Synthesis and Biological Evaluation of Bisthiazoles and Polythiazoles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biological Evaluations
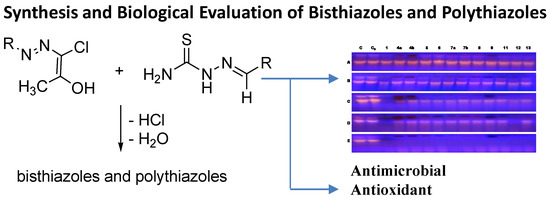
2.2.1. DNA Digestion Pattern
2.2.2. Antioxidant Activities
2.2.3. Antibacterial Activities
2.2.4. Cytotoxicity Evaluation
3. Experimental
3.1. Synthesis
3.2. Biological Evaluations
3.2.1. DNA Digestion Pattern
3.2.2. ABTS Antioxidant Assay
3.2.3. NO Scavenging Method
3.2.4. Determination of Total Antioxidant Activity in Linoleic Acid Emulsion
3.2.5. Antibacterial Activities
3.2.6. Minimum Inhibitory Concentrations (MIC)
3.2.7. Cytotoxicity Assay
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Jacques, P.; Andre, T.; Girault, P. Fungicidal 3-(Nitrophenoxy)-1,2,5-thiadiazole. FR 2215219 A1 1,974,0823, 23 August 1974. [Google Scholar]
- Noguchi, T.; Asada, M.; Ando, M.; Matsuda, K.; Uchiyama, Y. Insecticide-Acaricide Composition of the Hydrazine Series. JP 48008496 B 19730315, 15 March 1973. [Google Scholar]
- Quiroga, A.G.; Perez, J.M.; Lopez-Solera, I.; Masaguer, J.R.; Luque, A.; Raman, P.; Edwards, A.; Alonso, C.; Navarro-Ranninger, C. Novel Tetranuclear Orthometalated Complexes of Pd(II) and Pt(II) Derived from p-Isopropylbenzaldehyde Thiosemicarbazone with Cytotoxic Activity in cis-DDP Resistant Tumor Cell Lines. Interaction of These Complexes with DNA. J. Med. Chem. 1998, 41, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Offiong, O.E.; Martelli, S. New 1-[quinolyl(4)]-1,2,3-triazoles: Synthesis and evaluation of antiinflammatory and analgesic properties. II Farmaco 1994, 49, 513–518. [Google Scholar] [PubMed]
- Hadjipavlou-Litina, D. Structure-Activity Relationships of some 1,4-Benzodioxane Aryl-piperazine Derivatives as α-Blocking Agents. Pharmazie 1996, 51, 468–470. [Google Scholar] [PubMed]
- Bharti, S.K.; Nath, G.; Tilak, R.; Singh, S.K. Synthesis, anti-bacterial and anti-fungal activities of some novel Schiff bases containing 2,4-disubstituted thiazole ring. Eur. J. Med. Chem. 2010, 45, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.J.; Illig, C.R.; Subasinghe, N.; Hoffman, J.B.; Rudolph, M.J.; Soll, R.; Molloy, C.J.; Bone, R.; Green, D.; Randall, T.; et al. Synthesis of thiophene-2-carboxamidines containing 2-amino-thiazoles and their biological evaluation as urokinase inhibitors. Bioorgan. Med. Chem. Lett. 2001, 11, 915–918. [Google Scholar] [CrossRef]
- Haviv, F.; Ratajczyk, J.D.; DeNet, R.W.; Kerdesky, F.A.; Walters, R.L.; Schmidt, S.P.; Holms, J.H.; Young, P.R.; Carter, G.W. 3-[1-(2-Benzoxazolyl)hydrazino]-propanenitrile derivatives: Inhibitors of immune complex induced inflammation. J. Med. Chem. 1988, 31, 1719–1728. [Google Scholar] [CrossRef] [PubMed]
- Patt, W.C.; Hamilton, H.W.; Taylor, M.D.; Ryan, M.J.; Taylor, D.G.; Connolly, C.J., Jr.; Doherty, A.M.; Klutchko, S.R.; Sircar, I. Structure-activity relationships of a series of 2-amino-4-thiazole-containing renin inhibitors. J. Med. Chem. 1992, 35, 2562–2572. [Google Scholar] [CrossRef] [PubMed]
- Bell, F.W.; Cantrell, A.S.; Hoegberg, M.; Jaskunas, S.R.; Johansson, N.G.; Jordan, C.L.; Kinnick, M.D.; Lind, P.; Morin, J.M., Jr. Phenethylthiazolethiourea (PETT) Compounds, a New Class of HIV-1 Reverse Transcriptase Inhibitors. 1. Synthesis and Basic Structure-Activity Relationship Studies of PETT Analogs. J. Med. Chem. 1995, 38, 4929–4936. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.H.; Wan, X.Z.; Jiang, B. Syntheses and biological activities of bis(3-indolyl)thiazoles, analogues of marine bis(indole)alkaloid nortopsentins. Bioorgan. Med. Chem. Lett. 1999, 9, 569–572. [Google Scholar] [CrossRef]
- Medime, E.; Capan, G. Synthesis and anticonvulsant activity of new 4-thiazolidone and 4-thiazoline derivatives. II Farmaco 1994, 49, 449–451. [Google Scholar]
- Metzger, J.V. Comprehensive Heterocyclic Chemistry I; Pergamon: New York, NY, USA, 1984; Volume 6, p. 328. [Google Scholar]
- Breslow, R. On the Mechanism of Thiamine Action. IV.1 Evidence from Studies on Model Systems. J. Am. Chem. Soc. 1958, 80, 3719–3726. [Google Scholar] [CrossRef]
- Milne, G.W. Ashgate Handbook of Antineoplastic Agents; Gower: London, UK, 2000. [Google Scholar]
- De Souza, M.V.N.; De Almeida, M.V. Drogas anti-VIH: Passado, presente e perspectivas futuras. Quim. Nova 2003, 26, 366–372. [Google Scholar] [CrossRef]
- Lednicer, D.; Mitscher, L.A.; George, G.I. Organic Chemistry of Drug Synthesis; Wiley: New York, NY, USA, 1990; Volume 4, pp. 95–97. [Google Scholar]
- Knadler, M.P.; Bergstrom, R.F.; Callaghan, J.T.; Rubin, A. Nizatidine, an H2-blocker. Its metabolism and disposition in man. Drug Metab. Dispos. 1986, 14, 175–182. [Google Scholar] [PubMed]
- Borisenko, V.E.; Koll, A.; Kolmakov, E.E.; Rjasnyi, A.G. Hydrogen bonds of 2-aminothiazoles in intermolecular complexes (1:1 and 1:2) with proton acceptors in solutions. J. Mol. Struct. 2006, 783, 101–115. [Google Scholar] [CrossRef]
- Beuchet, P.; Varache-Lembege, M.; Neveu, A.; Leger, J.M.; Vercauteren, J.; Larrouture, S.; Deffieux, G.; Nuhrich, A. New 2-sulfonamidothiazoles substituted at C-4: Synthesis of polyoxygenated aryl derivatives and in vitro evaluation of antifungal activity. Eur. J. Med. Chem. 1999, 34, 773–779. [Google Scholar] [CrossRef]
- Fink, B.E.; Mortensen, D.S.; Stauffer, S.R.; Aron, Z.D.; Katzenellenbogen, J.A. Novel structural templates for estrogen-receptor ligands and prospects for combinatorial synthesis of estrogens. Chem. Biol. 1999, 6, 205–219. [Google Scholar] [CrossRef]
- Van Muijlwijk-Koezen, J.E.; Timmerman, H.; Vollinga, R.C.; von Drabbe Kunzel, J.F.; de Groote, M.; Visser, S.; IJzerman, A.P. Thiazole and Thiadiazole Analogues as a Novel Class of Adenosine Receptor Antagonists. J. Med. Chem. 2001, 44, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Hallinan, E.A.; Hagen, T.J.; Tsymbalov, S.; Stapelfeld, A.; Savage, M.A. 2,4-Disubstituted oxazoles and thiazoles as latent pharmacophores for diacylhydrazine of SC-51089, a potent PGE2 antagonist. Bioorgan. Med. Chem. 2001, 9, 1–6. [Google Scholar] [CrossRef]
- Buchanan, J.L.; Bohacek, R.S.; Luke, G.P.; Hatada, M.; Lu, X.; Dalgarno, D.C.; Narula, S.S.; Yuan, R.; Holt, D.A. Structure-based design and synthesis of a novel class of Src SH2 inhibitors. Bioorgan. Med. Chem. Lett. 1999, 9, 2353–2358. [Google Scholar] [CrossRef]
- Fekri, A.; Keshk, E.M. Utility involving thioacetoacetanilides as precursors for synthesis of new thiazole, thiadiazole and thiophene derivatives with antimicrobial activity. J. Sulfur Chem. 2016, 37, 148–161. [Google Scholar] [CrossRef]
- Dawood, K.M.; Gomha, S.M. Synthesis and Anti-cancer Activity of 1,3,4-Thiadiazole and 1,3-Thiazole Derivatives Having 1,3,4-Oxadiazole Moiety. J. Heerocycl. Chem. 2015, 52, 1400–1405. [Google Scholar] [CrossRef]
- El-Kady, M.; Abbas, E.M.H.; Salem, M.S.; Kassem, A.F.M.; Abd El-Moez, S.I. Synthesis and antimicrobial evaluation of novel 1,3-thiazoles and unsymmetrical azines. Res. Chem. Intermed. 2016, 4, 3333–3349. [Google Scholar] [CrossRef]
- Sayed, A.R. Synthesis of bis-thiazoles, bis-pyrazoles, bis-hydrazonates, and bis-triazolothiadiazoles based on bis-hydrazonoyl and bis-hydrazones. Turk. J. Chem. 2015, 39, 600–609. [Google Scholar] [CrossRef]
- Sayed, A.R. Synthesis of novel bis-thiadiazoles, bis-triazoles and polypyrazole derivatives based on hydrazonoyl halides. Tetrahedron 2013, 69, 5293–5298. [Google Scholar] [CrossRef]
- Maccioni, E.; Cardia, M.C.; Distinto, S.; Bonsignore, L.; De Logu, A. An investigation of the biological effect of structural modifications of isothiosemicarbazones and their cyclic analogues. II Farmaco 2003, 58, 951–959. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ismail, M.A.; Arafa, R.K.; Youssef, M.M.; El-Sayed, W.M. Anticancer, antioxidant activities, and DNA affinity of novel monocationic bithiophenes and analogues. Drug Des. Dev. Ther. 2014, 8, 1659–1672. [Google Scholar] [CrossRef] [PubMed]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Youssef, M.M.; Al-Omair, M.A.; Ismail, M.A. Synthesis, DNA affinity, and antimicrobial activity of 4-substituted phenyl-2,2′-bichalcophenes and aza-analogues. Med. Chem. Res. 2012, 21, 4074–4082. [Google Scholar] [CrossRef]
- Youssef, M.M.; Arafa, R.K.; Ismail, M.A. Synthesis, antimicrobial, and antiproliferative activities of substituted phenylfuranylnicotinamidines. Drug Des. Dev. Ther. 2016, 10, 1133–1146. [Google Scholar]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.S.S.; Youssef, M.M.; Aboelghar, M.F.; Hafez, A.M.; El-Ayaan, U. Synthesis and characterization of binary and ternary oxovanadium complexes of N,N′-(2-pyridyl)thiourea and curcumin: Catalytic oxidation potential, antibacterial, antimicrobial, antioxidant and DNA interaction studies. Appl. Organomet. Chem. 2017, 31, e3650. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard, 7th ed.; CLSI: Wayne, Pennsylvania, 2006. [Google Scholar]
- Elghamry, I.; Youssef, M.M.; Al-Omair, M.A.; Elsawy, H. Synthesis, antimicrobial, DNA degradation and antioxidant activities of tricyclic sultams derivatives from saccharin. Eur. J. Med. Chem. 2017, 139, 107–113. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1, 2a, 2b, 3, 4a, 4b, 6, 7a and 7b are available from the authors. |






| Compound No. | % Inhibition |
|---|---|
| Control of ABTS | - |
| Ascorbic acid | 86.2 |
| 1 | 67.4 |
| 4a | 61.2 |
| 4b | 53.7 |
| 5 | 51.9 |
| 6 | 60.1 |
| 7a | 51.2 |
| 7b | 50.3 |
| 8 | 48.1 |
| 9 | 49.4 |
| 11 | 58.5 |
| 12 | 63.7 |
| 13 | 56.1 |
| Compound | Gram-Negative | Gram-Positive | ||
|---|---|---|---|---|
| E. coli | P. aeruginosa | S. aureus | B. megaterium | |
| 1 | 17 | 16 | 17 | 16 |
| 4a | 16 | 15 | 16 | 16 |
| 4b | 12 | 11 | 14 | 12 |
| 5 | 12 | 12 | 11 | 10 |
| 6 | 15 | 15 | 14 | 15 |
| 7a | 13 | 12 | 13 | 12 |
| 7b | 12 | 12 | 11 | 11 |
| 8 | 10 | 10 | 11 | 11 |
| 9 | 15 | 15 | 14 | 14 |
| 11 | 15 | 14 | 17 | 12 |
| 12 | 16 | 15 | 16 | 14 |
| 13 | 15 | 14 | 13 | 14 |
| Ampicillin | 19 | 20 | 23 | 21 |
| Compound | E. coli | P. aeruginosa | B. megaterium | S. aureus |
|---|---|---|---|---|
| Ampicillin | 20 | 25 | 25 | 15 |
| 1 | 35 | 35 | 40 | 35 |
| 4a | 35 | 35 | 35 | 40 |
| 4b | 55 | 50 | 65 | 60 |
| 5 | 50 | 55 | 60 | 65 |
| 6 | 40 | 45 | 45 | 40 |
| 7a | 60 | 65 | 70 | 70 |
| 7b | 65 | 55 | 65 | 65 |
| 8 | 75 | 70 | 80 | 80 |
| 9 | 60 | 70 | 65 | 70 |
| 11 | 40 | 45 | 40 | 40 |
| 12 | 34 | 40 | 45 | 40 |
| 13 | 40 | 45 | 40 | 45 |
| Compounds | In Vitro Cytotoxicity IC50 (µM) • | ||
|---|---|---|---|
| HePG-2 | HCT-116 | MCF-7 | |
| DOX •• | 4.50 ± 0.2 | 5.23 ± 0.3 | 4.17 ± 0.2 |
| 1 | 8.92 ± 0.6 | 8.22 ± 0.9 | 6.89 ± 0.4 |
| 4a | 9.94 ± 0.5 | 8.87 ± 0.7 | 7.83 ± 0.6 |
| 4b | 25.49 ± 1.7 | 21.43 ± 1.5 | 37.64 ± 1.2 |
| 5 | 24.48 ± 1.3 | 33.24 ± 2.2 | 23.47 ± 2.0 |
| 6 | 10.41 ± 1.1 | 12.66 ± 1.3 | 16.57 ± 1.5 |
| 7a | 36.57 ± 3.2 | 48.52 ± 2.9 | 26.13 ± 2.8 |
| 7b | 35.53 ± 2.6 | 24.43 ± 1.7 | 27.92 ± 1.6 |
| 8 | 53.54 ± 2.1 | 61.41 ± 2.8 | 69.33 ± 2.6 |
| 9 | 83.56 ± 4.1 | 71.13 ± 3.6 | 53.91 ± 3.5 |
| 11 | 18.52 ± 1.3 | 14.72 ± 1.1 | 17.90 ± 1.4 |
| 12 | 11.25 ± 1.4 | 19.54 ± 1.7 | 12.66 ± 1.5 |
| 13 | 30.75 ± 2.3 | 41.37 ± 2.5 | 35.11 ± 2.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Omair, M.A.; Sayed, A.R.; Youssef, M.M. Synthesis and Biological Evaluation of Bisthiazoles and Polythiazoles. Molecules 2018, 23, 1133. https://doi.org/10.3390/molecules23051133
Al-Omair MA, Sayed AR, Youssef MM. Synthesis and Biological Evaluation of Bisthiazoles and Polythiazoles. Molecules. 2018; 23(5):1133. https://doi.org/10.3390/molecules23051133
Chicago/Turabian StyleAl-Omair, Mohammed A., Abdelwahed R. Sayed, and Magdy M. Youssef. 2018. "Synthesis and Biological Evaluation of Bisthiazoles and Polythiazoles" Molecules 23, no. 5: 1133. https://doi.org/10.3390/molecules23051133
APA StyleAl-Omair, M. A., Sayed, A. R., & Youssef, M. M. (2018). Synthesis and Biological Evaluation of Bisthiazoles and Polythiazoles. Molecules, 23(5), 1133. https://doi.org/10.3390/molecules23051133