Expression, Distribution, and Role of C-Type Lectin Receptors in the Human and Animal Middle Ear and Eustachian Tube: A Review
Abstract
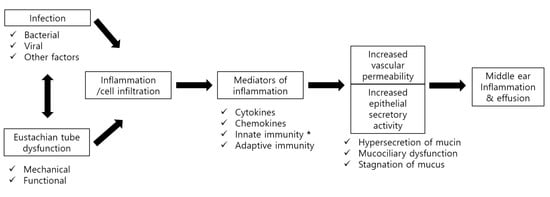
:1. Introduction
2. Classification of Lectin Families
3. Innate Immunity and C-Type Lectin Receptors (CLRs)
3.1. Innate Immunity and Pattern Recognition Receptors (PRRs)
3.2. C-Type Lectin Receptors (CLRs)
4. Types and Functions of CLRs Expressed in the Middle Ear and Eustachian Tube
4.1. Expression of CLRs in the Middle Ear and Eustachian Tube
4.2. Pathophysiologic Roles of CLRs Expressed in the Middle Ear and Eustachian Tubes
4.2.1. Mannose-Binding Lectin (MBL, Also Called Mannan-Binding Lectin)
4.2.2. Surfactant Proteins (SP)-A and -D
4.2.3. Type I Transmembrane CLRs
4.2.4. Type II Transmembrane CLRs Associated with OM
4.2.5. Selectin
4.2.6. Additional Experiments
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bluestone, C.D.; Klein, J.O. Otitis media and eustachian tube dysfunction. In Pediatric Otolaryngology, 4th ed.; Bluestone, C.D., Stool, S.E., Alper, C.M., Eds.; Saunders: Philadelphia, PA, USA, 2003; pp. 474–685. [Google Scholar]
- Teele, D.W.; Klein, J.O.; Rosner, B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: A prospective cohort study. J. Infect. Dis. 1989, 160, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Igarashi, M.; Jenkins, H.A. Volumetric analysis of the tympanic isthmus in human temporal bones. Arch. Otolaryngol. Head Neck Surg. 1987, 113, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Paparella, M.M.; Kim, C.S.; Goycoolea, M.V.; Giebink, S. Pathogenesis of otits media. Ann. Otol. Rhinol. Laryngol. 1977, 86, 481–492. [Google Scholar] [PubMed]
- Paparrella, M.M.; Schachern, P.A.; Yoon, T.H.; Abdelhammid, M.M.; Sahni, R.; da Costa, S.S. Otopathologic correlates of the continuum of otitis media. Ann. Otol. Rhinol. Laryngol. 1990, 148, 17–22. [Google Scholar] [CrossRef]
- Yilmaz, M.; Goksu, N.; Bayramoglu, I.; Bayazit, Y.A. Practical use of MENSA in atelectatic ears and adhesive otitis media. ORL 2006, 68, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Rovers, M.M.; Schilder, A.G.; Zielhuis, G.A.; Rosenfeld, R.M. Otitis media. Lancet 2004, 363, 465–473. [Google Scholar] [CrossRef]
- Rutishauser, U.; Sachs, L. Cell-to-cell binding induced by different lectins. J. Cell Biol. 1975, 65, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Matthew, B.; Marshall, K.; Calli, L.; Li, C.L.; Michael, Y.; Corinne, S.; Ashish, S.; Anna, S.M.; Reza, Z.; Damon, P.E.; et al. Lectin-Dependent Enhancement of Ebola Virus Infection via Soluble and Transmembrane C-type Lectin Receptors. PLoS ONE 2013, 8, e60838. [Google Scholar]
- Vigerust, D.J.; Shepherd, V.L. Virus glycosylation: Role in virulence and immune interactions. Trends Microbiol. 2007, 15, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Drickamer, K.; Taylor, M.E. Evolving views of protein glycosylation. Trends Biochem. Sci. 1998, 23, 321–324. [Google Scholar] [CrossRef]
- Varki1, A.; Angata, T. Siglec—The major subfamily of I-type lectins. Glycobiology 2006, 16, 1R–27R. [Google Scholar] [CrossRef] [PubMed]
- Guspa, G.S. Lectin: An overview. In Animal Lectins: Form, Function and Clinical Applications, 2012th ed.; Guspa, G.S., Ed.; Springer: Vienna, Austria, 2012; Chapter 1; pp. 3–25. [Google Scholar]
- Maverakis, E.; Kim, K.; Shimoda, M.; Gershwin, M.E.; Wilken, R.; Raychaudhuri, S.; Ruhaak, L.R.; Lebrilla, C.B. Glycans in the Immune system and The Altered Glycan Theory of Autoimmunity: A Critical Review. J. Autoimmun. 2015, 57, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004, 5, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Willart, M.A.; Deswarte, K.; Pouliot, P.; Braun, H.; Beyaert, R.; Lambrecht, B.N.; Hammad, H. Interleukin-1a controls allergic sensitization to inhaled house dust mite via the epithelial release of GM-CSF and IL-33. J. Exp. Med. 2012, 209, 1505–1517. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Kim, Y.I.; Lee, J.W.; Byun, J.Y.; Park, M.S.; Yeo, S.G. Decreased expression of TLR-9 and cytokines in the presence of bacteria in patients with otitis media with effusion. Clin. Exp. Otorhinolaryngol. 2013, 6, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Kigerl, K.A.; de Rivero Vaccari, J.P.; Dietrich, W.D.; Popovich, P.G.; Keane, R.W. Pattern recognition receptors and central nervous system repair. Exp. Neurol. 2014, 258, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Lepenies, B.; Lee, J.; Sonkaria, S. Targeting C-type lectin receptors with multivalent carbohydrate ligands. Adv. Drug Deliv. Rev. 2013, 65, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Drickmaer, K.; Taylor, M.E. Recent insights into structures and functions of C-type lectins in the immune system. Curr. Opin. Struct. Biol. 2015, 34, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Figdor, C.G.; van Kooyk, Y.; Adema, G.J. C-type lectin receptors on dendritic cells and Langerhans cells. Nat. Rev. Immunol. 2002, 2, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Van Asbeck Hoepelman, A.I.; Scharringa, J.; Herpers, B.L.; Verhoef, J. Mannose binding lectin plays a crucial role in innate immunity against yeast by enhanced complement activation and enhanced uptake of polymorphonuclear cells. BMC Microbiol. 2008, 18, 1471–2180. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Han, S.H.; Byun, J.Y.; Park, M.S.; Kim, Y.I.; Yeo, S.G. Expression of C-type lectin receptor mRNA in chronic otitis media with cholesteatoma. Acta Otolaryngol. 2017, 137, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Guo, X.; Olszewski, E.; Fan, Z.; Ai, Y.; Han, Y.; Xu, L.; Li, J.; Wang, H. Expression of Surfactant Protein-A during LPS-Induced Otitis Media with Effusion in Mice. Otolaryngol. Head Neck Surg. 2015, 153, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.G.; Park, D.C.; Oh, I.H.; Kim, Y.I.; Choi, S.A.; Jung, S.Y.; Kang, H.M.; Yeo, S.G. Increased expression of Dec-205, Bcl-10, Tim-3, and Trem-1 mRNA in chronic otitis media with cholesteatoma. Acta Otolaryngol. 2014, 134, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, D.C.; Oh, I.W.; Kim, Y.I.; Kim, J.B.; Yeo, S.G. C-type lectin receptors mRNA expression in patients with otitis media with effusion. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 1846–1851. [Google Scholar] [CrossRef] [PubMed]
- Pospiech, L.; Jaworska, M.; Kubacka, M. Soluble L-selectin and interleukin-8 in otitis media with effusion. Auris Nasus Larynx 2000, 27, 213–217. [Google Scholar] [CrossRef]
- Himi, T.; Kamimura, M.; Kataura, A.; Imai, K. Quantitative analysis of soluble cell adhesion molecules in otitis media with effusion. Acta Otolaryngol. 1994, 114, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Garred, P.; Brygge, K.; Sørensen, C.H.; Madsen, H.O.; Thiel, S.; Svejgaard, A. Mannan-binding protein-levels in plasma and upper-airways secretions and frequency of genotypes in children with recurrence of otitis media. Clin. Exp. Immunol. 1993, 94, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Konishi, M.; Nishitani, C.; Mitsuzawa, H.; Shimizu, T.; Sano, H.; Harimaya, A.; Fujii, N.; Himi, T.; Kuroki, Y. Alloiococcus otitidis is a ligand for collectins and Toll-like receptor 2, and its phagocytosis is enhanced by collectins. Eur. J. Immunol. 2006, 36, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, M.; Himi, T.; Kataura, A. Cell adhesion molecules of experimental otits media in the rat. Acta Otolaryngol. 1996, 116, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Tomaiuolo, R.; Ruocco, A.; Salapete, C.; Carru, C.; Baggio, G.; Franceschi, C.; Zinellu, A.; Vaupel, J.; Bellia, C.; Lo Sasso, B.; et al. Activity of mannose-binding lectin (MBL) in centenarians. Aging Cell 2012, 11, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Ogden, C.A.; de Cathelineau, A.; Hoffmann, P.R.; Bratton, D.; Ghebrehiwet, B.; Fadok, V.A.; Henson, P.M. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J. Exp. Med. 2001, 194, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Stuart, L.M.; Takahashi, K.; Shi, L.; Savill, J.; Ezekowitz, R.A. Mannose-binding lectin-deficient mice display defective apoptotic cell clearance but no autoimmune phenotype. J. Immunol. 2005, 174, 3220–3226. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Olinger, G.G.; Aris, S.; Chen, Y.; Gewurz, H.; Spear, G.T. Mannose-binding lectin binds to Ebola and Marburg envelope glycoproteins, resulting in blocking of virus interaction with DC-SIGN and complement-mediated virus neutralization. J. Gen. Virol. 2005, 86, 2535–2542. [Google Scholar] [CrossRef] [PubMed]
- Oberley, R.E.; Snyder, J.M. Recombinant human SP-A1 and SP-A2 proteins have different carbohydrate-binding characteristics. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 284, L871–L881. [Google Scholar] [CrossRef] [PubMed]
- Uemura, T.; Sano, H.; Katoh, T.; Nishitani, C.; Mitsuzawa, H.; Shimizu, T.; Kuroki, Y. Surfactant protein A without the interruption of Gly-X-Y repeats loses a kink of oligomeric structure and exhibits impaired phospholipid liposome aggregation ability. Biochemistry 2006, 45, 14543–14551. [Google Scholar] [CrossRef] [PubMed]
- Palaniyar, N.; Ridsdale, R.A.; Possmayer, F.; Harauz, G. Surfactant protein A (SP-A) forms a novel supraquaternary structure in the form of fibers. Biochem. Biophys. Res. Commun. 1998, 250, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Kostina, E.; Ofek, I.; Crouch, E.; Friedman, R.; Sirota, L.; Klinger, G.; Sahly, H.; Keisari, Y. Noncapsulated Klebsiella pneumoniae bearing mannose-containing O antigens is rapidly eradicated from mouse lung and triggers cytokine production by macrophages following opsonization with surfactant protein D. Infect. Immun. 2005, 73, 8282–8290. [Google Scholar] [CrossRef] [PubMed]
- Hawgood, S.; Brown, C.; Edmondson, J.; Stumbaugh, A.; Allen, L.; Goerke, J.; Clark, H.; Poulain, F. Pulmonary collectins modulate strain-specific influenza a virus infection and host responses. J. Virol. 2004, 78, 8565–8572. [Google Scholar] [CrossRef] [PubMed]
- Chiba, H.; Pattanajitvilai, S.; Evans, A.J.; Harbeck, R.J.; Voelker, D.R. Human surfactant protein D (SP-D) binds Mycoplasma pneumoniae by high affinity interactions with lipids. J. Biol. Chem. 2002, 277, 20379–20385. [Google Scholar] [CrossRef] [PubMed]
- Szolnoky, G.; Bata-Csörgö, Z.; Kenderessy, A.S.; Kiss, M.; Pivarcsi, A.; Novák, Z.; Nagy Newman, K.; Michel, G.; Ruzicka, T.; Maródi, L.; et al. A mannose-binding receptor is expressed on human keratinocytes and mediates killing of Candida albicans. J. Investig. Dermatol. 2001, 117, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, H.; Yarwood, H.; Ashworth, A.; Isacke, C.M. Endo180, an endocytic recycling glycoprotein related to the macrophage mannose receptor is expressed on fibroblasts, endothelial cells and macrophages and functions as a lectin receptor. J. Cell Sci. 2000, 113, 1021–1032. [Google Scholar] [PubMed]
- Umut, G.; Marcela, R.; Sonali, S.; Sigrid, H.; Imran, H.; Simon, J.; Gordon, D.B.; David, L.W.; Philip, R.T.; Luisa, M.P. Fungal Recognition Enhances Mannose Receptor Shedding through Dectin-1 Engagement. J. Biol. Chem. 2011, 286, 7822–7829. [Google Scholar]
- Gazi, U.; Martinez-Pormares, L. Influence of the mannose receptor in host immune responses. Immunobiology 2009, 214, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Stahl, P.D.; Ezekowitz, R.A. The mannose receptor is a pattern recognition receptor involved in host defense. Curr. Opin. Immunol. 1998, 10, 50–55. [Google Scholar] [CrossRef]
- East, L.; Isacke, C.M. The mannose receptor family. Biochim. Biophys. Acta 2002, 1572, 364–386. [Google Scholar] [CrossRef]
- Butler, M.; Morel, A.S.; Jordan, W.J.; Eren, E.; Hue, S.; Shrimpton, R.E.; Ritter, M.A. Altered expression and endocytic function of CD205 in human dendritic cells, and detection of a CD205-DCL-1 fusion protein upon dendritic cell maturation. Immunology 2007, 120, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Swiggard, W.J.; Heufler, C.; Peng, M.; Mirza, A.; Steinman, R.M.; Nussenzweig, M.C. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature 1995, 375, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Van Kooyk, Y.; Rabinovich, G.A. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat. Immunol. 2008, 9, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Herre, J.; Williams, D.L.; Willment, J.A.; Marshall, A.S.; Gordon, S. Dectin-1 mediates the biological effects of β-glucans. J. Exp. Med. 2003, 197, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Dennehy, K.M.; Brown, G.D. The role of the β-glucan receptor Dectin-1 in control of fungal infection. J. Leukoc. Biol. 2007, 82, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Gantner, B.N.; Simmons, R.M.; Canavera, S.J.; Akira, S.; Underhill, D.M. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med. 2003, 197, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Drummond, R.A.; Saijo, S.; Iwakura, Y.; Brown, G.D. The role of Syk/CARD9 coupled C-type lectins in antifungal immunity. Eur. J. Immunol. 2011, 41, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Sancho, D.; Reis, E.S.C. Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annu. Rev. Immunol. 2012, 30, 491–529. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, S.; Matsumoto, M.; Takeuchi, O.; Matsuzawa, T.; Ishikawa, E.; Sakuma, M.; Tateno, H.; Uno, J.; Hirabayashi, J.; Mikami, Y.; et al. C-type lectin Mincle is an activating receptor for pathogenic fungus, Malassezia. Proc. Natl. Acad. Sci. USA 2009, 106, 1897–1902. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, E.; Ishikawa, T.; Morita, Y.S.; Toyonaga, K.; Yamada, H.; Takeuchi, O.; Kinoshita, T.; Akira, S.; Yoshikai, Y.; Yamasaki, S. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J. Exp. Med. 2009, 206, 2879–2888. [Google Scholar] [CrossRef] [PubMed]
- Gringhuis, S.I.; den Dunnen, J.; Litjens, M.; van Het Hof, B.; van Kooyk, Y.; Geijtenbeek, T.B. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-κB. Immunity 2007, 26, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, S.; Zelenay, S.; Sancho, D.; Hanč, P.; Kjær, S.; Feest, C.; Fletcher, G.; Durkin, C.; Postigo, A.; Skehel, M.; et al. F-actin is an evolutionarily conserved damage-associated molecular pattern recognized by DNGR-1, a receptor for dead cells. Immunity 2012, 36, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Bouchon, A.; Dietrich, J.; Colonna, M. Cutting edge: Inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J. Immunol. 2000, 164, 4991–4995. [Google Scholar] [CrossRef] [PubMed]
- Frisancho-Kiss, S.; Nyland, J.F.; Davis, S.E.; Barrett, M.A.; Gatewood, S.J.; Njoku, D.B.; Cihakova, D.; Silbergeld, E.K.; Rose, N.R.; Fairweather, D. Tim-3, Cutting edge: T cell Ig mucin-3 reduces inflammatory heart disease by increasing CTLA-4 during innate immunity. J. Immunol. 2006, 176, 6411–6415. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Liu, Y.; Peng, J.; Chen, L.; Zou, T.; Xiao, H.; Liu, Z.; Li, W.; Bum, Y.; Qi, Y. The IRAK-1-BCL10-MALT1-TRAF6-TAK1 cascade mediates signaling to NF-κB from Toll-like receptor 4. J. Biol. Chem. 2006, 281, 26029–26040. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.B.; Springer, T.A. Leukocytes roll on a selectin at physiologic flow rates: Distinction from and prerequisite for adhesion through integrins. Cell 1991, 65, 859–873. [Google Scholar] [CrossRef]
- Springer, T.A. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell 1994, 76, 301–314. [Google Scholar] [CrossRef]
- Zimmaerman, G.A.; Prescot, S.M.; McIntyre, T.M. Endothelial cell interactions with granulocytes: Tethering and signalling molecules. Immunol. Today 1992, 13, 93–99. [Google Scholar] [CrossRef]
- Von Adrian, U.H.; Chambers, J.D.; Berg, E.L.; Michie, S.A.; Brown, D.A.; Karolak, D.; Ramezani, L.; Berger, E.M.; Arfors, K.E.; Butcher, E.C. L-selectin mediates neutrophil rolling in inflamed venules through sialyl-Lewis-dependent and independent recognition pathways. Blood 1993, 82, 182–191. [Google Scholar]
- Berg, M.; James, S.P. Human neutrophils release the Leu-8 lymphnode receptor during cell activation. Blood 1990, 76, 2381–2388. [Google Scholar] [PubMed]
- Tedder, T.F.; Steeber, D.A.; Pizcueta, P. L-selectin-deficient mice have impaired leukocute recruitment into inflammatory sites. J. Exp. Med. 1995, 181, 2259–2264. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, T.K.; Jutila, M.A.; Berg, E.L.; Butcher, E.C. Neutrophil mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science 1989, 245, 1238–1241. [Google Scholar] [CrossRef] [PubMed]
- Schleiffenbaum, B.E.; Spertini, O.; Tedder, T.F. Soluble L-selectin is present in human plasma at high levels and retains functional activity. J. Cell Biol. 1992, 119, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.R.; Fennie, C.; Lasky, L.A. Neutrophil influx into an inflammatory site inhibited by a soluble homing receptor-IgG chimaera. Nature 1991, 349, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Gamble, J.R.; Skinner, M.P.; Berndt, M.C.; Vadas, M.A. Prevention of activated neutrophil adhesion to endothelium by soluble adhesion protein GMP140. Science 1990, 249, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Tamatani, T.; Kotani, M.; Miyasaka, M. Characterization of rat LECAM-1 (L-selectin) by the use of monoclonal antibodies and evidence for the presence of soluble LECAM-1 in rat sera. Eur. J. Immunol. 1993, 23, 2181–2188. [Google Scholar] [CrossRef] [PubMed]
- Engleder, E.; Demmerer, E.; Wang, X.; Honeder, C.; Zhu, C.; Studenik, C.; Wirth, M.; Arnoldner, C.; Gabor, F. Determination of the glycosylation-pattern of the middle ear mucosa in guinea pigs. Int. J. Pharm. 2015, 484, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Sone, M.; Paparella, M.M.; Schachern, P.A.; Morizono, N.; Le, C.T.; Lin, J. Expression of glycoconjugates in human eustachian tubes with otitis media. Laryngoscope 1998, 108, 1474–1479. [Google Scholar] [CrossRef] [PubMed]
- Linder, T.E.; Daniels, R.L.; Lim, D.J.; DeMaria, T.F. Effect of intranasal inoculation of Streptococcus pneumoniae on the structure of the surface carbohydrates of the chinchilla eustachian tube and middle ear mucosa. Microb. Pathog. 1994, 16, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Linder, T.E.; Lim, D.J.; DeMaria, T.F. Changes in the structure of the cell surface carbohydrates of the chinchilla tubotympanum following Streptococcus pneumoniae-induced otitis media. Microb. Pathog. 1992, 13, 293–303. [Google Scholar] [CrossRef]
- Hamaguchi, Y.; Takeuchi, K.; Jin, C.S.; Majima, Y.; Suzumura, H.; Sakakura, Y.; Juhn, S.K. The relationship between proteases activity and glycoprotein levels in middle ear effusions from experimental otitis media in cats. Acta Otolaryngol. 1991, 483, 23–29. [Google Scholar] [CrossRef]
- Kirkeby, S.; Friis, M.; Mikkelsen, H.B.; Cayé-Thomasen, P. Bacterial adherence in otitis media: Determination of N-acetylgalactosamine (GalNAc) residues in the submucosal glands and surface epithelium of the normal and diseased Eustachian tube. Microb. Pathog. 2011, 51, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Mey, A.; Leffler, H.; Hmama, Z.; Normier, G.; Revillard, J.P. The animal lectin galectin-3 interacts with bacterial lipopolysaccharides via two independent sites. J. Immunol. 1996, 156, 1572–1577. [Google Scholar] [PubMed]
- Lasky, L.A. Selectin-carbohydrate interactions and the initiation of the inflammatory response. Ann. Rev. Biochem. 1995, 64, 113–139. [Google Scholar] [CrossRef] [PubMed]
- Rosen, S.D.; Singer, M.S.; Yednock, T.D. Involvement of sialic acid on endothelial cells in organ-specific lymphocyte recirculation. Science 1985, 228, 1005–1007. [Google Scholar] [CrossRef] [PubMed]
- Sharon, N. Bacterial lectins, cell-cell recognition and infectious disease. FEES Lett. 1987, 217, 145–157. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

| Characteristics | Explanation |
|---|---|
| Defining arrangement of amino acid residues involved in binding | Often typical for each group |
| Location of cognate residues within glycans | Typically in sequences at outer ends of glycan chains |
| Shared evolutionary origins | Yes (within each group) |
| Shared structural features | Yes (within each group) |
| Single-site binding affinity | Often low; high avidity generated by multivalency |
| Specificity for glycans recognized | Stereospecificity high for specific glycan structures |
| Subgroups | C-type lectins, galectins, Galectins (S-type lectin), P-type lectins (M6P receptors), I-type lectins, L-type lectins, R-type lectins, etc. |
| Type of glycans recognized | N-glycans, O-glycans, glycosphingo-lipids (a few also recognize sulfated glycosaminoglycans) |
| Types of glycans recognized within each group | May be similar (e.g., galectins) or different (e.g., C-type lectins) |
| Valency of binding sites | Multivalency common (either within native structures or by clustering) |
| Lectin Family | Typical Saccharide Ligands | Subcellular Location | Examples of Functions |
|---|---|---|---|
| Calnexin | Glc1Man9 | ER | Protein sorting in the endoplasmic reticulum. |
| Chitinase-like lectins | Chito-oligosaccharides | Extracellular | Collagen metabolism (YKL-40). |
| C-type lectins | Various | Cell membrane, extracellular | Cell adhesion (selectins), glycoprotein clearance, innate immunity (collectins). |
| F-box lectins | GlcNAc2 | Cytoplasm | Degradation of misfolded glycoproteins. |
| Ficolins | GlcNAc, GalNAc | Cell membrane, extracellular | Innate immunity. |
| F-type lectins | Fuc-terminating oligosaccharides | Extracellular | Innate immunity. |
| Galectins | β-Galactosides | Cytoplasm, extracellular | Glycan crosslinking in the extracellular matrix. |
| I-type lectins (siglecs) | Sialic acid | Cell membrane | Cell adhesion. |
| Intelectins | Gal, galactofuranose, pentoses | Extracellular/cell membrane | Innate immunity; fertilization and embryogenesis. |
| L-type lectins | Various | ER, ERGIC, Golgi | Protein sorting in the ER. |
| M-type lectins | Man8 | ER | ER-associated degradation of glycoproteins. |
| P-type lectins | Mannose 6-phosphate, others | Secretory pathway | Protein sorting post-Golgi, glycoprotein trafficking, ER-associated degradation of glycoproteins, enzyme targeting. |
| R-type lectins | Various | Golgi, Cell membrane | Enzyme targeting, glycoprotein hormone turnover. |
| Authors and References | Species | Experimental Conditions | Type of CLRs | Detection Methods |
|---|---|---|---|---|
| Kim et al. [23] | Human | Chole OM | MBL, CD206, DEC-205, DC-SIGN, Langerin, MGL, CLEC5A, Dectin-2, BDCA2, Mincle, DCIR, Dectin-1, MICL, CLEC2, DNGR1, CLEC12B | Real-time PCR |
| Li et al. [24] | Mouse | OME | SP-A, SP-D | Reverse transcription PCR, Real-time PCR |
| Kim et al. [25] | Human | Chole OM | DEC205, Bcl-10, Tim-3, Trem-1 | Real-time PCR |
| Lee et al. [26] | Human | OME | Dectin-1, MR1, MR2, DC-SIGN, Syk, Card-9, Bcl-10, Malt-1, Src, DEC205, Galectin-1, Tim-3, Trem-1, DAP-12 | Real-time PCR |
| Pospiech et al. [27] | Human | OME | Soluble L-selectin | ELISA, Bradford assay |
| Himi et al. [28] | Human | OME | Soluble ICAM-1, soluble GMP-140 | ELISA |
| Garred et al. [29] | Human | OME | MBL | EIA |
| Konishi et al. [30] | Human | OME | MBL, SP-A | Immunoblotting analysis |
| Kamimura et al. [31] | Rat | OME | L-selectin | Flow cytometry |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.Y.; Kim, S.S.; Kim, Y.I.; Chung, H.Y.; Kim, S.H.; Yeo, S.G. Expression, Distribution, and Role of C-Type Lectin Receptors in the Human and Animal Middle Ear and Eustachian Tube: A Review. Molecules 2018, 23, 734. https://doi.org/10.3390/molecules23040734
Jung SY, Kim SS, Kim YI, Chung HY, Kim SH, Yeo SG. Expression, Distribution, and Role of C-Type Lectin Receptors in the Human and Animal Middle Ear and Eustachian Tube: A Review. Molecules. 2018; 23(4):734. https://doi.org/10.3390/molecules23040734
Chicago/Turabian StyleJung, Su Young, Sung Su Kim, Young Il Kim, Hee Yong Chung, Sang Hoon Kim, and Seung Geun Yeo. 2018. "Expression, Distribution, and Role of C-Type Lectin Receptors in the Human and Animal Middle Ear and Eustachian Tube: A Review" Molecules 23, no. 4: 734. https://doi.org/10.3390/molecules23040734
APA StyleJung, S. Y., Kim, S. S., Kim, Y. I., Chung, H. Y., Kim, S. H., & Yeo, S. G. (2018). Expression, Distribution, and Role of C-Type Lectin Receptors in the Human and Animal Middle Ear and Eustachian Tube: A Review. Molecules, 23(4), 734. https://doi.org/10.3390/molecules23040734