Extraction and Analysis of Gigantol from Dendrobium officinale with Response Surface Methodology
Abstract
:1. Introduction
2. Results and Discussion
2.1. Single-Factor Experiment of Gigantol Extraction
2.2. Screening of Gigantol Extraction Factors by the Two-Level Factorial Design
2.3. Optimization of Gigantol Extraction by Response Surface
2.3.1. Analysis of the Response Surface Model
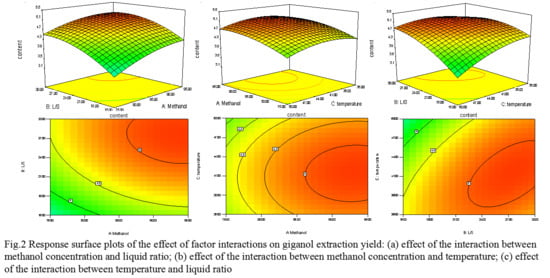
2.3.2. Interactions of Different Experimental Factors on the Effects of Response Variables
2.3.3. Validation of Optimal Conditions
2.4. Analysis of Gigantol in Dendrobium
2.4.1. Analysis of Gigantol in Dendrobium officinale Cultivation
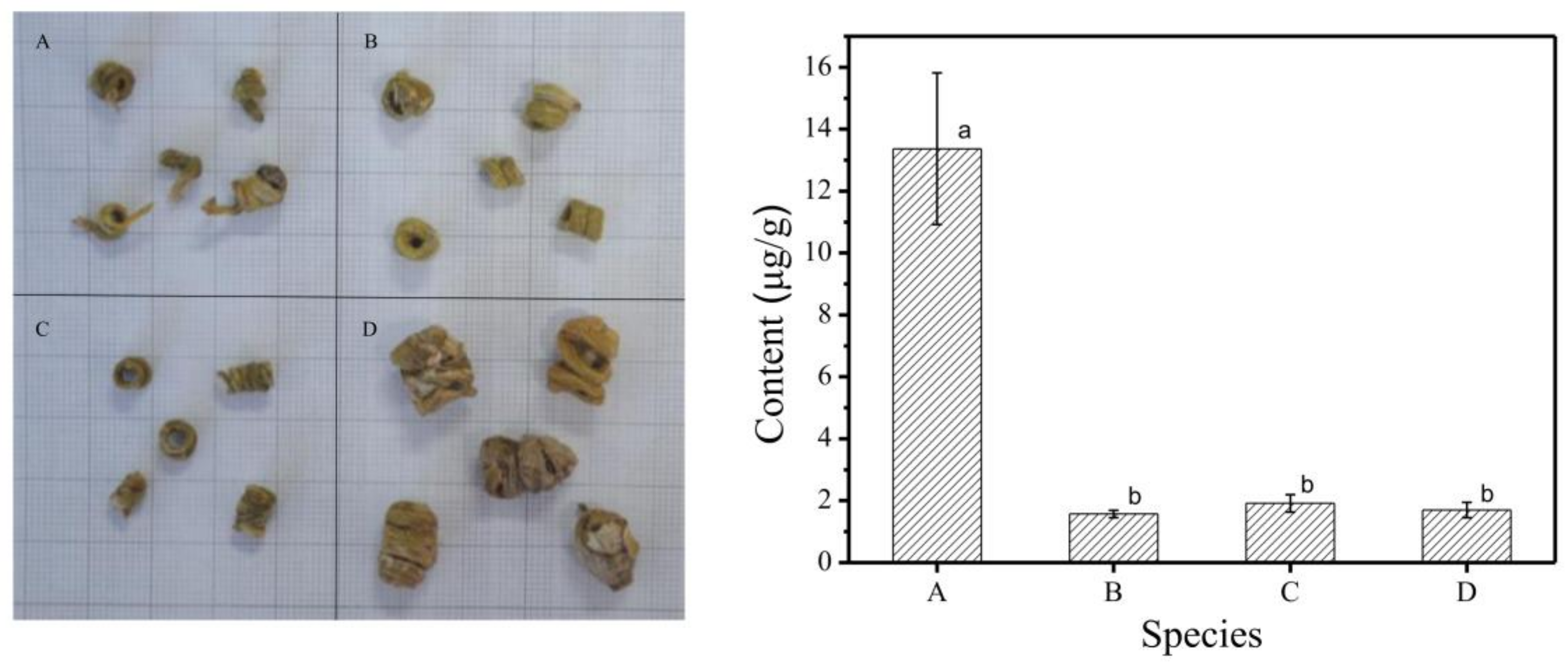
2.4.2. Analysis of Gigantol in Different Species of Fengdou
3. Materials and Methods
3.1. Herbal Samples
3.2. Chemicals and Solvents
3.3. Extraction Procedure
3.4. Reversed-Phase-HPLC Analysis
3.5. Experimental Design
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jin, Q.; Yao, Y.; Cai, Y.; Lin, Y. Molecular cloning and sequence analysis of a phenylalanine ammonia-lyase gene from Dendrobium. PLoS ONE 2013, 8, e62352. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Zhao, H.; Lu, J.; Liu, J.; Shen, B.; Wang, H. Preliminary genetic linkage maps of Chinese herb Dendrobium nobile and D. moniliforme. J. Genet. 2013, 92, 205. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.B.; Liu, J.; Wong, J.H.; Ye, X.; Wing Sze, S.C.; Tong, Y.; Zhang, K.Y. Review of research on Dendrobium, a prized folk medicine. Appl. Microbiol. Biotechnol. 2012, 93, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Bhummaphan, N.; Pithi, C. Gigantol suppresses cancer stem cell-like phenotypes in lung cancer cells. Evid.-Based Complement. Altern. Med. 2015, 2015, 836564. [Google Scholar] [CrossRef] [PubMed]
- Charoenrungruang, S.; Chanvorachote, P.; Sritularak, B.; Pongrakhananon, V. Gigantol, a bibenzyl from Dendrobium draconis, inhibits the migratory behavior of non-small cell lung cancer cells. J. Nat. Prod. 2014, 77, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lu, C.; Li, X.; Fang, H.; Wan, W.; Yang, Q.; Sun, X.; Wang, M.; Hu, X.; Chen, C.; et al. Synthesis and biological evaluation of novel gigantol derivatives as potential agents in prevention of diabetic cataract. PLoS ONE 2015, 10, e0141092. [Google Scholar] [CrossRef] [PubMed]
- Félix, A.; Yvesf, L.; Paul, L.; Augustin, A.; Farid, C.; Emilem, G. Optimization of anthocyanin, flavonol and phenolic acid extractions from Delonix regia tree flowers using ultrasound-assisted water extraction. Ind. Crop. Prod. 2010, 32, 439–444. [Google Scholar] [CrossRef]
- Lebovka, N.I.; Vorobiev, E.; Chemat, F. Enhancing Extraction Processes in the Food Industry; CRC Press: Boca Raton, FL, USA, 2011; Volume 15, pp. 25–83. [Google Scholar]
- Raviyan, P.; Zhang, Z.; Feng, H. Ultrasonication for tomato pectinmethylesterase inactivation: Effect of cavitation intensity and temperature on inactivation. J. Food Eng. 2005, 70, 189–196. [Google Scholar] [CrossRef]
- Proestos, C.; Boziaris, I.S.; Gje, N.; Komaitis, M. Analysis of flavonoids and phenolic acids in Greek aromatic plants: Investigation of their antioxidant capacity and antimicrobial activity. Food Chem. 2006, 95, 664–671. [Google Scholar] [CrossRef]
- Muhammad, U.; Lu, H.; Wang, J.; Han, J.; Zhu, X.; Lu, Z.; Tayyaba, S.; Hassan, Y.I. Optimizing the maximum recovery of Dihydromyricetin from Chinese vine tea, Ampelopsis grossedentata, using response surface methodology. Molecules 2017, 22, 2250. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zeng, H.; Xu, Z.; Zheng, B.; Lin, Y.; Gan, C.; MartinLo, Y. Ultrasonic-assisted extraction and antioxidant activity of polysaccharides recovered from white button mushroom (Agaricus bisporus). Carbohydr. Polym. 2012, 88, 522–529. [Google Scholar] [CrossRef]
- Afshari, K.; Samavati, V.; Shahidi, S.A. Ultrasonic-assisted extraction and in-vitro antioxidant activity of polysaccharide from hibiscus leaf. Int. J. Biol. Macromol. 2015, 74, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jiang, J.; Li, W.; Chen, J.; Wang, D.; Zhu, L. Optimum extraction process of polyphenols from the bark of Phyllanthus emblica L. based on the response surface methodology. J. Sep. Sci. 2009, 32, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Hayat, K.; Hussain, S.; Abbas, S.; Farooq, U.; Ding, B.; Xia, S.; Jia, C.; Zhang, X.; Xia, W. Optimized microwave-assisted extraction of phenolic acids from citrus mandarin peels and evaluation of antioxidant activity in vitro. Sep. Purif. Technol. 2010, 70, 63–70. [Google Scholar] [CrossRef]
- Prasad, K.N.; Yang, E.; Yi, C.; Zhao, M.M.; Jiang, Y.M. Effects of high pressure extraction on the extraction yield, total phenolic content and antioxidant activity of longan fruit pericarp. J. Pharm. Biomed. Anal. 2009, 10, 155–159. [Google Scholar] [CrossRef]
- Schmid, R. Recent advances in the description of the structure of water, the hydrophobic effect, and the like-dissolves-like rule. Monatsh. Chem. Mon. 2001, 132, 1295–1326. [Google Scholar] [CrossRef]
- Liyanapathirana, C.; Shahidi, F. Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chem. 2005, 93, 47–56. [Google Scholar] [CrossRef]
- Juntachote, T.; Berghofer, E.; Bauer, F.; Siebenhandl, S. The application of response surface methodology to the production of phenolic extracts of lemon grass, galangal, holy basil and rosemary. Int. J. Food Sci. Technol. 2006, 41, 121–133. [Google Scholar] [CrossRef]
- Roriz, C.L.; Barros, L.; Prieto, M.A.; Morales, P.; Ferreira, I.C. Floral parts of Gomphrena globosa L. as a novel alternative source of betacyanins: Optimization of the extraction using response surface methodology. Food Chem. 2017, 229, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Zhang, Z.; Sun, D.W. Kinetic modeling of ultrasound-assisted extraction of phenolic compounds from grape marc: Influence of acoustic energy density and temperature. Ultrason. Sonochem. 2014, 21, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Bimakr, M.; Rahman, R.A.; Taip, F.S.; Adzahan, N.M.; Sarker, M.Z.; Ganjloo, A. Supercritical carbon dioxide extraction of seed oil from winter melon (Benincasa hispida) and its antioxidant activity and fatty acid composition. Molecules 2013, 18, 997–1014. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Chen, N.; Dai, J.; Chen, N. Purification and cytotoxicity of glycoprotein isolated from Dendrobium huoshanense. China J. Chin. Mater. Med. 2017, 42, 130–134. [Google Scholar] [CrossRef]
- Wong, K.H.; Li, G.Q.; Li, K.M.; Razmovski-Naumovski, V.; Chan, K. Optimisation of Pueraria isoflavonoids by response surface methodology using ultrasonic-assisted extraction. Food Chem. 2017, 231, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Mazarei, F.; Jooyandeh, H.; Noshad, M.; Hojjati, M. Polysaccharide of caper (Capparis spinosa L.) leaf: Extraction optimization, antioxidant potential and antimicrobial activity. Int. J. Biol. Macromol. 2016, 95. [Google Scholar] [CrossRef] [PubMed]
- Kala, H.K.; Mehta, R.; Sen, K.K.; Tandey, R.; Mandal, V. Critical analysis of research trends and issues in microwave assisted extraction of phenolics: Have we really done enough. TrAc-Trends Anal. Chem. 2016, 85, 140–152. [Google Scholar] [CrossRef]
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. TrAc-Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |



| Run | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Response |
|---|---|---|---|---|---|
| Methanol (%; X1) | Liquid Ratio (mL/g; X2) | Temperature (°C; X3) | Time (min; X4) | Gigantol (μg/g; Y) | |
| 1 | 75 | 15 | 35 | 30 | 4.5111 |
| 2 | 75 | 30 | 35 | 30 | 4.3416 |
| 3 | 75 | 15 | 35 | 50 | 4.1946 |
| 4 | 95 | 30 | 35 | 50 | 4.0619 |
| 5 | 75 | 15 | 50 | 50 | 6.1446 |
| 6 | 95 | 30 | 50 | 30 | 6.5708 |
| 7 | 75 | 30 | 50 | 30 | 6.6438 |
| 8 | 95 | 15 | 35 | 50 | 2.5290 |
| 9 | 95 | 15 | 50 | 30 | 4.4223 |
| 10 | 75 | 30 | 35 | 50 | 6.5937 |
| 11 | 75 | 15 | 50 | 30 | 5.0497 |
| 12 | 95 | 30 | 50 | 50 | 6.2677 |
| 13 | 95 | 30 | 35 | 30 | 3.5000 |
| 14 | 75 | 30 | 50 | 50 | 8.3087 |
| 15 | 95 | 15 | 35 | 30 | 3.6740 |
| 16 | 95 | 15 | 50 | 50 | 5.1009 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 33.22 | 9 | 3.69 | 28.12 | 0.0003 | Significant |
| X1 | 5.83 | 1 | 5.83 | 44.45 | 0.0006 | |
| X2 | 7.10 | 1 | 7.10 | 54.13 | 0.0003 | |
| X3 | 14.26 | 1 | 14.26 | 108.62 | < 0.0001 | |
| X4 | 1.26 | 1 | 1.26 | 9.59 | 0.0212 |
| Run | Factor 1 | Factor 2 | Factor 3 | Response |
|---|---|---|---|---|
| Methanol (%; X1) | Liquid Ratio (mL/g; X2) | Temperature (°C; X3) | Gigantol (μg/g; Y) | |
| 1 | 95.00 (1) | 30.00 (1) | 35.00 (−1) | 4.8982 |
| 2 | 75.00 (−1) | 15.00 (−1) | 35.00 (−1) | 3.8299 |
| 3 | 85.00 (0) | 22.50 (0) | 55.11 (1.682) | 3.7676 |
| 4 | 101.82 (1.682) | 22.50 (0) | 42.50 (0) | 4.6509 |
| 5 | 85.00 (0) | 22.50 (0) | 42.50 (0) | 5.2345 |
| 6 | 85.00 (0) | 35.11 (1.682) | 42.50 (0) | 4.8230 |
| 7 | 85.00 (0) | 22.50 (0) | 29.89 (−1.682) | 4.4272 |
| 8 | 68.18 (−1.682) | 22.50 (0) | 42.50 (0) | 3.8907 |
| 9 | 75.00 (−1) | 30.00 (1) | 50.00 (1) | 4.1397 |
| 10 | 95.00 (1) | 15.00 (−1) | 50.00 (1) | 3.6150 |
| 11 | 85.00 (0) | 22.50 (0) | 42.50 (0) | 4.5767 |
| 12 | 95.00 (1) | 15.00 (−1) | 35.00 (−1) | 4.6886 |
| 13 | 75.00 (−1) | 30.00 (1) | 35.00 (−1) | 4.1879 |
| 14 | 85.00 (0) | 9.89 (−1.682) | 42.50 (0) | 3.1357 |
| 15 | 75.00 (−1) | 15.00 (−1) | 50.00 (1) | 2.5093 |
| 16 | 85.00 (0) | 22.50 (0) | 42.50 (0) | 4.4102 |
| 17 | 95.00 (1) | 30.00 (1) | 50.00 (1) | 4.8041 |
| 18 | 85.00 (0) | 22.50 (0) | 42.50 (0) | 5.2327 |
| 19 | 85.00 (0) | 22.50 (0) | 42.50 (0) | 4.2105 |
| 20 | 85.00 (0) | 22.50 (0) | 42.50 (0) | 5.0548 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 8.140 | 9 | 0.900 | 7.60 | 0.0020 | Significant |
| X1 | 1.560 | 1 | 1.560 | 13.12 | 0.0047 | |
| X2 | 2.840 | 1 | 2.840 | 23.84 | 0.0006 | |
| X3 | 0.970 | 1 | 0.970 | 8.18 | 0.0170 | |
| X1X2 | 0.043 | 1 | 0.043 | 0.37 | 0.5591 | |
| X1X3 | 0.005 | 1 | 0.005 | 0.04 | 0.8409 | |
| X2X3 | 0.630 | 1 | 0.630 | 5.33 | 0.0437 | |
| X12 | 0.470 | 1 | 0.470 | 3.94 | 0.0752 | |
| X22 | 1.160 | 1 | 1.160 | 9.73 | 0.0109 | |
| X32 | 0.840 | 1 | 0.840 | 7.08 | 0.0239 | |
| Residual | 1.190 | 10 | 0.120 | |||
| Lack of Fit | 0.200 | 5 | 0.040 | 0.20 | 0.9474 | Not significant |
| Pure Error | 0.990 | 5 | 0.200 | |||
| Cor Total | 9.330 | 19 |
| Category | Run | Yield (μg/g) | STDEV (%) | |
|---|---|---|---|---|
| Predictive | Experimental | |||
| Gigantol | 1 | 5.0838 | 4.6107 | −0.8354 |
| 2 | 5.0838 | 5.0319 | ||
| 3 | 5.0838 | 5.3264 | ||
| 4 | 5.0838 | 5.3549 | ||
| 5 | 5.0838 | 5.3072 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, S.; Zhu, Y.; Jiao, C.; Shi, M.; Wei, L.; Zhou, Y.; Jin, Q.; Cai, Y. Extraction and Analysis of Gigantol from Dendrobium officinale with Response Surface Methodology. Molecules 2018, 23, 818. https://doi.org/10.3390/molecules23040818
Zheng S, Zhu Y, Jiao C, Shi M, Wei L, Zhou Y, Jin Q, Cai Y. Extraction and Analysis of Gigantol from Dendrobium officinale with Response Surface Methodology. Molecules. 2018; 23(4):818. https://doi.org/10.3390/molecules23040818
Chicago/Turabian StyleZheng, Siyan, Yingpeng Zhu, Chunyan Jiao, Mengyao Shi, Lianping Wei, Yang Zhou, Qing Jin, and Yongping Cai. 2018. "Extraction and Analysis of Gigantol from Dendrobium officinale with Response Surface Methodology" Molecules 23, no. 4: 818. https://doi.org/10.3390/molecules23040818