Therapeutic Perspectives of 8-Prenylnaringenin, a Potent Phytoestrogen from Hops
Abstract
:1. Introduction
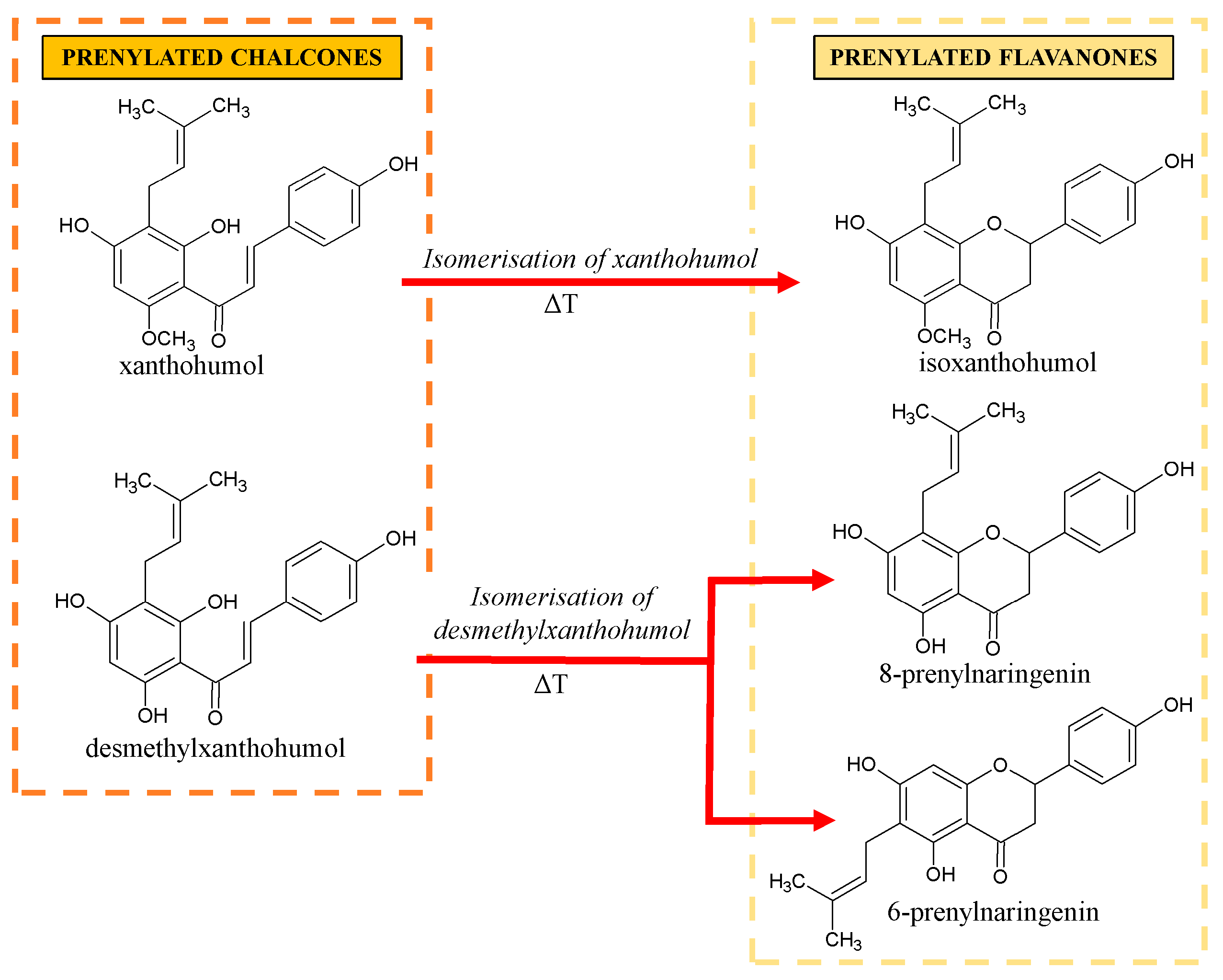
2. Prenylated Flavonoids
3. Mechanism of Action, Pharmacokinetics, and Biotransformation
4. Menopause Therapy
5. Prevention of Osteoporosis
6. Anticancer and Other Miscellaneous Effects of 8-PN
7. Safety Issues
8. Conclusions
Acknowledgments
Conflicts of Interest
References
- Zanoli, P.; Zavatti, M. Pharmacognostic and pharmacological profile of Humulus lupulus L. J. Ethnopharmacol. 2008, 116, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Karabin, M.; Hudcova, T.; Jelinek, L.; Dostalek, P. Biologically active compounds from hops and prospects for their use. Compr. Rev. Food Sci. Food Saf. 2016, 15, 542–567. [Google Scholar] [CrossRef]
- Poluzzi, E.; Piccinni, C.; Raschi, E.; Rampa, A.; Recanatini, M.; De Ponti, F. Phytoestrogens in postmenopause: The state of the art from a chemical, pharmacological and regulatory perspective. Curr. Med. Chem. 2014, 21, 417–436. [Google Scholar] [CrossRef] [PubMed]
- Bartmanska, A.; Tronina, T.; Poplonski, J.; Huszcza, E. Biotransformations of prenylated hop flavonoids for drug discovery and production. Curr. Drug Metab. 2013, 14, 1083–1097. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, L.; Pauli, G.; Farnsworth, N. The pharmacognosy of Humulus lupulus L. (hops) with an emphasis on estrogenic properties. Phytomedicine 2006, 13, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Bolego, C.; Poli, A.; Cignarella, A.; Paoletti, R. Phytoestrogens: Pharmacological and therapeutic perspectives. Curr. Drug Targets 2003, 4, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Ososki, A.L.; Kennelly, E.J. Phytoestrogens: A review of the present state of research. Phytother. Res. 2003, 17, 845–869. [Google Scholar] [CrossRef] [PubMed]
- Terao, J.; Mukai, R. Prenylation modulates the bioavailability and bioaccumulation of dietary flavonoids. Arch. Biochem. Biophys. 2014, 559, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Ohn Mar, S.; Malhi, F.S.; Syed Rahim, S.H.; Soe, M.M. Chinese and Indian women's experience with alternative medications for menopause related symptoms: A qualitative analysis. Chin. J. Integr. Med. 2017, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.D.; Manson, J.E.; Rossouw, J.E.; Siscovick, D.S.; Mouton, C.P.; Rifai, N.; Wallace, R.B.; Jackson, R.D.; Pettinger, M.B.; Ridker, P.M. Inflammatory biomarkers, hormone replacement therapy, and incident coronary heart disease: Prospective analysis from the women's health initiative observational study. JAMA 2002, 288, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Rossouw, J.E.; Prentice, R.L.; Manson, J.E.; Wu, L.; Barad, D.; Barnabei, V.M.; Ko, M.; LaCroix, A.Z.; Margolis, K.L.; Stefanick, M.L. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007, 297, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Folsom, A.R.; Anderson, J.P.; Ross, J.A. Estrogen replacement therapy and ovarian cancer. Epidemiology 2004, 15, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Heyerick, A.; Vervarcke, S.; Depypere, H.; Bracke, M.; De Keukeleire, D. A first prospective, randomized, double-blind, placebo-controlled study on the use of a standardized hop extract to alleviate menopausal discomforts. Maturitas 2006, 54, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Aghamiri, V.; Mirghafourvand, M.; Mohammad-Alizadeh-Charandabi, S.; Nazemiyeh, H. The effect of hop (Humulus lupulus L.) on early menopausal symptoms and hot flashes: A randomized placebo-controlled trial. Complement. Ther. Clin. Pract. 2016, 23, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Erkkola, R.; Vervarcke, S.; Vansteelandt, S.; Rompotti, P.; De Keukeleire, D.; Heyerick, A. A randomized, double-blind, placebo-controlled, cross-over pilot study on the use of a standardized hop extract to alleviate menopausal discomforts. Phytomedicine 2010, 17, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Van Duursen, M.B.; Smeets, E.E.; Rijk, J.C.; Nijmeijer, S.M.; van den Berg, M. Phytoestrogens in menopausal supplements induce ER-dependent cell proliferation and overcome breast cancer treatment in an in vitro breast cancer model. Toxicol. Appl. Pharmacol. 2013, 269, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Venturelli, S.; Burkard, M.; Biendl, M.; Lauer, U.M.; Frank, J.; Busch, C. Prenylated chalcones and flavonoids for the prevention and treatment of cancer. Nutrition 2016, 32, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Stompor, M.; Uram, L.; Podgorski, R. In vitro effect of 8-prenylnaringenin and naringenin on fibroblasts and glioblastoma cells-cellular accumulation and cytotoxicity. Molecules 2017, 22, 1092. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.S.; Choi, H.S.; Choi, H.S.; Choi, Y.K.; Um, J.Y.; Choi, I.; Shin, Y.C.; Ko, S.G. Phytoestrogens induce apoptosis via extrinsic pathway, inhibiting nuclear factor-κB signaling in HER2-overexpressing breast cancer cells. Anticancer Res. 2011, 31, 3301–3313. [Google Scholar] [PubMed]
- Busch, C.; Noor, S.; Leischner, C.; Burkard, M.; Lauer, U.M.; Venturelli, S. Anti-proliferative activity of hop-derived prenylflavonoids against human cancer cell lines. Wien. Med. Wochenschr. 2015, 165, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Keiler, A.M.; Helle, J.; Bader, M.I.; Ehrhardt, T.; Nestler, K.; Kretzschmar, G.; Bernhardt, R.; Vollmer, G.; Nikolic, D.; Bolton, J.L.; et al. A standardized Humulus lupulus (L.) ethanol extract partially prevents ovariectomy-induced bone loss in the rat without induction of adverse effects in the uterus. Phytomedicine 2017, 34, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Żołnierczyk, A.K.; Mączka, W.K.; Grabarczyk, M.; Wińska, K.; Woźniak, E.; Anioł, M. Isoxanthohumol—Biologically active hop flavonoid. Fitoterapia 2015, 103, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Karabin, M.; Hudcova, T.; Jelinek, L.; Dostalek, P. Biotransformations and biological activities of hop flavonoids. Biotechnol. Adv. 2015, 33, 1063–1090. [Google Scholar] [CrossRef] [PubMed]
- Karabin, M.; Hudcova, T. Význam chmelových prenylflavonoidů pro lidské zdraví. Chem. Listy 2012, 106, 1095–1103. [Google Scholar]
- Nikolic, D.; van Breemen, R.B. Analytical methods for quantitation of prenylated flavonoids from hops. Curr. Anal. Chem. 2013, 9, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.F.; Taylor, A.W.; Deinzer, M.L. Quantitative analysis of xanthohumol and related prenylflavonoids in hops and beer by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 1999, 832, 97–107. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, Y.; Yang, J.; He, J.; Sun, J.; Chen, F.; Zhang, M.; Yang, B. Prenylated flavonoids, promising nutraceuticals with impressive biological activities. Trends Food Sci. Technol. 2015, 44, 93–104. [Google Scholar] [CrossRef]
- Milligan, S.R. Reproductive and estrogenic effects of 8-prenylnaringenin in hops. In Beer in Health and Disease Prevention; Preedy, V.R., Ed.; Elsevier: San Diego, CA, USA, 2008; pp. 711–723. [Google Scholar]
- Koch, W.; Heim, G. Estrogens in hops and beer; preliminary report. Munch. Med. Wochenschr. 1953, 95, 845. [Google Scholar] [PubMed]
- Milligan, S.; Kalita, J.; Heyerick, A.; Rong, H.; De Cooman, L.; De Keukeleire, D. Identification of a potent phytoestrogen in hops (Humulus lupulus L.) and beer. J. Clin. Endocrinol. Metab. 1999, 84, 2249. [Google Scholar] [CrossRef] [PubMed]
- Rietjens, I.M.; Louisse, J.; Beekmann, K. The potential health effects of dietary phytoestrogens. Br. J. Pharmacol. 2017, 174, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.O. Overview of in vitro tools to assess the estrogenic and antiestrogenic activity of phytoestrogens. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2002, 777, 155–165. [Google Scholar] [CrossRef]
- Gruber, C.J.; Tschugguel, W.; Schneeberger, C.; Huber, J.C. Production and actions of estrogens. N. Engl. J. Med. 2002, 346, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Farzaneh, S.; Zarghi, A. Estrogen receptor ligands: A review (2013–2015). Sci. Pharm. 2016, 84, 409. [Google Scholar] [CrossRef] [PubMed]
- Simons, R.; Gruppen, H.; Bovee, T.F.; Verbruggen, M.A.; Vincken, J.-P. Prenylated isoflavonoids from plants as selective estrogen receptor modulators (phytoSERMs). Food Funct. 2012, 3, 810–827. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.F.; Page, J.E. Xanthohumol and related prenylflavonoids from hops and beer: To your good health! Phytochemistry 2004, 65, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, H.D.; Shih, E.; Thacker, H.L. ERAAs for menopause treatment: Welcome the ‘designer estrogens’. Clevel. Clin. J. Med. 2017, 84, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Hadji, P. The evolution of selective estrogen receptor modulators in osteoporosis therapy. Climacteric 2012, 15, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.; Besselink, E.; Henning, S.; Go, V.; Heber, D. Phytoestrogens induce differential estrogen receptor alpha-or beta-mediated responses in transfected breast cancer cells. Exp. Biol. Med. 2005, 230, 558–568. [Google Scholar] [CrossRef]
- Roelens, F.; Heldring, N.; Dhooge, W.; Bengtsson, M.; Comhaire, F.; Gustafsson, J.A.; Treuter, E.; De Keukeleire, D. Subtle side-chain modifications of the hop phytoestrogen 8-prenylnaringenin result in distinct agonist/antagonist activity profiles for estrogen receptors α and β. J. Med. Chem. 2006, 49, 7357–7365. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, G.; Zierau, O.; Wober, J.; Tischer, S.; Metz, P.; Vollmer, G. Prenylation has a compound specific effect on the estrogenicity of naringenin and genistein. J. Steroid Biochem. Mol. Biol. 2010, 118, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mukai, R. Prenylation enhances the biological activity of dietary flavonoids by altering their bioavailability. Biosci. Biotechnol. Biochem. 2018, 82, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.W.; Cooney, J.; Jensen, D.; Li, Y.; Paxton, J.W.; Birch, N.P.; Scheepens, A. Hop-derived prenylflavonoids are substrates and inhibitors of the efflux transporter breast cancer resistance protein (BCRP/ABCG2). Mol. Nutr. Food Res. 2014, 58, 2099–2110. [Google Scholar] [CrossRef] [PubMed]
- Rad, M.; Hümpel, M.; Schaefer, O.; Schoemaker, R.; Schleuning, W.D.; Cohen, A.; Burggraaf, J. Pharmacokinetics and systemic endocrine effects of the phyto-oestrogen 8-prenylnaringenin after single oral doses to postmenopausal women. Br. J. Clin. Pharmacol. 2006, 62, 288–296. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, M.B. Pharmacokinetic and pharmacologic variation between different estrogen products. J. Clin. Pharmacol. 1995, 35, 18S–24S. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Castro, L.A.; Burkard, M.; Sus, N.; Scheubeck, G.; Leischner, C.; Lauer, U.M.; Bosy-Westphal, A.; Hund, V.; Busch, C.; Venturelli, S. The oral bioavailability of 8-prenylnaringenin from hops (Humulus lupulus L.) in healthy women and men is significantly higher than that of its positional isomer 6-prenylnaringenin in a randomized crossover trial. Mol. Nutr. Food Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Van Breemen, R.B.; Yuan, Y.; Banuvar, S.; Shulman, L.P.; Qiu, X.; Alvarenga, R.F.R.; Chen, S.N.; Dietz, B.M.; Bolton, J.L.; Pauli, G.F. Pharmacokinetics of prenylated hop phenols in women following oral administration of a standardized extract of hops. Mol. Nutr. Food Res. 2014, 58, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, D.; Li, Y.; Chadwick, L.R.; Grubjesic, S.; Schwab, P.; Metz, P.; Van Breemen, R.B. Metabolism of 8-prenylnaringenin, a potent phytoestrogen from hops (Humulus lupulus), by human liver microsomes. Drug Metab. Dispos. 2004, 32, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, S.-H.; Kang, B.Y.; Lee, I.-S. Microbial metabolites of 8-prenylnaringenin, an estrogenic prenylflavanone. Arch. Pharm. Res. 2008, 31, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Possemiers, S.; Bolca, S.; Grootaert, C.; Heyerick, A.; Decroos, K.; Dhooge, W.; De Keukeleire, D.; Rabot, S.; Verstraete, W.; Van de Wiele, T. The prenylflavonoid isoxanthohumol from hops (Humulus lupulus L.) is activated into the potent phytoestrogen 8-prenylnaringenin in vitro and in the human intestine. J. Nutr. 2006, 136, 1862–1867. [Google Scholar] [CrossRef] [PubMed]
- Abdi, F.; Mobedi, H.; Roozbeh, N. Hops for menopausal vasomotor symptoms: Mechanisms of action. J. Menopausal Med. 2016, 22, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Ramírez, B.A.; Lamuela-Raventós, R.M.; Estruch, R.; Sasot, G.; Doménech, M.; Tresserra-Rimbau, A. Beer polyphenols and menopause: Effects and mechanisms—A review of current knowledge. Oxid. Med. Cell. Longev. 2017, 2017, 4749131. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.R.; Franks, R.B.; Fox, C. Review of efficacy of complementary and alternative medicine treatments for menopausal symptoms. J. Midwifery Womens Health 2017, 62, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Abdi, F.; Mobedi, H.; Mosaffa, N.; Dolatian, M.; Ramezani Tehrani, F. Hormone therapy for relieving postmenopausal vasomotor symptoms: A systematic review. Arch. Iran. Med. 2016, 19, 141–146. [Google Scholar] [PubMed]
- Miller, V.M.; Harman, S.M. An update on hormone therapy in postmenopausal women: Mini-review for the basic scientist. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H1013–H1021. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.D. The evidence base for HRT: What can we believe? Climacteric 2017, 20, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Lobo, R.A.; Pickar, J.H.; Stevenson, J.C.; Mack, W.J.; Hodis, H.N. Back to the future: Hormone replacement therapy as part of a prevention strategy for women at the onset of menopause. Atherosclerosis 2016, 254, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M. Complementary and alternative approaches to menopause. Endocrinol. Metab. Clin. N. Am. 2015, 44, 619–648. [Google Scholar] [CrossRef] [PubMed]
- Tonob, D.; Melby, M.K. Broadening our perspectives on complementary and alternative medicine for menopause: A narrative review. Maturitas 2017, 99, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Locklear, T.D.; Doyle, B.J.; Perez, A.L.; Wicks, S.M.; Mahady, G.B. Menopause in Latin America: Symptoms, attitudes, treatments and future directions in Costa Rica. Maturitas 2017, 104, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Depypere, H.T.; Comhaire, F.H. Herbal preparations for the menopause: Beyond isoflavones and black cohosh. Maturitas 2014, 77, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Bowe, J.; Li, X.F.; Kinsey-Jones, J.; Heyerick, A.; Brain, S.; Milligan, S.; O’Byrne, K. The hop phytoestrogen, 8-prenylnaringenin, reverses the ovariectomy-induced rise in skin temperature in an animal model of menopausal hot flushes. J. Endocrinol. 2006, 191, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, L.A.; Potthoff, P.; Schneider, H.P. International versions of the menopause rating scale (MRS). Health Qual. Life Outcomes 2003, 1, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keiler, A.M.; Zierau, O.; Kretzschmar, G. Hop extracts and hop substances in treatment of menopausal complaints. Planta Med. 2013, 79, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Effenberger, K.E.; Johnsen, S.A.; Monroe, D.G.; Spelsberg, T.C.; Westendorf, J.J. Regulation of osteoblastic phenotype and gene expression by hop-derived phytoestrogens. J. Steroid Biochem. Mol. Biol. 2005, 96, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Humpel, M.; Isaksson, P.; Schaefer, O.; Kaufmann, U.; Ciana, P.; Maggi, A.; Schleuning, W.D. Tissue specificity of 8-prenylnaringenin: Protection from ovariectomy induced bone loss with minimal trophic effects on the uterus. J. Steroid Biochem. Mol. Biol. 2005, 97, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Ming, L.G.; Lv, X.; Ma, X.N.; Ge, B.F.; Zhen, P.; Song, P.; Zhou, J.; Ma, H.P.; Xian, C.J.; Chen, K.M. The prenyl group contributes to activities of phytoestrogen 8-prenynaringenin in enhancing bone formation and inhibiting bone resorption in vitro. Endocrinology 2013, 154, 1202–1214. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Kang, L.; Ma, Y.; Chen, H.; Kuang, H.; Huang, Q.; He, M.; Peng, W. Effects and mechanisms of 8-prenylnaringenin on osteoblast MC3T3-E1 and osteoclast-like cells RAW264.7. Food Sci. Nutr. 2014, 2, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Hemachandra, L.; Madhubhani, P.; Chandrasena, R.; Esala, P.; Chen, S.-N.; Main, M.; Lankin, D.C.; Scism, R.A.; Dietz, B.M.; Pauli, G.F. Hops (Humulus lupulus) inhibits oxidative estrogen metabolism and estrogen-induced malignant transformation in human mammary epithelial cells (MCF-10A). Cancer Prev. Res. 2012, 5, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Dunlap, T.L.; Howell, C.E.; Mbachu, O.C.; Rue, E.A.; Phansalkar, R.; Chen, S.N.; Pauli, G.F.; Dietz, B.M.; Bolton, J.L. Hop (Humulus lupulus L.) extract and 6-prenylnaringenin induce P450 1A1 catalyzed estrogen 2-hydroxylation. Chem. Res. Toxicol. 2016, 29, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Rodrigues, I.; Guardao, L.; Rocha-Rodrigues, S.; Silva, C.; Magalhaes, J.; Ferreira-de-Almeida, M.; Negrao, R.; Soares, R. Xanthohumol and 8-prenylnaringenin ameliorate diabetic-related metabolic dysfunctions in mice. J. Nutr. Biochem. 2017, 45, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Fugh-Berman, A. “Bust enhancing” herbal products. Obstet. Gynecol. 2003, 101, 1345–1349. [Google Scholar] [PubMed]
- Di Vito, C.; Bertoni, A.; Nalin, M.; Sampietro, S.; Zanfa, M.; Sinigaglia, F. The phytoestrogen 8-prenylnaringenin inhibits agonist-dependent activation of human platelets. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 1724–1733. [Google Scholar] [CrossRef] [PubMed]
- Myasoedova, V.A.; Kirichenko, T.V.; Melnichenko, A.A.; Orekhova, V.A.; Ravani, A.; Poggio, P.; Sobenin, I.A.; Bobryshev, Y.V.; Orekhov, A.N. Anti-atherosclerotic effects of a phytoestrogen-rich herbal preparation in postmenopausal women. Int. J. Mol. Sci. 2016, 17, 1318. [Google Scholar] [CrossRef] [PubMed]
- De Cremoux, P.; This, P.; Leclercq, G.; Jacquot, Y. Controversies concerning the use of phytoestrogens in menopause management: Bioavailability and metabolism. Maturitas 2010, 65, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, G.; De Cremoux, P.; This, P.; Jacquot, Y. Lack of sufficient information on the specificity and selectivity of commercial phytoestrogens preparations for therapeutic purposes. Maturitas 2011, 68, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Keiler, A.M.; Dorfelt, P.; Chatterjee, N.; Helle, J.; Bader, M.I.; Vollmer, G.; Kretzschmar, G.; Kuhlee, F.; Thieme, D.; Zierau, O. Assessment of the effects of naringenin-type flavanones in uterus and vagina. J. Steroid Biochem. Mol. Biol. 2015, 145, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Helle, J.; Kraker, K.; Bader, M.I.; Keiler, A.M.; Zierau, O.; Vollmer, G.; Welsh, J.; Kretzschmar, G. Assessment of the proliferative capacity of the flavanones 8-prenylnaringenin, 6-(1.1-dimethylallyl)naringenin and naringenin in MCF-7 cells and the rat mammary gland. Mol. Cell. Endocrinol. 2014, 392, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Keiler, A.M.; Macejova, D.; Dietz, B.M.; Bolton, J.L.; Pauli, G.F.; Chen, S.N.; van Breemen, R.B.; Nikolic, D.; Goerl, F.; Muders, M.H.; et al. Evaluation of estrogenic potency of a standardized hops extract on mammary gland biology and on MNU-induced mammary tumor growth in rats. J. Steroid Biochem. Mol. Biol. 2017, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Bolca, S.; Li, J.; Nikolic, D.; Roche, N.; Blondeel, P.; Possemiers, S.; De Keukeleire, D.; Bracke, M.; Heyerick, A.; Van Breemen, R.B. Disposition of hop prenylflavonoids in human breast tissue. Mol. Nutr. Food Res. 2010, 54, S284–S294. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, G.; Jacquot, Y. Interactions of isoflavones and other plant derived estrogens with estrogen receptors for prevention and treatment of breast cancer-considerations concerning related efficacy and safety. J. Steroid Biochem. Mol. Biol. 2014, 139, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Van Duursen, M.B. Modulation of estrogen synthesis and metabolism by phytoestrogens in vitro and the implications for women’s health. Toxicol. Res. 2017, 6, 772–794. [Google Scholar] [CrossRef]
- Dietz, B.M.; Hajirahimkhan, A.; Dunlap, T.L.; Bolton, J.L. Botanicals and their bioactive phytochemicals for women’s health. Pharmacol. Rev. 2016, 68, 1026–1073. [Google Scholar] [CrossRef] [PubMed]
- Solak, K.A.; Santos, R.R.; van den Berg, M.; Blaauboer, B.J.; Roelen, B.A.; van Duursen, M.B. Naringenin (NAR) and 8-prenylnaringenin (8-PN) reduce the developmental competence of porcine oocytes in vitro. Reprod. Toxicol. 2014, 49, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Qiu, X.; Nikolic, D.; Chen, S.N.; Huang, K.; Li, G.; Pauli, G.F.; van Breemen, R.B. Inhibition of human cytochrome P450 enzymes by hops (Humulus lupulus) and hop prenylphenols. Eur. J. Pharm. Sci. 2014, 53, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Lynch, T.; Price, A. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am. Fam. Phys. 2007, 76, 391–396. [Google Scholar]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Štulíková, K.; Karabín, M.; Nešpor, J.; Dostálek, P. Therapeutic Perspectives of 8-Prenylnaringenin, a Potent Phytoestrogen from Hops. Molecules 2018, 23, 660. https://doi.org/10.3390/molecules23030660
Štulíková K, Karabín M, Nešpor J, Dostálek P. Therapeutic Perspectives of 8-Prenylnaringenin, a Potent Phytoestrogen from Hops. Molecules. 2018; 23(3):660. https://doi.org/10.3390/molecules23030660
Chicago/Turabian StyleŠtulíková, Kateřina, Marcel Karabín, Jakub Nešpor, and Pavel Dostálek. 2018. "Therapeutic Perspectives of 8-Prenylnaringenin, a Potent Phytoestrogen from Hops" Molecules 23, no. 3: 660. https://doi.org/10.3390/molecules23030660
APA StyleŠtulíková, K., Karabín, M., Nešpor, J., & Dostálek, P. (2018). Therapeutic Perspectives of 8-Prenylnaringenin, a Potent Phytoestrogen from Hops. Molecules, 23(3), 660. https://doi.org/10.3390/molecules23030660





