N-Confused Porphyrin Immobilized on Solid Supports: Synthesis and Metal Ions Sensing Efficacy
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Immobilization of NCTPP
2.2. Photophysical and Morphological Properties
2.2.1. UV-Vis Spectroscopy
2.2.2. SEM
2.3. Zeta Potential
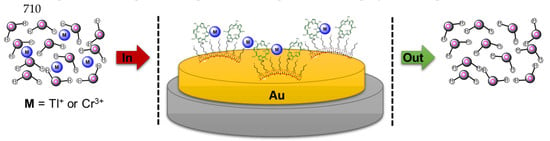
2.4. Metal-Receptor Interaction
3. Materials and Methods
3.1. General Remarks
3.2. NCTPP Immobilization on Functionalized Silica (Si) and on Merrifield Resin (MF)
3.2.1. Synthesis of NCTPP-Si
3.2.2. Synthesis of NCTPP-MF
3.2.3. Synthesis of NCTPP-Si-Py+ and NCTPP-MF-Py+
3.2.4. Si and MF Cationization
3.3. Photophysical and Morphological Properties
SEM
3.4. Zeta Potential
3.5. Sensing Ability Towards Metal Cations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Youssef, Z.; Arnoux, P.; Colombeau, L.; Toufaily, J.; Hamieh, T.; Frochot, C.; Roques-Carmes, T. Comparison of two procedures for the design of dye-sensitized nanoparticles targeting photocatalytic water purification under solar and visible light. J. Photochem. Photobiol. A Chem. 2018, 356, 177–192. [Google Scholar] [CrossRef]
- Phani Madhavi, T.; Srimurali, M.; Nagendra Prasad, K. Color Removal From Industrial Waste Water Using Alum. J. Environ. Res. Dev. 2014, 8, 890–894. [Google Scholar]
- Tewari, A.; Dubey, A.; Singh, P. Impact of Tanning Industries on Ground Water Resources. J. Environ. Res. Dev. 2012, 26, 1747–1761. [Google Scholar]
- Torma, C.Z.; Cséfalvay, E. Nanofiltration: A Final Step in Industrial Process Water Treatment. Period. Polytech. Chem. Eng. 2017, 62, 68–75. [Google Scholar] [CrossRef]
- Tabish, T.A.; Memon, F.A.; Gomez, D.E.; Horsell, D.W.; Zhang, S. A facile synthesis of porous graphene for efficient water and wastewater treatment. Sci. Rep. 2018, 8, 1817. [Google Scholar] [CrossRef] [PubMed]
- Vedhi, C.; Selvanathan, G.; Arumugam, P.; Manisankar, P. Electrochemical sensors of heavy metals using novel polymer-modified glassy carbon electrodes. Ionics 2009, 15, 377–383. [Google Scholar] [CrossRef]
- Liu, M.; Guan, Q.; Liu, S. Nitrogen-doped hollow carbon spheres for electrochemical detection of heavy metal ions. Ionics 2017, 1–11. [Google Scholar] [CrossRef]
- Pandey, G. Heavy Metals Causing Toxicity in Animals and Fishes. Res. J. Anim. Vet. Fish. Sci. Rev. 2014, 2, 17–23. [Google Scholar]
- Radi, S.; Abiad, C.E.; Moura, N.M.M.; Faustino, M.A.F.; Neves, M.G.P.M.S. New hybrid adsorbent based on porphyrin functionalized silica for heavy metals removal: Synthesis, characterization, isotherms, kinetics and thermodynamics studies. J. Hazard. Mater. 2017. [Google Scholar] [CrossRef] [PubMed]
- Elkady, A.A.; Sweet, S.T.; Wade, T.L.; Klein, A.G. Distribution and assessment of heavy metals in the aquatic environment of Lake Manzala, Egypt. Ecol. Indic. 2015, 58, 445–457. [Google Scholar] [CrossRef]
- Pechova, A.; Pavlata, L. Chromium as an essential nutrient: A review. Vet. Med. 2007, 52, 1–18. [Google Scholar] [CrossRef]
- Djoko, K.Y.; Ong, C.Y.; Walker, M.J.; McEwan, A.G. The Role of Copper and Zinc Toxicity in Innate Immune Defense against Bacterial Pathogens. J. Biol. Chem. 2015, 290, 18954–18961. [Google Scholar] [CrossRef] [PubMed]
- Bost, M.; Houdart, S.; Oberli, M.; Kalonji, E.; Huneau, J.F.; Margaritis, I. Dietary copper and human health: Current evidence and unresolved issues. J. Trace Elem. Med. Biol. 2016, 35, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Zinc in cellular regulation: The nature and significance of “zinc signals”. Int. J. Mol. Sci. 2017, 18, 2285. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Gupta, R.; Saraf, S.A.; Saraf, S.K. Zinc: The metal of life. Compr. Rev. Food Sci. Food Saf. 2014, 13, 358–376. [Google Scholar] [CrossRef]
- Wei, X.; Wang, Q.; Tang, W.; Zhao, S.; Xie, Y. Combination of pyrrole and pyridine for constructing selective and sensitive Zn2+ probes. Dyes Pigments 2017, 140, 320–327. [Google Scholar] [CrossRef]
- Ding, Y.; Li, X.; Li, T.; Zhu, W.; Xie, Y. α-Monoacylated and α,α′- and α,β′-diacylated dipyrrins as highly sensitive fluorescence “turn-on” Zn2+ probes. J. Org. Chem. 2013, 78, 5328–5338. [Google Scholar] [CrossRef] [PubMed]
- Samadi, N.; Ansari, R.; Khodaverdiloo, B. Synthesized some copolymer derivative of poly (Styrene–alternative-Maleic Anhydride) (SMA) for removal Cobalt (II) ions from aqueous solutions and determination residual cobalt (II) ions by using spectrophotometric method. Chem. Solid Mater. 2017, 2, 1–16. [Google Scholar]
- Alexander, J.A.; Zaini, M.A.A.; Abdulsalam, S.; Aliyu El-Nafaty, U.; Aroke, U.O. Isotherm studies of lead(II), manganese(II), and cadmium(II) adsorption by Nigerian bentonite clay in single and multimetal solutions. Part. Sci. Technol. 2018, 1–11. [Google Scholar] [CrossRef]
- Efome, J.E.; Rana, D.; Matsuura, T.; Lan, C.Q. Metal–organic frameworks supported on nanofibers to remove heavy metals. J. Mater. Chem. A 2018, 1–6. [Google Scholar] [CrossRef]
- Yu, J.; Lu, J.; Kang, Y. Removal of sulfate from wet FGD wastewater by co-precipitation with calcium hydroxide and sodium aluminate. Water Sci. Technol. 2018, wst2018019. [Google Scholar] [CrossRef] [PubMed]
- Corbett, P.J.; McIntosh, A.J.S.; Gee, M.; Hallett, J.P. Use of ionic liquids to remove harmful M2+ contaminants from hydrocarbon streams. Mol. Syst. Des. Eng. 2018. [Google Scholar] [CrossRef]
- Gomes, M.T.S.R. Application of piezoelectric quartz crystals to the analysis of trace metals in solution: A review. IEEE Sens. J. 2001, 1, 109–118. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Costa, L.D.; Costa, J.I.T.; Tomé, A.C. Porphyrin macrocycle modification: Pyrrole ring-contracted or -Expanded porphyrinoids. Molecules 2016, 21, 320. [Google Scholar] [CrossRef] [PubMed]
- Fallah, A.H.; Endud, S.; Alizadeh, A.; Chien, L.S. Metalloporphyrin/dendrimer-decorated MCM-41 biomimetic hybrid catalysts: High stability combined with facile catalyst recyclability. J. Porous Mater. 2018, 1–11. [Google Scholar] [CrossRef]
- Pereira, C.; Simões, M.; Tomé, J.; Almeida Paz, F. Porphyrin-Based Metal-Organic Frameworks as Heterogeneous Catalysts in Oxidation Reactions. Molecules 2016, 21, 1348. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, S.L.H.; Silva, A.M.N.; Medforth, C.J.; Freire, C. Iron(III) fluorinated porphyrins: Greener chemistry from synthesis to oxidative catalysis reactions. Molecules 2016, 21, 481. [Google Scholar] [CrossRef] [PubMed]
- Vasapollo, G.; Mele, G.; Del Sole, R.; Pio, I.; Li, J.; Mazzetto, S.E. Use of Novel Cardanol-Porphyrin Hybrids and Their TiO2-Based Composites for the Photodegradation of 4-Nitrophenol in Water. Molecules 2011, 16, 5769–5784. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-T.; Ji, H.-B.; Huang, X.-J. Photocatalytic Degradation of Methyl Orange over Metalloporphyrins Supported on TiO2 Degussa P25. Molecules 2012, 17, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Wang, J.; Mei, L.; Tao, W.; Barrett, A.; Su, Z.; Wang, S.; Ma, G.; Shi, J.; Zhang, S. Porphyrin/SiO2/Cp*Rh(bpy)Cl Hybrid Nanoparticles Mimicking Chloroplast with Enhanced Electronic Energy Transfer for Biocatalyzed Artificial Photosynthesis. Adv. Funct. Mater. 2018, 28, 1705083. [Google Scholar] [CrossRef]
- Martínez-Klimov, M.E.; Organista-Mateos, U.; Borja-Miranda, A.; Rivera, M.; Amelines-Sarria, O.; Martínez-García, M. Electrical properties of multi-pyrene/porphyrin-dendrimers. Molecules 2015, 20, 17533–17543. [Google Scholar] [CrossRef] [PubMed]
- Syu, Y.K.; Tingare, Y.; Lin, S.Y.; Yeh, C.Y.; Wu, J.J. Porphyrin dye-sensitized zinc oxide aggregated anodes for use in solar cells. Molecules 2016, 21, 1025. [Google Scholar] [CrossRef] [PubMed]
- Berna, J.F.; Seetharaman, S.; Martin-Gomis, L.; Charalambidis, G.; Trapali, A.; Karr, P.A.; Coutsolelos, A.G.G.; Fernandez-Lazaro, F.; DSouza, F.; Sastre-Santos, A. Supramolecular Complex of a Fused Zinc Phthalocyanine-Zinc Porphyrin Dyad Assembled by Two Imidazole-C60 Units: Ultrafast Energy Transfer Followed by Electron Transfer. Phys. Chem. Chem. Phys. 2018, 20, 7798–7807. [Google Scholar] [CrossRef]
- Cerqueira, A.F.R.; Almodôvar, V.A.S.; Neves, M.G.P.M.S.; Tomé, A.C. Coumarin-tetrapyrrolic macrocycle conjugates: Synthesis and applications. Molecules 2017, 22, 994. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Lu, N.; Gu, Y.; Li, C.; Zhang, T.; Liu, H.; Zhang, Z.; Zhai, S. Catalytic activity of biomimetic model of cytochrome P450 in oxidation of dopamine. Talanta 2018, 179, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Hernández Anzaldo, S.; Arroyo Abad, U.; León García, A.; Ramírez Rosales, D.; Zamorano Ulloa, R.; Reyes Ortega, Y. Spectroscopic and Kinetic Characterization of Peroxidase-Like π-Cation Radical Pinch-Porphyrin-Iron(III) Reaction Intermediate Models of Peroxidase Enzymes. Molecules 2016, 21, 804. [Google Scholar] [CrossRef] [PubMed]
- Guleria, M.; Kumar, C.; Das, T.; Amirdhanayagam, J.; Sharma, R.; Sarma, H.D.; Dash, A. Studies towards elucidating the potential of 5,10,15,20-tetrakis(p-carboxymethyleneoxyphenyl)porphyrin as a theranostic agent for applications in PET and PDT. MedChemComm 2018. [Google Scholar] [CrossRef]
- Boscencu, R.; Manda, G.; Radulea, N.; Socoteanu, R.P.; Ceafalan, L.C.; Neagoe, I.V.; MacHado, I.F.; Basaga, S.H.; Ferreira, L.F.V. Studies on the synthesis, photophysical and biological evaluation of some unsymmetrical meso-tetrasubstituted phenyl porphyrins. Molecules 2017, 22, 1815. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Jiang, Y.Y.; Jiang, T.; Yin, W.; Yang, J.P.; Cao, M.L.; Fang, Y.Q.; Liu, H.Y. Water-soluble manganese and iron mesotetrakis(carboxyl)porphyrin: DNA binding, oxidative cleavage, and cytotoxic activities. Molecules 2017, 22, 1084. [Google Scholar] [CrossRef] [PubMed]
- De Simone, B.C.; Mazzone, G.; Russo, N.; Sicilia, E.; Toscano, M. Metal atom effect on the photophysical properties of Mg(II), Zn(II), Cd(II), and Pd(II) tetraphenylporphyrin complexes proposed as possible drugs in photodynamic therapy. Molecules 2017, 22, 1093. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.D.; De E Silva, J.A.; Fonseca, S.M.; Arranja, C.T.; Urbano, A.M.; Sobral, A.J.F.N. Photophysical Characterization and in Vitro Phototoxicity Evaluation of 5,10,15,20-Tetra(quinolin-2-yl)porphyrin as a Potential Sensitizer for Photodynamic Therapy. Molecules 2016, 21, 439. [Google Scholar] [CrossRef] [PubMed]
- Viana, O.S.; Ribeiro, M.S.; Rodas, A.C.D.; Rebouças, J.S.; Fontes, A.; Santos, B.S. Comparative study on the efficiency of the photodynamic inactivation of candida albicans Using CdTe Quantum Dots, Zn(II) Porphyrin and Their Conjugates as Photosensitizers. Molecules 2015, 20, 8893–8912. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, A.F.R.; Moura, N.M.M.; Serra, V.V.; Faustino, M.A.F.; Tomé, A.C.; Cavaleiro, J.A.S.; Neves, M.G.P.M.S. β-Formyl- and β-Vinylporphyrins: Magic building blocks for novel porphyrin derivatives. Molecules 2017, 22, 1290. [Google Scholar] [CrossRef] [PubMed]
- Prabphal, J.; Vilaivan, T.; Praneenararat, T. Fabrication of a Paper-Based Turn-Off Fluorescence Sensor for Cu2+ Ion from a Pyridinium Porphyrin. ChemistrySelect 2018, 3, 894–899. [Google Scholar] [CrossRef]
- Ding, Y.; Zhu, W.H.; Xie, Y. Development of Ion Chemosensors Based on Porphyrin Analogues. Chem. Rev. 2017, 117, 2203–2256. [Google Scholar] [CrossRef] [PubMed]
- Paolesse, R.; Nardis, S.; Monti, D.; Stefanelli, M.; Di Natale, C. Porphyrinoids for Chemical Sensor Applications. Chem. Rev. 2017, 117, 2517–2583. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.M.M.; Farinha, A.S.F.; Muteto, P.V.; Woranovicz-Barreira, S.M.; Almeida Paz, F.A.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Tomé, A.C.; Gomes, M.T.S.R.; Sessler, J.L.; et al. New porphyrin derivatives for phosphate anion sensing in both organic and aqueous media . Chem. Commun. 2014, 50, 1359–1361. [Google Scholar] [CrossRef] [PubMed]
- Figueira, F.; Rodrigues, J.M.M.; Farinha, A.A.S.; Cavaleiro, J.A.S.; Tomé, J.P.C. Synthesis and anion binding properties of porphyrins and related compounds. J. Porphyr. Phthalocyanines 2016, 20, 950–965. [Google Scholar] [CrossRef]
- Sessler, J.L.; Gale, P.A.; Cho, W.-S. Anion Receptor Chemistry, 1st ed.; Thomas Graham House, Ed.; The Royal Society of Chemistry: Cambridge, UK, 2006; ISBN 978-0-85404-974-5. [Google Scholar]
- Ding, Y.; Tang, Y.; Zhu, W.; Xie, Y. Fluorescent and colorimetric ion probes based on conjugated oligopyrroles. Chem. Soc. Rev. 2015, 44, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Moura, N.M.M.; Núñez, C.; Faustino, M.A.F.; Cavaleiro, J.A.S.; Neves, M.G.P.M.S.; Capelo, J.L.; Lodeiro, C. Preparation and ion recognition features of porphyrin–chalcone type compounds as efficient red-fluorescent materials. J. Mater. Chem. C 2014, 2, 4772–4783. [Google Scholar] [CrossRef]
- Moura, N.M.M.; Núñez, C.; Santos, S.M.; Faustino, M.A.F.; Cavaleiro, J.A.S.; Almeida Paz, F.A.; Neves, M.G.P.M.S.; Capelo, J.L.; Lodeiro, C. A New 3,5-Bisporphyrinylpyridine Derivative as a Fluorescent Ratiometric Probe for Zinc Ions. Chemistry 2014, 20, 6684–6692. [Google Scholar] [CrossRef] [PubMed]
- Moura, N.M.M.; Núñez, C.; Santos, S.M.; Faustino, M.A.F.; Cavaleiro, J.A.S.; Neves, M.G.P.M.S.; Capelo, J.L.; Lodeiro, C. Synthesis, Spectroscopy Studies, and Theoretical Calculations of New Fluorescent Probes Based on Pyrazole Containing Porphyrins for Zn(II), Cd(II), and Hg(II) Optical Detection. Inorg. Chem. 2014, 53, 6149–6158. [Google Scholar] [CrossRef] [PubMed]
- Furuta, H.; Asano, T.; Ogawa, T. “N-Confused Porphyrin”: A New Isomer of Tetraphenylporphyrin. J. Am. Chem. Soc. 1994, 116, 767–768. [Google Scholar] [CrossRef]
- Toganoh, M.; Miyachi, H.; Akimaru, H.; Ito, F.; Nagamura, T.; Furuta, H. Anion responsive dyad system of porphyrin and N-confused porphyrin. Org. Biomol. Chem. 2009, 7, 3027–3030. [Google Scholar] [CrossRef]
- Won, D.-H.; Toganoh, M.; Uno, H.; Furuta, H. Pt(II) N-confused porphyrin: An expanded pyrrole that affords a stable π-anion. Dalton Trans. 2009, 705209, 6151–6158. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Morimoto, T.; Furuta, H. SnIV complexes of N-confused porphyrins and oxoporphyrins—Unique fluorescence “switch-on” halide receptors. Angew. Chem. Int. Ed. 2006, 45, 6907–6910. [Google Scholar] [CrossRef] [PubMed]
- Geier, R.G.; Lindsey, J.S. N-Confused Tetraphenylporphyrin and Tetraphenylsapphyrin Formation in One-Flask Syntheses of Tetraphenylporphyrin. J. Org. Chem. 1999, 64, 1596–1603. [Google Scholar] [CrossRef]
- Xiao, Z.; Patrick, B.O.; Dolphin, D. Inner C-cyanide addition and nucleophilic addition to Ni (II) N-confused porphyrins. Chem. Commun. 2003, 2, 1062–1063. [Google Scholar] [CrossRef]
- Toganoh, M.; Furuta, H. Blooming of confused porphyrinoids—Fusion, expansion, contraction, and more confusion. Chem. Commun. 2012, 48, 937–954. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.D.; Ziegler, C.J. The metal complexes of N-confused porphyrin as heme model compounds. J. Inorg. Biochem. 2006, 100, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.D.; Ziegler, C.J. Developments in the metal chemistry of N-confused porphyrin. Coord. Chem. Rev. 2003, 247, 1–19. [Google Scholar] [CrossRef]
- Yamamoto, T.; Toganoh, M.; Mori, S.; Uno, H.; Furuta, H. Rhenium complexes of peripherally π-extended N-confused porphyrins. Chem. Sci. 2012, 3, 3241–3248. [Google Scholar] [CrossRef]
- Qu, W.; Ding, T.; Cetin, A.; Harvey, J.D.; Taschner, M.J.; Ziegler, C.J. Facile Peripheral Modification of N-Confused Porphyrin. J. Org. Chem. 2006, 71, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, M.Q.; Menezes, J.C.J.M.D.S.; Pires, S.M.G.; Neves, M.G.P.M.S.; Simões, M.M.Q.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Daniel-da-Silva, A.L.; Almeida, A.; et al. Pyrrolidine-fused chlorin photosensitizer immobilized on solid supports for the photoinactivation of Gram negative bacteria. Dyes Pigments 2014, 110, 123–133. [Google Scholar] [CrossRef]
- Gomes, M.T.S.R.; Costa, J.R.M.L.; Oliveira, J.A.B.P. The quantification of sodium in mineral waters using a quartz crystal microbalance. Talanta 2003, 59, 247–252. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals, 7th ed.; Butterworth-Heinemann, Ed.; Elsevier: Oxford, UK, 2013; ISBN 978-0-12-382161-4. [Google Scholar]
Sample Availability: Samples of the compounds NCTPP-Si, NCTPP-MF, NCTPP-Si-Py+ and NCTPP-MF-Py+ are available from the authors. |










| Material | Metal | Sensitivity (103 Hz L mol−1) |
|---|---|---|
| NCTPP-Pr+ | Tl (I) | 28.3 |
| Cr(III) | 99.7 | |
| NCTPP-Si | Tl (I) | 35.4 |
| Cr(III) | 98.9 | |
| NCTPP-Si-Py+ | Tl (I) | 21.9 |
| Cr(III) | 15.0 | |
| NCTPP-MF | Tl (I) | 21.9 |
| Cr(III) | 96.1 | |
| NCTPP-MF-Py+ | Tl (I) | 26.3 |
| Cr(III) | 60.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamelas, S.R.D.; Gomes, A.T.P.C.; Moura, N.M.M.; Faustino, M.A.F.; Cavaleiro, J.A.S.; Lodeiro, C.; Veríssimo, M.I.S.; Fernandes, T.; Daniel-da-Silva, A.L.; Gomes, M.T.S.R.; et al. N-Confused Porphyrin Immobilized on Solid Supports: Synthesis and Metal Ions Sensing Efficacy. Molecules 2018, 23, 867. https://doi.org/10.3390/molecules23040867
Gamelas SRD, Gomes ATPC, Moura NMM, Faustino MAF, Cavaleiro JAS, Lodeiro C, Veríssimo MIS, Fernandes T, Daniel-da-Silva AL, Gomes MTSR, et al. N-Confused Porphyrin Immobilized on Solid Supports: Synthesis and Metal Ions Sensing Efficacy. Molecules. 2018; 23(4):867. https://doi.org/10.3390/molecules23040867
Chicago/Turabian StyleGamelas, Sara R. D., Ana T. P. C. Gomes, Nuno M. M. Moura, Maria A. F. Faustino, José A. S. Cavaleiro, Carlos Lodeiro, Marta I. S. Veríssimo, Tiago Fernandes, Ana L. Daniel-da-Silva, M. Teresa S. R. Gomes, and et al. 2018. "N-Confused Porphyrin Immobilized on Solid Supports: Synthesis and Metal Ions Sensing Efficacy" Molecules 23, no. 4: 867. https://doi.org/10.3390/molecules23040867
APA StyleGamelas, S. R. D., Gomes, A. T. P. C., Moura, N. M. M., Faustino, M. A. F., Cavaleiro, J. A. S., Lodeiro, C., Veríssimo, M. I. S., Fernandes, T., Daniel-da-Silva, A. L., Gomes, M. T. S. R., & Neves, M. G. P. M. S. (2018). N-Confused Porphyrin Immobilized on Solid Supports: Synthesis and Metal Ions Sensing Efficacy. Molecules, 23(4), 867. https://doi.org/10.3390/molecules23040867