Synthesis and Evaluation of C15 Triene Urushiol Derivatives as Potential Anticancer Agents and HDAC2 Inhibitor
Abstract
1. Introduction
2. Results
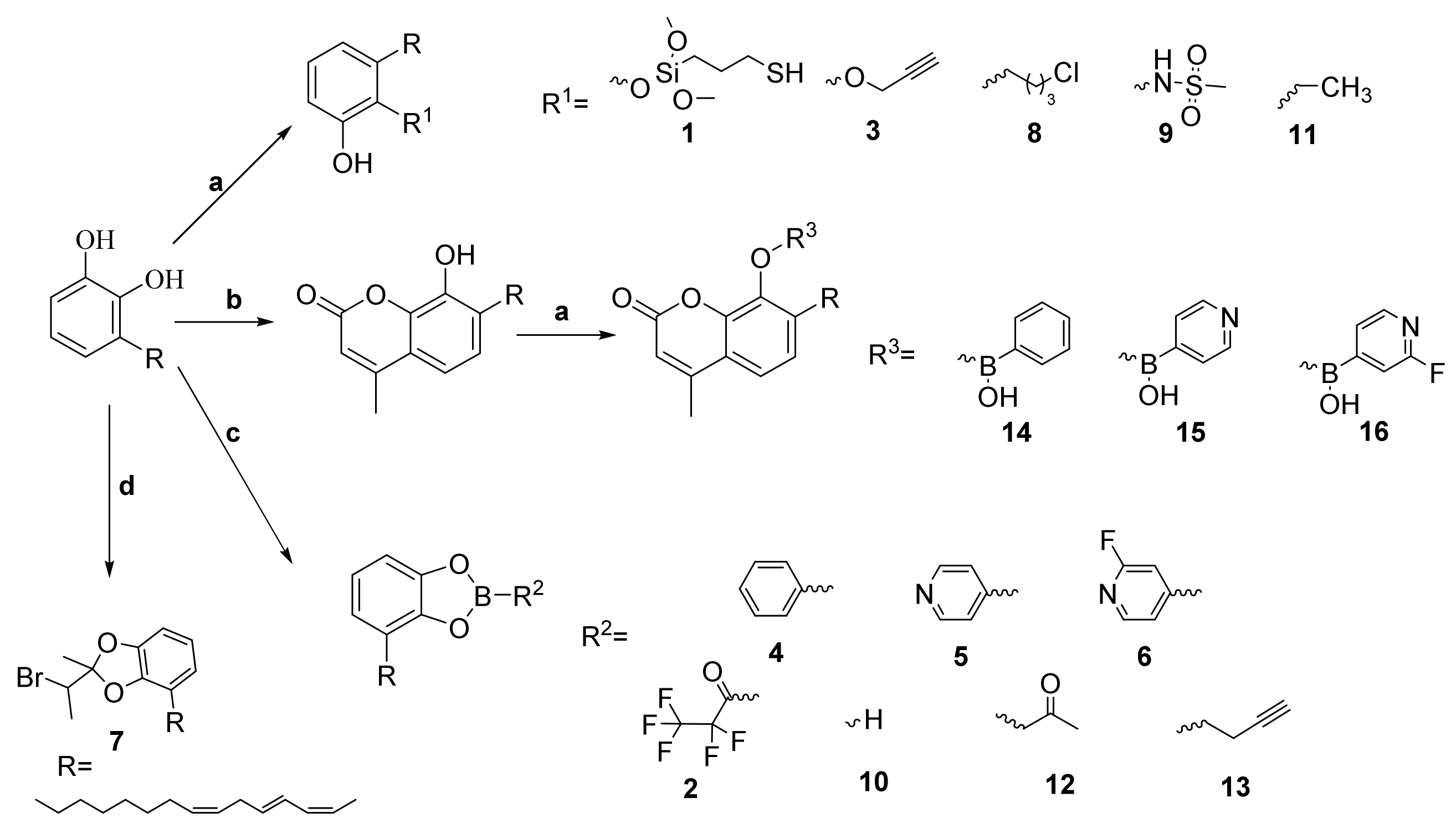
2.1. Chemistry
2.2. Anti-Tumor Activity
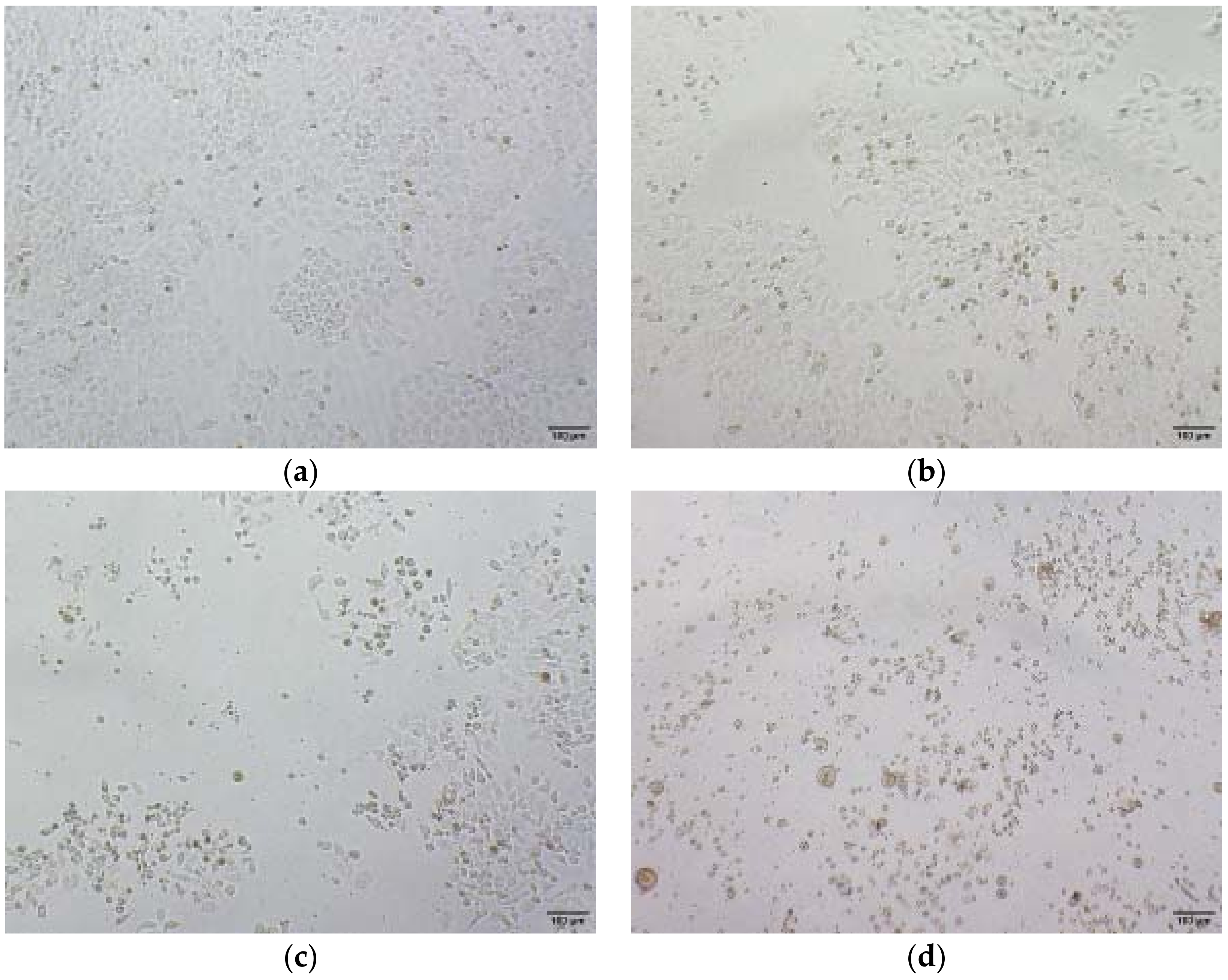
2.3. Inhibition of Cell Migration
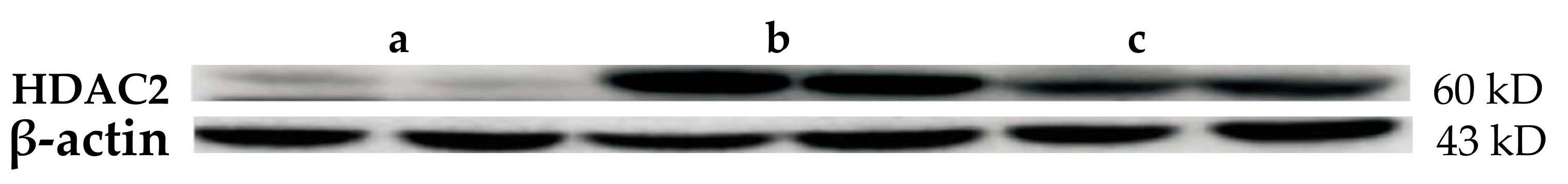
2.4. Western Blot Results
2.5. FCM-Flow Cytometry Analysis
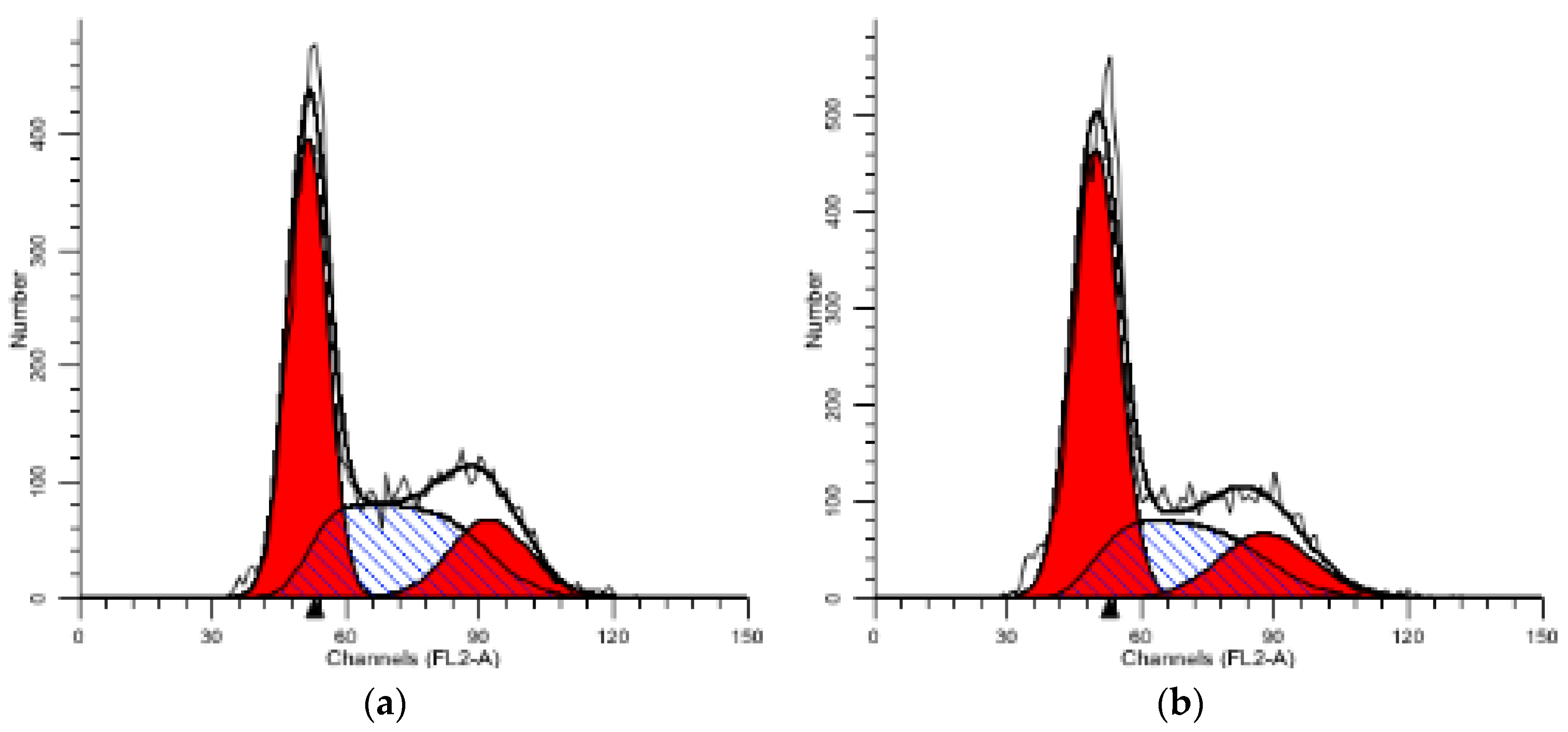
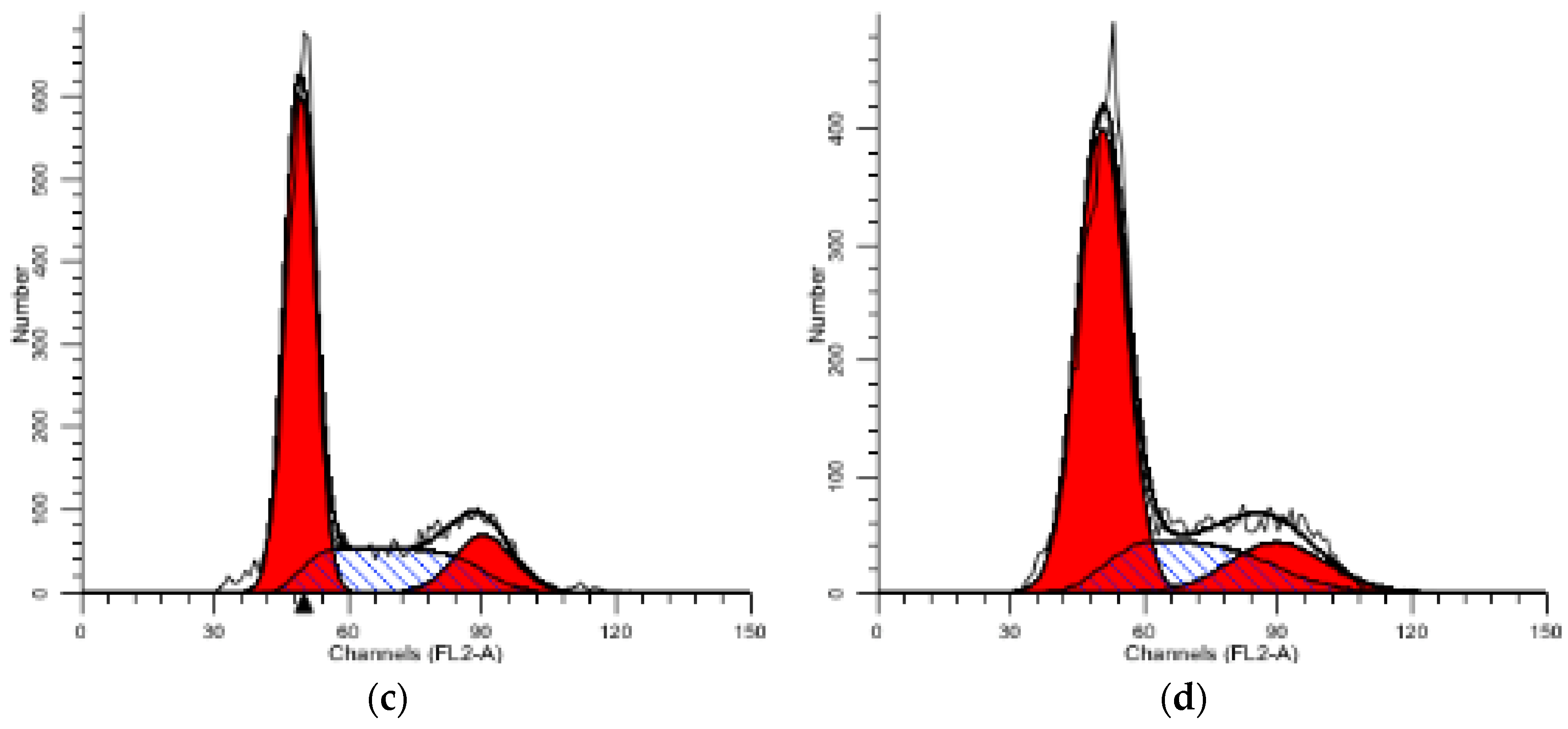
2.5.1. PI Single Staining Assay for Cell Cycle
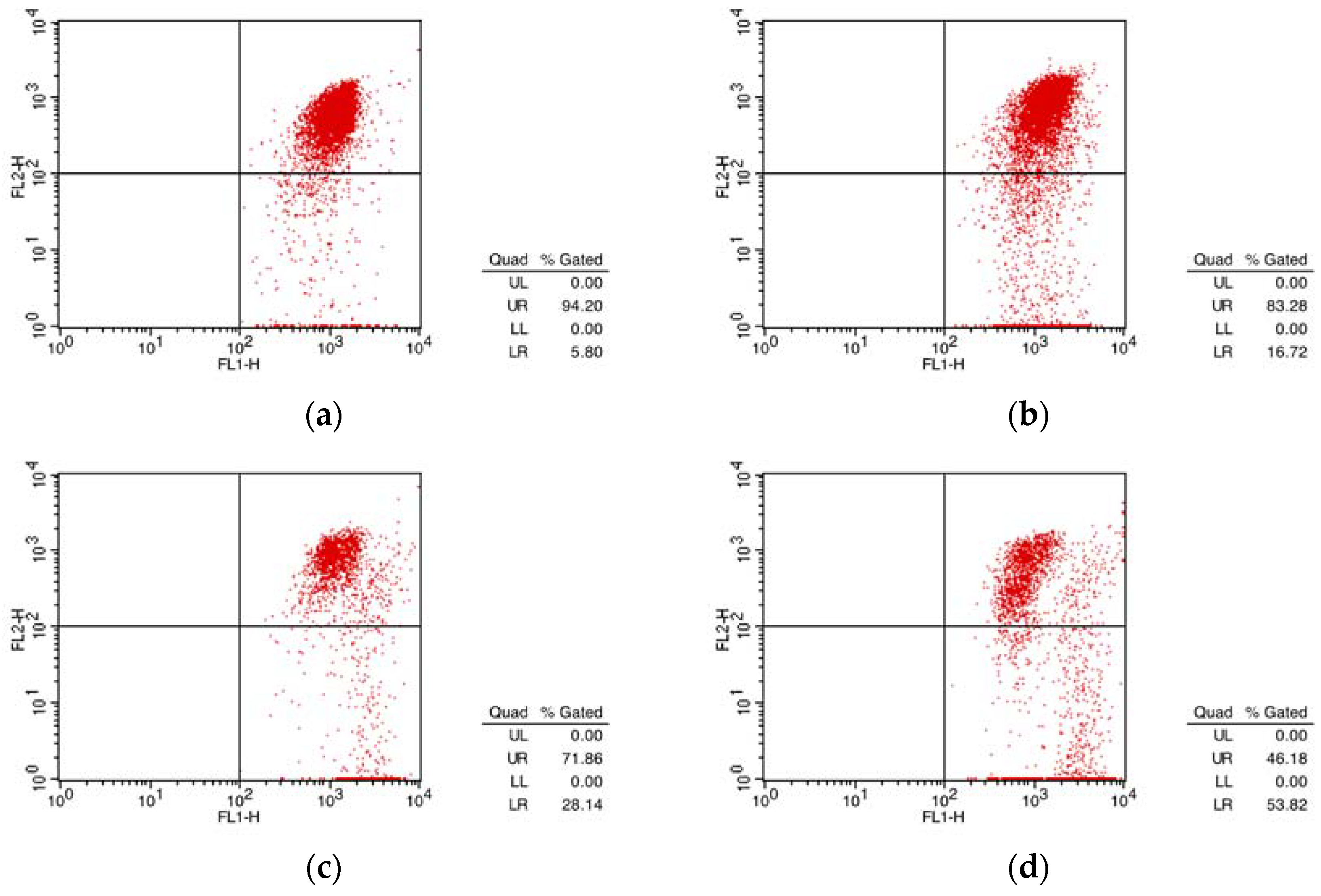
2.5.2. Annexin-V FITC/PI Double Staining for Apoptosis
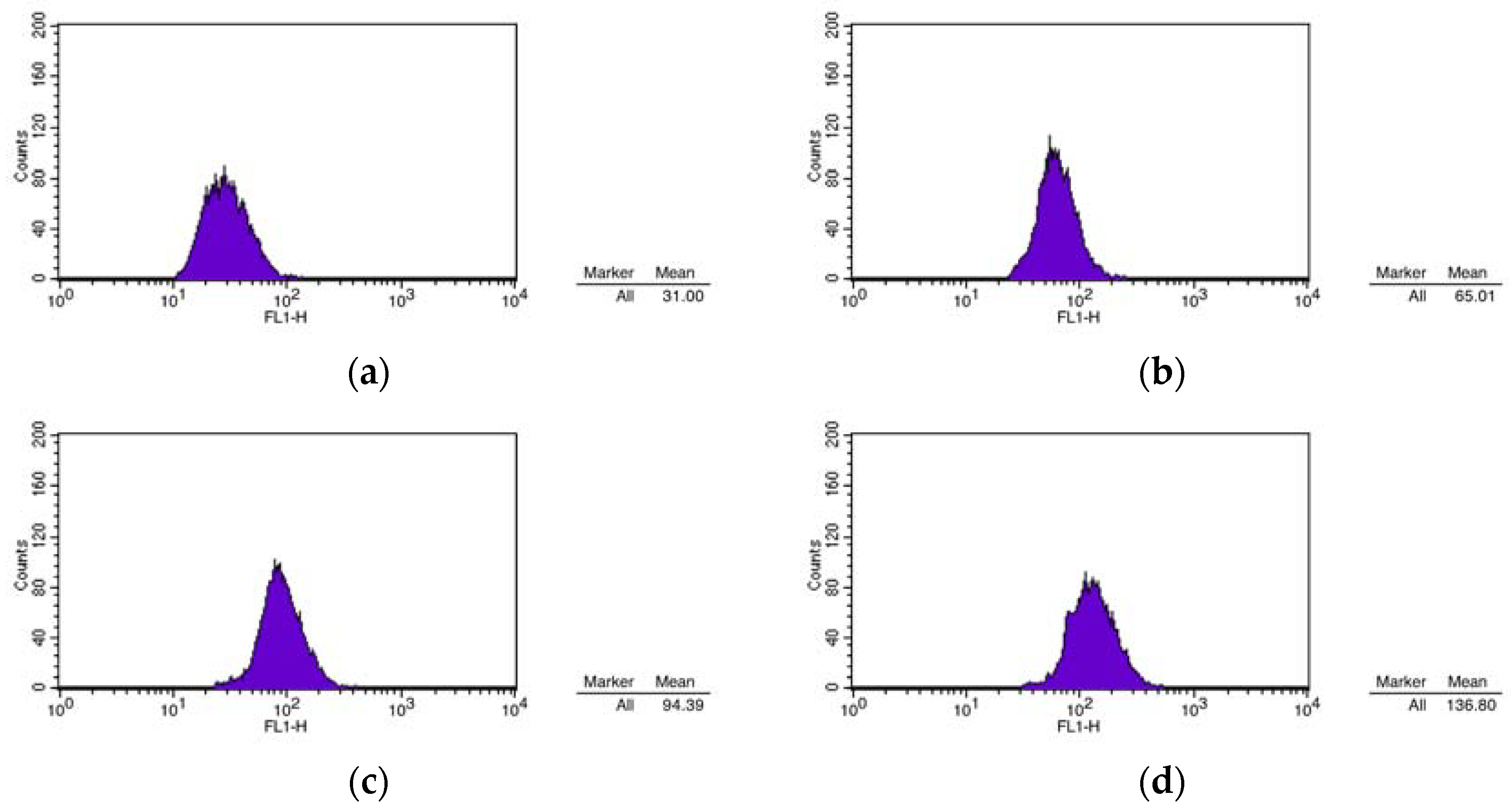
2.5.3. JC-1 Staining Assay Mitochondrial Membrane Potential
2.5.4. Calcium Content Detection
2.6. Molecular Docking
2.6.1. Conjunction Conformation Score
2.6.2. Binding Pattern Analysis
3. Materials and Methods
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. synthesis of Triene Uruhsiol Hydroxyl Protection Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Watanabe, H.; Fujimoto, A.; Takahara, A. Characterization of catechol-containing natural thermosetting polymer “urushiol” thin film. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 3688–3692. [Google Scholar] [CrossRef]
- Ryckewaert, L.; Sacconnay, L.; Carrupt, P.A.; Nurisso, A.; Simoes-Pires, C. Non-specific SIRT inhibition as a mechanism for the cytotoxicity of ginkgolic acids and urushiols. Toxicol. Lett. 2014, 229, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Jang, I.S.; Park, J.W.; Jo, E.B.; Cho, C.K.; Lee, Y.W.; Yoo, H.S.; Park, J.; Kim, J.; Jang, B.C.; Choi, J.S. Growth inhibitory and apoptosis-inducing effects of allergen-free Rhus verniciflua Stokes extract on A549 human lung cancer cells. Oncol. Rep. 2016, 36, 3037–3043. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ki Tae Suk, S.K.B. Antibacterial Effects of the Urushiol Component in the Sap of the Lacquer Tree (Rhus verniciflua Stokes) on Helicobacter pylori. Helicobacter 2011, 16, 434–443. [Google Scholar]
- Hong, S.H.; Suk, K.T.; Choi, S.H.; Lee, J.W.; Sung, H.T.; Kim, C.H.; Kim, E.J.; Kim, M.J.; Han, S.H.; Kim, M.Y.; et al. Anti-oxidant and natural killer cell activity of Korean red ginseng (Panax ginseng) and urushiol (Rhus vernicifera Stokes) on non-alcoholic fatty liver disease of rat. Food Chem. Toxicol. 2013, 55, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.S.; Shin, D.H. Antibacterial capacity of cavity disinfectants against Streptococcus mutans and their effects on shear bond strength of a self-etch adhesive. Dent. Mater. J. 2016, 35, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Park, K.Y.; Kim, S.J.; Oh, S.; Moon, J.H. Antimicrobial activity of the synthesized non-allergenic urushiol derivatives. Biosci. Biotechnol. Biochem. 2015, 79, 1915–1918. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, C.; Ye, J.; Chen, H.; Tao, R. Design, virtual screening, molecular docking and molecular dynamics studies of novel urushiol derivatives as potential HDAC2 selective inhibitors. Gene 2017, 637, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, C.; Deng, T.; Tao, R.; Li, W. Novel urushiol derivatives as HDAC8 inhibitors: Rational design, virtual screening, molecular docking and molecular dynamics studies. J. Biomol. Struct. Dyn. 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Bi, Y.; He, Y.; Huang, W.; Liu, L.; Li, Y.; Zhang, S.; Liu, X.; Yu, N. Design, synthesis and biological evaluation of di-substituted cinnamic hydroxamic acids bearing urea/thiourea unit as potent histone deacetylase inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 6432–6435. [Google Scholar] [CrossRef] [PubMed]
- Hildmann, C.; Riester, D.; Schwienhorst, A. Histone deacetylases—An important class of cellular regulators with a variety of functions. Appl. Microbiol. Biotechnol. 2007, 75, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Drummond, D.C.; Noble, C.O.; Kirpotin, D.B.; Guo, Z.; Scott, G.K.; Benz, C.C. Clinical development of histone deacetylase inhibitors as anticancer agents. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 495–528. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Jalla, A.; Abou Shama, M.; Stafford, J.; Cao, B. Chromatography-Free and Eco-Friendly Synthesis of Aryl Tosylates and Mesylates. Synthesis 2015, 47, 2578–2585. [Google Scholar] [CrossRef]
- Chen, D.Z.; Jing, C.X.; Cai, J.Y.; Wu, J.B.; Wang, S.; Yin, J.L.; Li, X.N.; Li, L.; Hao, X.J. Design, Synthesis, and Structural Optimization of Lycorine-Derived Phenanthridine Derivatives as Wnt/beta-Catenin Signaling Pathway Agonists. J. Nat. Prod. 2016, 79, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.-F.; Huang, W.-B.; Guo, Y.; Jiang, J.-A.; Pan, X.-D.; Liao, D.-H. An Efficient Strategy for Protecting Dihydroxyl Groups of Catechols. Synlett 2013, 24, 741–746. [Google Scholar] [CrossRef]
- Rezayati, S.; Sheikholeslami-Farahani, F.; Rostami-Charati, F.; Abad, S.A.S. One-pot synthesis of coumarine derivatives using butylenebispyridinium hydrogen sulfate as novel ionic liquid catalyst. Res. Chem. Intermed. 2015, 42, 4097–4107. [Google Scholar] [CrossRef]
- Potdar, M.K. Coumarin syntheses via Pechmann condensation in Lewis acidic chloroaluminate ionic liquid. Tatrahedron Lett. 2001, 42, 9285–9287. [Google Scholar] [CrossRef]
- Khandekar, A.C.; Khadilkar, B.M. Pechmann Reaction in Chloroaluminate Ionic Liquid. Synlett 2002, 1, 152–154. [Google Scholar] [CrossRef]
- Kaupp, G.; Naimi-Jamal, M.R.; Stepanenko, V. Waste-free and facile solid-state protection of diamines, anthranilic acid, diols, and polyols with phenylboronic acid. Chemistry 2003, 9, 4156–4161. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Jiang, Z.; Zhang, G.; Xin, N.; Gan, L. Facile preparation of fullerenyl boronic esters. Tetrahedron 2012, 68, 5193–5196. [Google Scholar] [CrossRef]
- Ohtsuka, K.; Inagi, S.; Fuchigami, T. Electrochemical Properties and Reactions of Oxygen-Containing Organotrifluoroborates and Their Boronic Acid Esters. ChemElectroChem 2017, 4, 183–187. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, D.; Tan, W.; Wang, Z.; Zhu, D. Formation of the intermediate nitronyl nitroxide–anthracene dyad sensing saccharides. Bioorg. Med. Chem. Lett. 2007, 17, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, D.; Zhu, D. A New Saccharide Sensor Based on a Tetrathiafulvalene—Anthracene Dyad with a Boronic Acid Group. J. Org. Chem. 2005, 70, 5729–5732. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Wang, Z.; Zhang, D.; Zhu, D. A New Saccharides and Nnucleosides Sensor Based on Tetrathiafulvalene-anthracene Dyad with Two Boronic Acid Groups. Sensors 2006, 6, 954–961. [Google Scholar] [CrossRef]
- Pasa, S.; Aydın, S.; Kalaycı, S.; Boğa, M.; Atlan, M.; Bingul, M.; Şahin, F.; Temel, H. The synthesis of boronic-imine structured compounds and identification of their anticancer, antimicrobial and antioxidant activities. J. Pharm. Anal. 2016, 6, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Fisicaro, E.; Compari, C.; Bacciottini, F.; Contardi, L.; Pongiluppi, E.; Barbero, N.; Viscardi, G.; Quagliotto, P.; Donofrio, G.; Krafft, M.P. Nonviral gene-delivery by highly fluorinated gemini bispyridinium surfactant-based DNA nanoparticles. J. Colloid Interface Sci. 2017, 487, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Raju, T.B.; Vaghasiya, J.V.; Afroz, M.A.; Soni, S.S.; Iyer, P.K. Influence of m-fluorine substituted phenylene spacer dyes in dye-sensitized solar cells. Org. Electron. 2016, 39, 371–379. [Google Scholar] [CrossRef]
- Verma, R.; Awasthi, K.K.; Rajawat, N.K.; Soni, I.; John, P.J. Curcumin modulates oxidative stress and genotoxicity induced by a type II fluorinated pyrethroid, beta-cyfluthrin. Food Chem. Toxicol. 2016, 97, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Dale, L.; Boger, J.H.; Hikota, M.; Ishida, M. Total Synthesis of Phomazarin. J. Am. Chem. Soc. 1999, 121, 2471–2477. [Google Scholar]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Jakalian, A.; Bush, B.L.; Jack, D.B.; Bayly, C.I. Fast, Efficient Generation of High-Quality Atomic Charges. AM1-BCC Model: I. Method. J. Comput. Chem. 2000, 21, 132–146. [Google Scholar] [CrossRef]
- Jakalian, A.; Jack, D.B.; Bayly, C.I. Fast, efficient generation of high-quality atomic charges. AM1-BCC model: II. Parameterization and validation. J. Comput. Chem. 2002, 23, 1623–1641. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.T.; Brozell, S.R.; Mukherjee, S.; Pettersen, E.F.; Meng, E.C.; Thomas, V.; Rizzo, R.C.; Case, D.A.; James, T.L.; Kuntz, I.D. DOCK 6: Combining techniques to model RNA-small molecule complexes. RNA 2009, 15, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Sudipto Mukherjee, T.E.B.; Rizzo, R.C. Docking Validation Resources: Protein Family and Ligand Flexibility Experiments. J. Chem. Inf. Model. 2010, 50, 1986–2000. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–16 are available from the authors. |
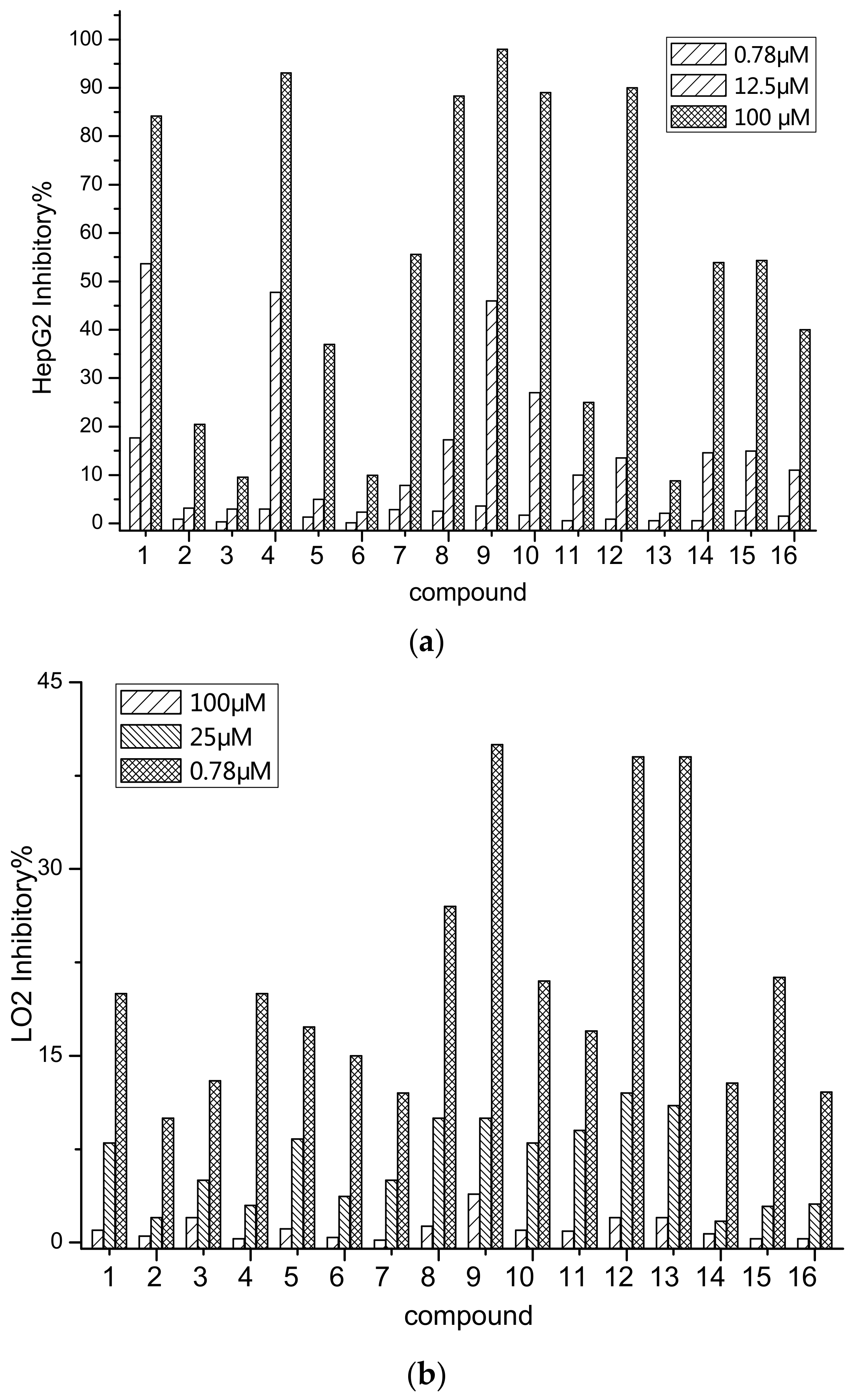


| Compound | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| HepG2 a/IC50 | 7.88 | 197.94 | >200 b | 15.33 | 180 | >200 | 89.66 | 37.25 |
| LO2/IC50 | 150.59 | >200 | >200 | 180.78 | >200 | >200 | >200 | 180.51 |
| Compound | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| HepG2 a/IC50 | 15.01 | 28.75 | >200 | 50.57 | 65.43 | 78.66 | 67.04 | 150.62 |
| LO2/IC50 | 120.81 | 198.36 | >200 | 120.54 | 150.84 | >200 | >200 | >200 |
| Compound | Group/μM | G1 (%) | S (%) | G2 (%) |
|---|---|---|---|---|
| 1 | CON | 48.06 | 35.8 | 16.14 |
| 1 | 54.48 | 29.72 | 15.79 | |
| 3 | 60.17 | 26.19 | 13.64 | |
| 12 | 65.80 | 20.40 | 13.79 |
| Compound | Group | UL (%) | UR (%) | LL (%) | LR (%) | Apoptosis (%) |
|---|---|---|---|---|---|---|
| 1 | CON | 0.63 | 2.44 | 94.78 | 2.16 | 4.6 |
| 1 μm | 1.56 | 6.35 | 82.71 | 9.38 | 15.73 | |
| 3 μm | 0.07 | 16.18 | 67.33 | 16.42 | 32.60 | |
| 12 μm | 3.24 | 10.19 | 48.91 | 37.68 | 47.87 |
| Compound | Group | Green Fluorescence (%) |
|---|---|---|
| 1 | CON | 5.80 |
| 1 μm | 16.72 | |
| 3 μm | 28.14 | |
| 12 μm | 53.82 |
| Compound | Group | Mean |
|---|---|---|
| 1 | CON | 31.00 |
| 1 μm | 65.01 | |
| 3 μm | 94.39 | |
| 12 μm | 136.80 |
| Compound | Pose | Grid Score | Grid_vdw | Grid_es | Int_energy |
|---|---|---|---|---|---|
| Ligand 1 | 1 | −54.809010 | −47.656796 | −7.152214 | 13.308063 |
| 2 | −53.249386 | −46.243793 | −7.005592 | 15.280022 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Z.; Wang, C.; Jiang, J. Synthesis and Evaluation of C15 Triene Urushiol Derivatives as Potential Anticancer Agents and HDAC2 Inhibitor. Molecules 2018, 23, 1074. https://doi.org/10.3390/molecules23051074
Qi Z, Wang C, Jiang J. Synthesis and Evaluation of C15 Triene Urushiol Derivatives as Potential Anticancer Agents and HDAC2 Inhibitor. Molecules. 2018; 23(5):1074. https://doi.org/10.3390/molecules23051074
Chicago/Turabian StyleQi, Zhiwen, Chengzhang Wang, and Jianxin Jiang. 2018. "Synthesis and Evaluation of C15 Triene Urushiol Derivatives as Potential Anticancer Agents and HDAC2 Inhibitor" Molecules 23, no. 5: 1074. https://doi.org/10.3390/molecules23051074
APA StyleQi, Z., Wang, C., & Jiang, J. (2018). Synthesis and Evaluation of C15 Triene Urushiol Derivatives as Potential Anticancer Agents and HDAC2 Inhibitor. Molecules, 23(5), 1074. https://doi.org/10.3390/molecules23051074






