Preconcentration of Trace Neonicotinoid Insecticide Residues Using Vortex-Assisted Dispersive Micro Solid-Phase Extraction with Montmorillonite as an Efficient Sorbent
Abstract
:1. Introduction
2. Result and Discussion
2.1. Optimization of the VA-d-µ-SPE Procedure
2.1.1. Effect of the Sorbent Amount
2.1.2. Effect of Sample Volume
2.1.3. Ionic Strength
2.1.4. Effect of Type and Volume of the Desorption Solvent
2.1.5. Effect of Vortex Time
2.1.6. Reusability of Adsorbent
2.2. Analytical Performance of the Proposed Method
2.3. Real Samples Analysis
2.4. Comparison of the Proposed VA-d-μ-SPE Method with Other Sample Preparation Methods
3. Experimental
3.1. Chemicals and Reagents
3.2. Apparatus
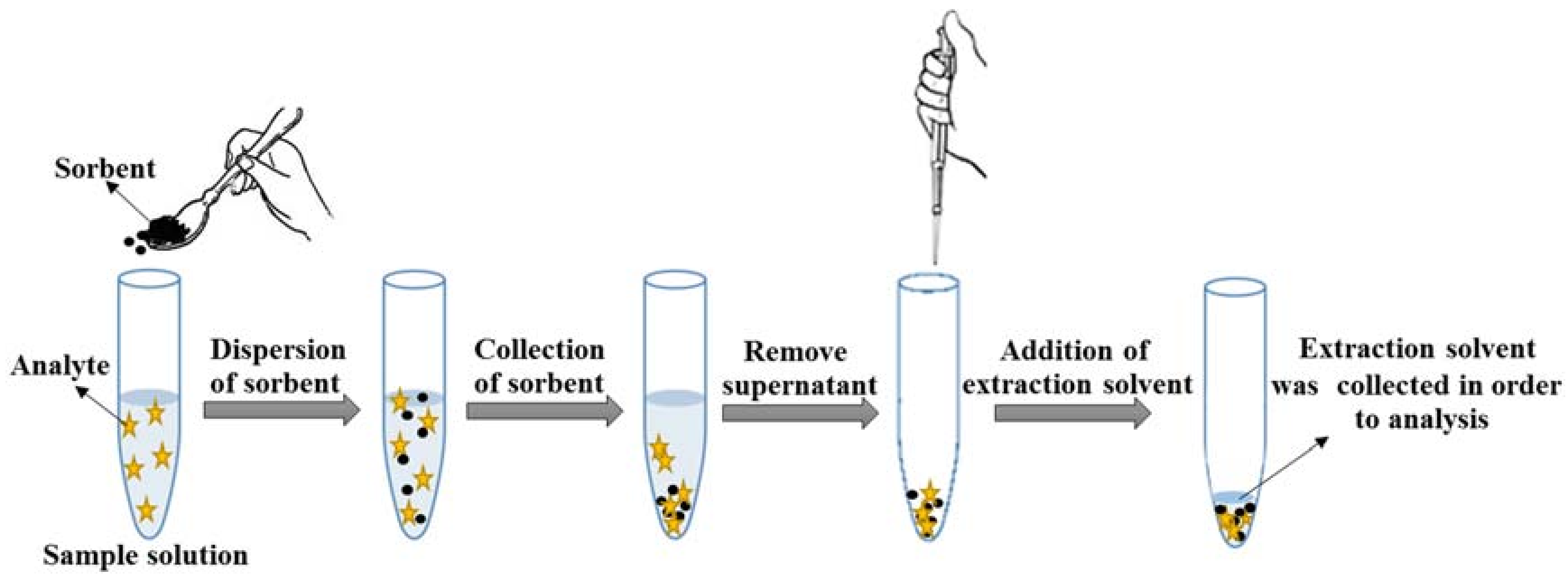
3.3. VA-d-µ-SPE Procedure
3.4. Preparation of Samples
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Di, A.; Fidente, P.; Attard, D.; Dommarco, R.; Seccia, S.; Morrica, P. Application of solid-phase extraction and liquid chromatography-mass spectrometry to the determination of neonicotinoid pesticide residues in fruit and vegetables. J. Chromatogr. A. 2006, 1108, 1–6. [Google Scholar]
- Lazic, S.; Franko, M.; Sakac, M.; Jovanov, P.; Kos, J. Development of HPLC-DAD method for determination of neonicotinoids in honey. J. Food Compos. Anal. 2015, 40, 106–113. [Google Scholar]
- Biever, M.P.; Hoberg, J.R.; Jacobson, B.; Dionne, E.; Sulaiman, M. Icon rice seed treatment toxicity to crayfish (Procambarus clarkii) in experimental rice paddies. Environ. Toxicol. Chem. 2003, 22, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, X.; Yin, X.; Wang, C.; Wang, Z. Dispersive liquid-liquid microextraction combined with sweeping micellar electrokinetic chromatography for the determination of some neonicotinoid insecticides in cucumber samples. Food Chem. 2012, 133, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Casado, J.; Rodríguez, I.; Ramil, M.; Cela, R. Selective extraction and determination of neonicotinoid insecticides in wine by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A. 2016, 1460, 9–15. [Google Scholar]
- EU Pesticides Database. Available online: http://ec.europa.eu/sanco_pesticides/public/?event-activesubstance.selection (accessed on 21 March 2018).
- Zhou, Q.; Ding, Y.; Xiao, J. Sensitive determination of thiamethoxam, imidacloprid and acetamiprid in environmental water samples with solid-phase extraction packed with multiwalled carbon nanotubes prior to high-performance liquid chromatography. Anal. Bioanal. Chem. 2006, 385, 1520–1525. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Bamorowat, M.; Reza, M.; Mogaddam, A. Ringer tablet-based ionic liquid phase microextraction: Application in extraction and preconcentration of neonicotinoid insecticides from fruit juice and vegetable samples. Talanta 2016, 160, 211–216. [Google Scholar]
- Valverde, R.S.; Galanti, A.; Girotti, S.; Garc, M.D.G.; Mart, M. Column switching liquid chromatography and post-column photochemically fluorescence detection to determine imidacloprid and 6-chloronicotinic acid in honeybees. J. Chromatogr. A. 2007, 1147, 17–23. [Google Scholar]
- Abdel-Ghany, M.F.; Hussein, L.A.; El, N.F.; El–khatib, A.H.; Linscheid, M.W. Simultaneous determination of eight neonicotinoid insecticide residues and two primary metabolites in cucumbers and soil by liquid chromatography-tandem mass spectrometry coupled with QuEChERS. J. Chromatogr. B. 2016, 1031, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Belda, M.; Garrido, I.; Campillo, N.; Viñas, P.; Hellín, P.; Flores, P.; Fenoll, J. Determination of spirocyclic tetronic/tetramic acid derivatives and neonicotinoid insecticides in fruits and vegetables by liquid chromatography and mass spectrometry after dispersive liquid-liquid microextraction. Food Chem. 2016, 202, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Vichapong, J.; Burakham, R.; Srijaranai, S. Vortex-assisted surfactant-enhanced-emulsification liquid-liquid microextraction with solidification of floating organic droplet combined with HPLC for the determination of neonicotinoid pesticides. Talanta 2013, 117, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Han, C.; Qian, Y.; Ding, H.; Chen, X.; Xi, J. Determination of neonicotinoid pesticides residues in agricultural samples by solid-phase extraction combined with liquid chromatography-tandem mass spectrometry. J. Chromatogr. A. 2011, 1218, 4426–4433. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yang, X.; Wang, J.; Cui, J.; Dong, A.J.; Zhao, H.T.; Zhang, L.W.; Wang, Z.Y.; Xu, R.B.; Li, W.J.; et al. Multi-residue method for determination of seven neonicotinoid insecticides in grains using dispersive solid-phase extraction and dispersive liquid-liquid micro-extraction by high performance liquid chromatography. Food Chem. 2012, 134, 1691–1698. [Google Scholar] [CrossRef] [PubMed]
- Jansson, C.; Pihlström, T.; Österdahl, B.; Markides, K.E. A new multi-residue method for analysis of pesticide residues in fruit and vegetables using liquid chromatography with tandem mass spectrometric detection. J. Chromatogr. A. 2004, 1023, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Grujic, S.; Vasiljevic, T.; Lauševic, M.; Radišic, M. Determination of selected pesticides in fruit juices by matrix solid-phase dispersion and liquid chromatography-tandem mass spectrometry. Food Chem. 2009, 113, 712–719. [Google Scholar]
- Cai, L.; Xing, J.; Dong, L.; Wu, C. Application of polyphenylmethylsiloxane coated fiber for solid-phase microextraction combined with microwave-assisted extraction for the determination of organochlorine pesticides in Chinese teas. J. Chromatogr. A. 2003, 1015, 11–21. [Google Scholar] [CrossRef]
- Zohrabi, P.; Shamsipur, M.; Hashemi, M.; Hashemi, B. Liquid-phase microextraction of organophosphorus pesticides using supramolecular solvent as a carrier for ferrofluid. Talanta 2016, 160, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Cazorla-Reyes, R.; Fernández-Moreno, J.L.; Romero-González, R.; Garrido, A.; Luis, J.; Vidal, M. Single solid phase extraction method for the simultaneous analysis of polar and non-polar pesticides in urine samples by gas chromatography and ultrahigh-pressure liquid chromatography coupled to tandem mass spectrometry. Talanta 2011, 85, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Arthur, C.L.; Pawliszyn, J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- He, Y.; Lee, H.K. Liquid-Phase Microextraction in a Single Drop of Organic Solvent by Using a Conventional Microsyringe. Anal. Chem. 1997, 69, 4634–4640. [Google Scholar] [CrossRef]
- Stanisz, E.; Werner, J.; Zgoła-Grzes, A. Liquid-phase microextraction techniques based on ionic liquids for preconcentration and determination of metals. Trends Anal. Chem. 2014, 61, 54–66. [Google Scholar] [CrossRef]
- Khezeli, T.; Daneshfar, A. Development of dispersive micro-solid phase extraction based on micro and nano sorbents. Trends Anal. Chem. 2017, 89, 99–118. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenck, F. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [PubMed]
- Huck, C.W.; Bonn, G.K. Recent Developments in Polymer-Based Sorbents for Solid-Phase Extraction. J. Chromatogr. A. 2000, 885, 51–72. [Google Scholar] [CrossRef]
- Zielke, R.C.; Pinnavaia, T.J. Modified clays for the adsorption of environmental toxicants: binding of chlorophenols to pillared, delaminated, and hydroxy-interlayered smectities. Clay Clay Miner. 1998, 36, 403–408. [Google Scholar] [CrossRef]
- Zhao, G.; Zhou, L.; Li, Y.; Liu, X.; Ren, X.; Liu, X. Enhancement of phenol degradation using immobilized microorganisms and organic modified montmorillonite in a two-phase partitioning bioreactor. J. Hazard. Mater. 2009, 169, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Salehinia, S.; Ghoreishi, S.M.; Maya, F.; Cerdà, V. Hydrophobic magnetic montmorillonite composite material for the efficient adsorption and microextraction of bisphenol A from water samples. J. Environ. Chem. Eng. 2016, 4, 4062–4071. [Google Scholar] [CrossRef]
- Pelit, F.O.; Pelit, L.; Dizdaş, T.N.; Aftafa, C.; Ertaş, H.; Yalçınkaya, E.E.; Turkmen, H.; Ertaş, F.N. A novel polythiophene-ionic liquid modified clay composite solid phase microextraction fiber: Preparation, characterization and application to pesticide analysis. Anal. Chim. Acta 2015, 859, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Aftafa, C.; Pelit, F.O.; Yalçinkaya, E.E.; Turkmen, H.; Kapdan, I.; Nil Ertaş, F. Ionic liquid intercalated clay sorbents for micro solid phase extraction of steroid hormones from water samples with analysis by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2014, 1361, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.T.; Saraji, M.; Sherafatmand, H. Polypyrrole/montmorillonite nanocomposite as a new solid phase microextraction fiber combined with gas chromatography-corona discharge ion mobility spectrometry for the simultaneous determination of diazinon and fenthion organophosphorus pesticides. Anal. Chim. Acta 2014, 814, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Naing, N.N.; Li, S.F.Y.; Lee, H.K. Graphene oxide-based dispersive solid-phase extraction combined with in situ derivatization and gas chromatography-mass spectrometry for the determination of acidic pharmaceuticals in water. J. Chromatogr. A. 2015, 1426, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, M.; Rajabi, M.; Asghari, A. Ultrasound-promoted dispersive micro solid-phase extraction of trace anti-hypertensive drugs from biological matrices using a sonochemically synthesized conductive polymer nanocomposite. Ultrason. Sonochem. 2017, 39, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Seebunrueng, K.; Santaladchaiyakit, Y.; Srijaranai, S. Vortex-assisted low density solvent based demulsified dispersive liquid-liquid microextraction and high-performance liquid chromatography for the determination of organophosphorus pesticides in water samples. Chemosphere 2014, 103, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Jalilian, N.; Ebrahimzadeh, H.; Asgharinezhad, A.A. Dispersive micro-solid phase extraction of aromatic amines based on an efficient sorbent made from poly (1,8-diaminonaphtalen) and magnetic multiwalled carbon nanotubes composite. J. Chromatogr. A. 2017, 1499, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Sarafraz–Yazdi, A.; Mofazzeli, F.; Es’haghi, Z. A new high-speed hollow fiber based liquid phase microextraction method using volatile organic solvent for determination of aromatic amines in environmental water samples prior to high-performance liquid chromatography. Talanta 2009, 79, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Salisaeng, P.; Arnnok, P.; Patdhanagul, N.; Burakham, R. Vortex-Assisted Dispersive Micro-Solid Phase Extraction Using CTAB-Modified Zeolite NaY Sorbent Coupled with HPLC for the Determination of Carbamate Insecticides. J. Agric. Food Chem. 2016, 64, 2145–2152. [Google Scholar] [CrossRef] [PubMed]
- Seccia, S.; Fidente, P.; Attard, D.; Morrica, P. Multiresidue determination of nicotinoid insecticide residues in drinking water by liquid chromatography with electrospray ionization mass spectrometry. Anal. Chim. Acta 2005, 553, 21–26. [Google Scholar] [CrossRef]
- Jovanov, P.; Guzsvány, V.; Franko, M.; Lazić, S.; Sakač, M.; Šarić, B.; Banjac, V. Multi-residue method for determination of selected neonicotinoid insecticides in honey using optimized dispersive liquid–liquid microextraction combined with liquid chromatography-tandem mass spectrometry. Talanta 2013, 111, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Campillo, N.; Viñas, P.; Férez–Melgarejo, G.; Hernández–Córdoba, M. Liquid chromatography with diode array detection and tandem mass spectrometry for the determination of neonicotinoid insecticides in honey samples using dispersive liquid-liquid microextraction. J. Agric. Food Chem. 2013, 61, 4799–4805. [Google Scholar] [CrossRef] [PubMed]







| Insecticide | Linear Equation | Linearity (ng mL−1) | Coefficient of Determination (R2) | LOD (ng mL−1) | LOQ (ng mL−1) | Intra-Day (n = 3) %RSD | Inter-Days (n = 3 days × 3) %RSD | EF | ||
|---|---|---|---|---|---|---|---|---|---|---|
| tR | Peak Area | tR | Peak Area | |||||||
| Thiamethoxam | y = 1894030x + 256 | 0.5–1000 | 0.9951 | 0.009 | 0.031 | 0.46 | 2.75 | 0.21 | 4.58 | 73 |
| Clothianidin | y = 3990993x − 3842 | 0.5–1000 | 0.9972 | 0.013 | 0.042 | 0.30 | 2.97 | 0.16 | 5.71 | 176 |
| Imidacloprid | y = 1385642x – 6607 | 0.5–1000 | 0.9940 | 0.006 | 0.010 | 0.30 | 2.85 | 0.16 | 5.10 | 8 |
| Acetamiprid | y = 945451x – 4414 | 0.5–1000 | 0.9963 | 0.005 | 0.008 | 0.28 | 3.24 | 0.44 | 4.99 | 8 |
| Thiacloprid | y = 2249620x + 43266 | 0.5–1000 | 0.9980 | 0.065 | 0.263 | 0.23 | 3.26 | 0.14 | 7.17 | 16 |
| Sample | Market | Amount Found ± SD, μg mL−1 (n = 2) | ||||
|---|---|---|---|---|---|---|
| Thiamethoxam | Clothianidin | Imidacloprid | Acetamiprid | Thiacloprid | ||
| Watermelon | Local Market | 0.03 ± 0.007 | 0.05 ± 0.003 | - | - | 0.007 ± 0.01 |
| Super Market | 0.11 ± 0.08 | 0.01 ± 0.007 | 0.07 ± 0.1 | 0.27 ± 0.1 | 0.005 ± 0.01 | |
| Grape | Local Market | 0.04 ± 0.01 | 0.04 ± 0.002 | - | 0.06 ± 0.03 | 0.01 ± 0.01 |
| Super Market | 0.26 ± 0.02 | 0.03 ± 0.003 | - | - | - | |
| Longan | Local Market | - | - | - | - | - |
| Super Market | - | - | - | - | - | |
| Water sample | Water I | - | - | - | - | - |
| Water II | - | - | - | - | - | |
| Water III | - | - | - | - | - | |
| Method | Analytes | Sample | Analytical Technique | Linearity | LOD | Recovery (%) | Reference |
|---|---|---|---|---|---|---|---|
| DSPE-DLLME | Nitenpyram, Dinotefuran, Clothianidin, Thiamethoxam, Acetamiprid, Imidacloprid, Thiacloprid | Grain | HPLC-DAD | 0.02–4.5 μg mL−1 | 0.002–0.005 mg kg−1 | 76–123 | [14] |
| VSLLME-SFO | Acetamiprid, Clotianidin, Nitenpyram, Imidacloprid, Thiamathoxam | Water and fruit juice | HPLC-DAD | 0.0005-5 µg mL−1 | 0.1–0.5 μg L−1 | 85–105 | [12] |
| SPE | Acetamiprid, Imidacloprid, Thiacloprid, Thiamethoxam | Drinking water | LC-ESI-MS | 0–1 mg L−1 | 0.01 µg L−1 | 95–104 | [38] |
| DLLME | Acetamiprid, Clothianidin, Thiamethoxam, Imidacloprid, Dinotefuran, Thiacloprid, Nitenpyram | Honey | LC-MS/MS | 1.5–100.0 µg kg−1 | 0.5–1.0 μg kg−1 | 74.3–113.9 | [39] |
| SPE-DLLME | Thiamethoxam, Clothianidin, Imidacloprid, Acetamiprid, Thiacloprid | Honey | LC-APCI-IT-MS/MS | 0.1–7500 ng g−1 | 0.2–1.0 ng g−1 | 90–104 | [40] |
| DLLME | Thiacloprid, Acetamiprid, Imidaclothiz, Imidacloprid | Cucumber | MEKC | 2.7–200 ng g−1 | 0.8–1.2 ng g−1 | 79.7–98 | [4] |
| VA-D-µ-SPE | Imidacloprid, Acetamiprid, Clothianidin, Thiacloprid, Thiamethoxam | Fruit juice and natural surface water | HPLC-DAD | 0.5–1000 ng mL−1 | 0.005–0.065 ng mL−1 | 8–176 | This study |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moyakao, K.; Santaladchaiyakit, Y.; Srijaranai, S.; Vichapong, J. Preconcentration of Trace Neonicotinoid Insecticide Residues Using Vortex-Assisted Dispersive Micro Solid-Phase Extraction with Montmorillonite as an Efficient Sorbent. Molecules 2018, 23, 883. https://doi.org/10.3390/molecules23040883
Moyakao K, Santaladchaiyakit Y, Srijaranai S, Vichapong J. Preconcentration of Trace Neonicotinoid Insecticide Residues Using Vortex-Assisted Dispersive Micro Solid-Phase Extraction with Montmorillonite as an Efficient Sorbent. Molecules. 2018; 23(4):883. https://doi.org/10.3390/molecules23040883
Chicago/Turabian StyleMoyakao, Khwankaew, Yanawath Santaladchaiyakit, Supalax Srijaranai, and Jitlada Vichapong. 2018. "Preconcentration of Trace Neonicotinoid Insecticide Residues Using Vortex-Assisted Dispersive Micro Solid-Phase Extraction with Montmorillonite as an Efficient Sorbent" Molecules 23, no. 4: 883. https://doi.org/10.3390/molecules23040883
APA StyleMoyakao, K., Santaladchaiyakit, Y., Srijaranai, S., & Vichapong, J. (2018). Preconcentration of Trace Neonicotinoid Insecticide Residues Using Vortex-Assisted Dispersive Micro Solid-Phase Extraction with Montmorillonite as an Efficient Sorbent. Molecules, 23(4), 883. https://doi.org/10.3390/molecules23040883





