han, S.S.; Yoon, W.S.; Park, M.S.; Ji, B.C.; Cho, J. Preparation of organo-soluble poly[(2,2′-m-phenylene)-5,5′-bibenzimidazole] with high yield by homogeneous nitration reaction. J. Appl. Polym. Sci. 2000, 78, 438–445. [Google Scholar] [CrossRef]
- Dalpozzo, R.; De Nino, A.; Maiuolo, L.; Procopio, A.; Romeo, R.; Sindona, G. A convenient method for the synthesis of N-vinyl derivatives of nucleobases. Synthesis 2002, 2, 172–174. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Lan, J.; You, J.; Chen, L. Synthesis and biological applications of imidazolium-based polymerized ionic liquid as a gene delivery vector. Chem. Biol. Drug Des. 2009, 74, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Kizhnyaev, V.N.; Pokatilov, F.A.; Tsypina, N.A.; Ratovskii, G.V.; Vereshchagin, L.I.; Smirnov, A.I. Synthesis of N-vinyl-1,2,3-triazole derivatives. Russ. J. Org. Chem. 2002, 38, 1056–1059. [Google Scholar] [CrossRef]
- Kimura, J.; Nakamichi, S.; Ogawa, S.; Obora, Y. Iridium-catalyzed vinylation of carbazole derivatives with vinyl acetate. Synlett 2017, 28, 719–723. [Google Scholar] [CrossRef]
- Digenis, G.A.; McClanah, J.S.; Chen, P.-L. Synthesis of [2,3,4,5-14C]-1-vinyl-2-pyrrolidinone. J. Label. Compd. Radiopharm. 1992, 33, 11–17. [Google Scholar] [CrossRef]
- Arsenyan, P.; Petrenko, A.; Belyakov, S. Vinylation of pyridylcarboxamides with vinyltrimethoxysilane. Chem. Heterocycl. Compd. 2012, 47, 1527–1532. [Google Scholar] [CrossRef]
- Song, R.-J.; Deng, C.-L.; Xie, Y.-X.; Li, J.-H. Solvent-free copper/iron co-catalyzed N-arylation reactions of nitrogen-containing heterocycles with trimethoxysilanes in air. Tetrahedron Lett. 2007, 48, 7845–7848. [Google Scholar] [CrossRef]
- Han, S.-H.; Hwang, S.-H.; Kim, Y.-K.; Jung, H.-J.; Lim, J.-O.; Kim, S.-Y.; Jeong, E.-J.; Park, J.-H.; Lee, E.-Y.; Kim, K.-H.; et al. Heterocyclic Compound And Organic Light-Emitting Diode Including The Same. U.S. Patent 20150048343 A1, 19 February 2015. [Google Scholar]
- Arsenyan, P.; Petrenko, A.; Paegle, E.; Belyakov, S. Direct N- and C-vinylation with trimethoxyvinylsilane. Mendeleev Commun. 2011, 21, 326–328. [Google Scholar] [CrossRef]
- Bolshan, Y.; Batey, R.A. Copper-catalyzed cross-coupling of amides and potassium alkenyltrifluoroborate salts: A general approach to the synthesis of enamides. Tetrahedron 2010, 66, 5283–5294. [Google Scholar] [CrossRef]
- Lebedev, A.Y.; Izmer, V.V.; Kazyul’kin, D.N.; Beletskaya, I.P.; Voskoboynikov, A.Z. Palladium-catalyzed stereocontrolled vinylation of azoles and phenothiazine. Org. Lett. 2002, 4, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Clemo, G.R.; Perkin, W.H. CCXXVIII.—Vinyl derivatives especially of carbazole and tetrahydrocarbazole and their behaviour with acids. Chem. Soc. Trans. 1924, 125, 1804–1814. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Mason, R.; Bu, X.R.; Harrison, J. Combined phase transfer catalysis and ultrasound to enhance tandem alkylation of azo dyes. Tetrahedron 2002, 58, 3747–3753. [Google Scholar] [CrossRef]
- Abele, E.; Dzenitis, O.; Rubina, K.; Lukevics, E. Synthesis of N- and S-vinyl derivatives of heteroaromatic compounds using phase-transfer catalysis. Chem. Heterocycl. Compd. 2002, 38, 682–685. [Google Scholar] [CrossRef]
- Weber, W.P.; Gokil, G.W. Phase-Transfer Catalysis in Organic Synthesis, 1st ed.; Springer: Berlin, Germany, 1977; ISBN 978-3-642-46357-0. [Google Scholar]
- Iddon, B.; Khan, N.; Lim, B.L. Azoles. Part 4. Nucleophilic substitution reactions of halogenoimidazoles. J. Chem. Soc. Perkin Trans. 1 1987, 1437–1443. [Google Scholar] [CrossRef]
- Zakaryan, G.B.; Hayotsyan, S.S.; Attaryan, H.S.; Hasratyan, G.V. Dehydrochlorination of 1-(2-chloroethyl)azoles in aqueous solution of N-methylmorpholine N-oxide. Russ. J. Gen. Chem. 2016, 86, 414–416. [Google Scholar] [CrossRef]
- Veeravagu, P.; Arnold, R.T.; Eigenmann, E.W. Competitive elimination-substitution reactions. Some dramatic differences between bromides and tosylates. J. Am. Chem. Soc. 1964, 86, 3072–3075. [Google Scholar] [CrossRef]
- Huber, T.; Kaiser, D.; Rickmeier, J.; Magauer, T. Experimental studies on the selective β-C–H halogenation of enones. J. Org. Chem. 2015, 80, 2281–2294. [Google Scholar] [CrossRef] [PubMed]
- Hanley, P.S.; Hartwig, J.F. Intermolecular migratory insertion of unactivated olefins into palladium–nitrogen bonds. Steric and electronic effects on the rate of migratory insertion. J. Am. Chem. Soc. 2011, 133, 15661–15673. [Google Scholar] [CrossRef] [PubMed]
- Bian, J.; Li, X.; Wang, N.; Wu, X.; You, Q.; Zhang, X. Discovery of quinone-directed antitumor agents selectively bioactivated by NQO1 over CPR with improved safety profile. Eur. J. Med. Chem. 2017, 129, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Reppe, W.; Keyssner, E. Production of N-vinyl Compounds. U.S. Patent 2,066,160, 29 December 1936. [Google Scholar]
- Shmidt, E.Y.; Protsuk, N.I.; Vasil’tsov, A.M.; Ivanov, A.V.; Mikhaleva, A.I.; Trofimov, B.A. Improved method for the synthesis of 1-vinylindole. Chem. Heterocycl. Compd. 2013, 49, 404–407. [Google Scholar] [CrossRef]
- Lin, J.W.-P. Synthesis, characterization, and polymerization of N-vinylarylamines. J. Polym. Sci. Polym. Chem. Ed. 1979, 17, 3797–3810. [Google Scholar] [CrossRef]
- Schobert, H. Production of acetylene and acetylene-based chemicals from coal. Chem. Rev. 2014, 114, 1743–1760. [Google Scholar] [CrossRef] [PubMed]
- Rodygin, K.S.; Werner, G.; Kucherov, F.A.; Ananikov, V.P. Calcium carbide: A unique reagent for organic synthesis and nanotechnology. Chem. Asian J. 2016, 11, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Teong, S.P.; Lim, J.; Zhang, Y. Vinylation of aryl ether (lignin β-O-4 linkage) and epoxides with calcium carbide through C-O Bond cleavage. ChemSusChem 2017, 10, 3198–3201. [Google Scholar] [CrossRef] [PubMed]
- Ledovskaya, M.S.; Rodygin, K.S.; Ananikov, V.P. Calcium-mediated one-pot preparation of isoxazoles with deuterium incorporation. Org. Chem. Front. 2018, 5, 226–231. [Google Scholar] [CrossRef]
- Hosseni, A.; Pilevar, A.; Hogan, E.; Mogwitz, B.; Schulze, A.S.; Schreiner, P.R. Calcium carbide catalytically activated with tetra-n-butyl ammonium fluoride for Sonogashira cross coupling reactions. Org. Biomol. Chem. 2017, 15, 6800–6807. [Google Scholar] [CrossRef] [PubMed]
- Rodygin, K.S.; Ananikov, V.P. An efficient metal-free pathway to vinyl thioesters with calcium carbide as the acetylene source. Green Chem. 2016, 18, 482–486. [Google Scholar] [CrossRef]
- Rodygin, K.S.; Kostin, A.A.; Ananikov, V.P. Calcium carbide as a convenient acetylene source in the synthesis of unsaturated sulfides, promising functionalized monomers. Mendeleev Commun. 2015, 25, 415–416. [Google Scholar] [CrossRef]
- Werner, G.; Rodygin, K.S.; Kostin, A.A.; Gordeev, E.G.; Kashin, A.S.; Ananikov, V.P. A solid acetylene reagent with enhanced reactivity: Fluoride-mediated functionalization of alcohols and phenols. Green Chem. 2017, 19, 3032–3041. [Google Scholar] [CrossRef]
- Teong, S.P.; Chua, A.Y.H.; Deng, S.; Li, X.; Zhang, Y. Direct vinylation of natural alcohols and derivatives with calcium carbide. Green Chem. 2017, 19, 1659–1662. [Google Scholar] [CrossRef]
- Rodygin, K.S.; Werner, I.; Ananikov, V.P. A green and sustainable route to carbohydrate vinyl ethers for accessing bioinspired materials with a unique microspherical morphology. ChemSusChem 2018, 11, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Rattanangkool, E.; Vilaivan, T.; Sukwattanasinitt, M.; Wacharasindhu, S. An atom-economic approach for vinylation of indoles and phenols using calcium carbide as acetylene surrogate. Eur. J. Org. Chem. 2016, 25, 4347–4353. [Google Scholar] [CrossRef]
- Reppe, W. Vinylierung. Liebigs Ann. 1956, 601, 81–138. [Google Scholar] [CrossRef]
- Hegedus, L.S.; Winton, P.M.; Sudarsanan, V. Palladium-assisted N-alkylation of indoles: Attempted application to polycyclization. J. Org. Chem. 1981, 46, 2215–2221. [Google Scholar] [CrossRef]
- Natansohn, A. Two-dimensional NMR spectra of poly(N-vinylcarbazole). J. Polym. Sci. A 1989, 27, 4257–4265. [Google Scholar] [CrossRef]
- Bymaster, F.P.; Nelson, D.L.; Delapp, N.W.; Falcone, J.F.; Eckols, K.; Truex, L.L.; Foreman, M.M. Antagonism by olanzapine of dopamine D1, serotonin2, muscarinic, histamine H1 and α1-adrenergic receptors in vitro. Schizophr. Res. 1999, 37, 107–122. [Google Scholar] [CrossRef]
- Marazziti, D.; Piccinni, A.; Baroni, S.; Mungai, F.; Presta, S.; Mucci, F.; Dell’Osso, L. Current trends on antipsychotics: Focus on asenapine. Curr. Med. Chem. 2016, 23, 2204–2216. [Google Scholar] [CrossRef] [PubMed]
- A Process for Preparing N-vinyl Compounds. FR Patent 801519, 6 August 1936.
Sample Availability: Samples of the compounds are not available from the authors. |




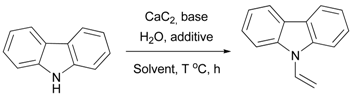
| Entry | Solvent | Base + Additive, equiv. | CaC2, equiv. | Water, equiv. | T, °C | Time, h | Yield, % 2 |
|---|---|---|---|---|---|---|---|
| 1 | Hexane | KOH (1.1), KF (1) | 2 | 4 | 130 | 4 | trace |
| 2 | Toluene | KOH (1.1), KF (1) | 2 | 4 | 130 | 4 | trace |
| 3 | DMSO | KOH (1.1), KF (1) | 2 | 4 | 130 | 8 | 38 |
| 4 3 | DMSO | KOH (1.1), KF (1) | 2 | 4 | 130 | 4 | 80 |
| 5 | DMSO | KOH (1.1), KF (1) | 2 | 8 | 130 | 4 | 62 |
| 6 | DMSO | KOH (1.1), KF (1) | 4 | 8 | 130 | 4 | 57 |
| 7 | DMSO | KF (1) | 2 | 4 | 130 | 4 | 54 |
| 8 | DMSO | KOH (1.1) | 2 | 4 | 130 | 4 | 25 |
| 9 4 | DMSO | KOH (1.1), KF (1) | 2 | 4 | 130 | 4 | 88 |
| 10 | DMSO | KOH (1.1), KF (1) | 2 | 4 | 110 | 4 | 66 |
| 11 | DMSO | KOH (1.1), KF (1) | 2 | 4 | 150 | 4 | 50 |
| 12 | DMSO | KOH (1.5), KF (1.5) | 2 | 4 | 130 | 4 | 52 |
| 13 5 | DMSO | KOH, KF | 2 | - | 130 | 4 | trace |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodygin, K.S.; Bogachenkov, A.S.; Ananikov, V.P. Vinylation of a Secondary Amine Core with Calcium Carbide for Efficient Post-Modification and Access to Polymeric Materials. Molecules 2018, 23, 648. https://doi.org/10.3390/molecules23030648
Rodygin KS, Bogachenkov AS, Ananikov VP. Vinylation of a Secondary Amine Core with Calcium Carbide for Efficient Post-Modification and Access to Polymeric Materials. Molecules. 2018; 23(3):648. https://doi.org/10.3390/molecules23030648
Chicago/Turabian StyleRodygin, Konstantin S., Alexander S. Bogachenkov, and Valentine P. Ananikov. 2018. "Vinylation of a Secondary Amine Core with Calcium Carbide for Efficient Post-Modification and Access to Polymeric Materials" Molecules 23, no. 3: 648. https://doi.org/10.3390/molecules23030648
APA StyleRodygin, K. S., Bogachenkov, A. S., & Ananikov, V. P. (2018). Vinylation of a Secondary Amine Core with Calcium Carbide for Efficient Post-Modification and Access to Polymeric Materials. Molecules, 23(3), 648. https://doi.org/10.3390/molecules23030648