A Microdialysis in Adjuvant Arthritic Rats for Pharmacokinetics–Pharmacodynamics Modeling Study of Geniposide with Determination of Drug Concentration and Efficacy Levels in Dialysate
Abstract
:1. Introduction
2. Results
2.1. Establishment of PK Study of GE in Articular Cavities of AA Rats
2.2. Establishment of PD Index Detection Method of PGE2 in Articular Cavities of AA Rats
2.3. In Vitro and In Vivo Probe Recovery
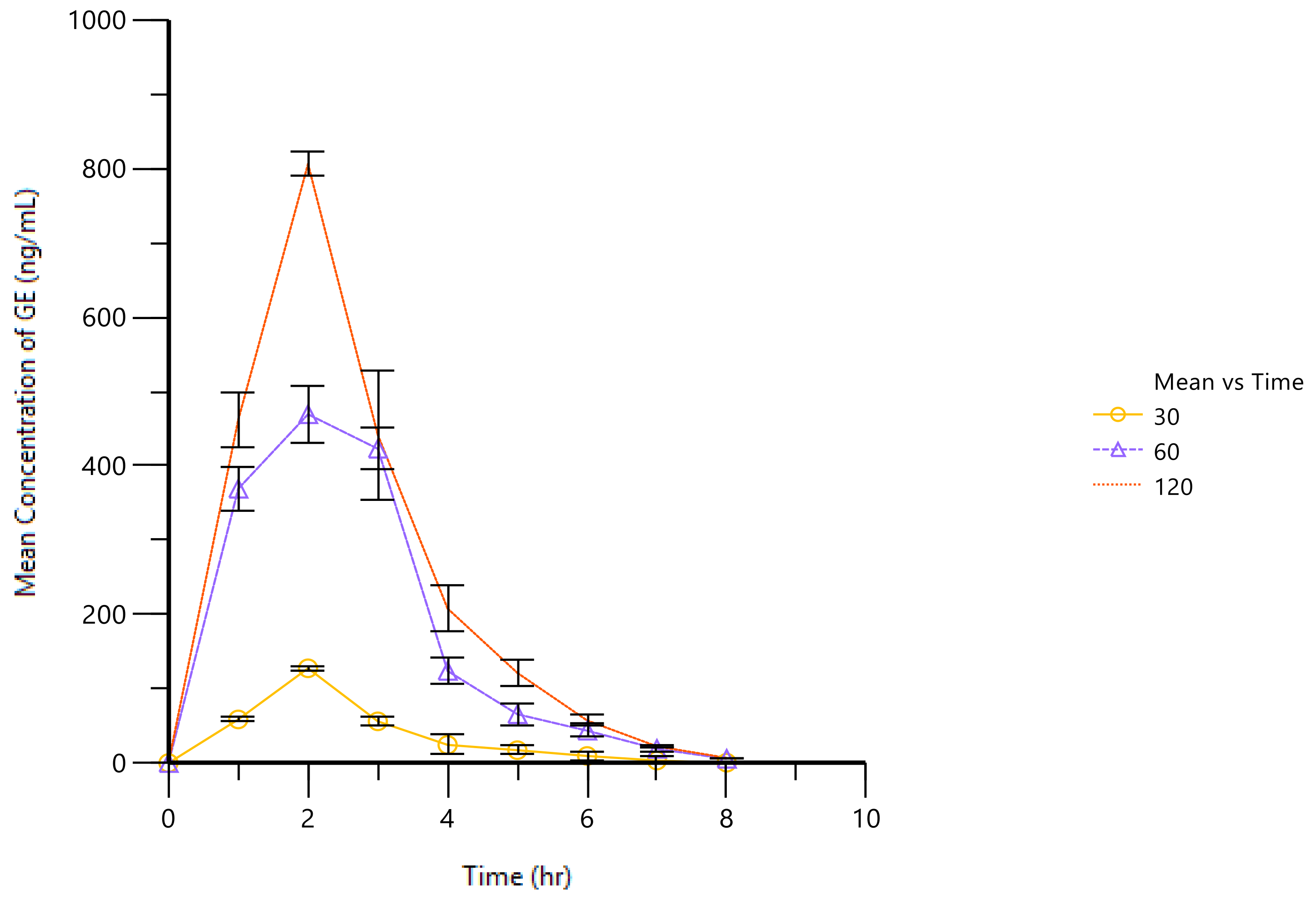
2.4. PK of GE in AA Rat Joint Cavities
2.5. PGE2 Concentrations in Microdialysate in Articular Cavities of AA Rats
2.6. PK–PD Modeling
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. GE and PGE2 Assay
4.3. Animals
4.4. Experimental Grouping and Administration
4.5. Microdialysis Sampling
4.6. Study on in Vitro and in Vivo Recovery of Microdialysis Probe
4.7. GE PK Parameters
4.8. PK–PD Modeling
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Smolen, J.S.; Redlich, K. The Autoimmune Diseases, 5th ed.; United States Academic Press: Waltham, MA, USA, 2014; pp. 511–523. [Google Scholar]
- Gasparyan, A.Y.; Stavropoulos-Kalinoglou, A.; Mikhailidis, D.P.; Douglas, K.M.; Kitas, G.D. Platelet function in rheumatoid arthritis: Arthritic and cardiovascular implications. Rheumatol. Int. 2011, 31, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Mankia, K.; Emery, P. Is localized autoimmunity the trigger for rheumatoid arthritis? Unravelling new targets for prevention. Discov. Med. 2016, 20, 129–135. [Google Scholar]
- He, X.J.; Ding, Y.; Xiang, W.; Dang, X.Q. Roles of 1.25(OH)2D3 and Vitamin D Receptor in the Pathogenesis of Rheumatoid Arthritis and Systemic Lupus Erythematosus by Regulating the Activation of CD4+ T Cells and the PKCδ/ ERK Signaling Pathway. Cell. Physiol. Biochem. 2016, 40, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Marra, C.A.; Bansback, N.; Anis, A.H.; Shojania, K. Introduction to economic modeling for clinical rheumatologists: Application to biologic agents in rheumatoid arthritis. Clin. Rheumatol. 2011, 30, S9–S18. [Google Scholar] [CrossRef] [PubMed]
- See, L.C.; Kuo, C.F.; Chou, I.J.; Chiou, M.J.; Yu, K.H. Sex- and age-specific incidence of autoimmune rheumatic diseases in the Chinese population: A Taiwan population-based study. Semin. Arthritis Rheum. 2013, 43, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, X.; Xu, M.; Wu, X.; Zhao, F.; Zhao, C. Quercetin attenuates collagen-induced arthritis by restoration of Th17/Treg balance and activation of Heme Oxygenase 1-mediated anti-inflammatory effect. Int. Immunopharmacol. 2018, 54, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.S.; Lee, E.G.; Jeon, H.S.; Chae, H.J.; Park, S.J.; Lee, Y.C.; Yoo, W.H. Quercetin inhibits IL-1β-induced proliferation and production of MMPs, COX-2, and PGE2 by rheumatoid synovial fibroblast. Inflammation 2012, 35, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.Y.; Lee, E.G.; Lee, H.; Cho, I.J.; Choi, Y.J.; Sung, M.S.; Yoo, H.G.; Yoo, W.H. Kaempferol inhibits IL-1β-induced proliferation of rheumatoid arthritis synovial fibroblasts and the production of COX-2, PGE2 and MMPs. Int. J. Mol. Med. 2013, 32, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.N.; Liu, C.Y.; Yin, F.; Zhang, Y.; Liu, J. 5′-AMP-activated protein kinase plays an essential role in geniposide-regulated glucose-stimulated insulin secretion in rat pancreatic INS-1β cells. J. Nat. Med. 2016, 71, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.L.; Zhang, X.M.; Jin, G.X.; Shi, Z.; Sun, W.; Chen, F. Geniposide reduces development of streptozotocin-induced diabetic nephropathy via regulating NF-κB signaling pathways. Fundam. Clin. Pharmacol. 2017, 31, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Wang, G.F.; Liu, Z.Q.; Rao, J.J.; Lü, L.; Xu, W.; Wu, S.G.; Zhang, J.J. Effect of geniposide, a hypoglycemic glucoside, on hepatic regulating enzymes in diabetic mice induced by a high-fat diet and streptozotocin. Acta Pharmacol. Sin. 2009, 30, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Xiaofeng, Y.; Qinren, C.; Jingping, H.; Xiao, C.; Miaomiao, W.; Xiangru, F.; Xianxing, X.; Meixia, H.; Jing, L.; Jingyuan, W.; et al. Geniposide, an Iridoid Glucoside Derived from Gardenia jasminoides, Protects against Lipopolysaccharide-Induced Acute Lung Injury in Mice. Planta Med. 2012, 78, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.H.; Liu, B.; Liu, Z.; Liang, D.; Li, F.; Li, D.; Cao, Y.; Zhang, X.; Zhang, N.; Yang, Z. Geniposide, from Gardenia jasminoides Ellis, inhibits the inflammatory response in the primary mouse macrophages and mouse models. Int. Immunopharmacol. 2012, 14, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.M.; Wu, H.; Chen, J.; Chen, J.Y.; Hu, S.L.; Shen, C. Effects and mechanisms of Geniposide on rats with adjuvant arthritis. Int. Immunopharmacol. 2014, 20, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Cai, L.; Tang, W.J.; Lei, C.; Hu, C.M.; Yu, F. Apoptotic Effect of Geniposide on Fibroblast-Like Synoviocytes in Rats with Adjuvant-Induced Arthritis via Inhibiting ERK Signal Pathway In Vitro. Inflammation 2016, 39, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Gao, X.; Lei, C.; Hu, C.M.; Yu, F. Effect of geniposide on serum IL-1beta and TNF-α of rheumatoid arthritis rats. China J. Chin. Mater. Med. 2005, 30, 708–711. [Google Scholar]
- Hansen, D.K.; Davies, M.I.; Lunte, S.M.; Lunte, C.E. Lunte, Pharmacokinetic andmetabolism studies using microdialysis sampling. Pharm. Sci. 1999, 88, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C.; Araújo, L.G.; Pigatto, M.C.; Haas, S.E.; Costa, T.D.; Araújo, B.V. Validation of LC-MS/MS method applied to evaluation of free tissue concentrations of vildagliptin in diabetic rats by microdialysis. Biomed. Chromatogr. 2014, 28, 1722–1727. [Google Scholar] [CrossRef] [PubMed]
- Greco, S.; Danysz, W.; Zivkovic, A.; Gross, R.; Stark, H. Microdialysate analysis of monoamine neurotransmitters-A versatile and sensitive LC–MS/MS method. Anal. Chim. Acta 2013, 771, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Fang, C.; Smagin, G. Derivatization for the simultaneous LC/MS quantification of multiple neurotransmitters in extracellular fluid from rat brain microdialysis. J. Pharm. Biomed. Anal. 2014, 100, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.M.; Hou, M.L.; Lin, L.C.; Tsai, T.H. Development of a microdialysis system to monitor lamivudine in blood and liver for the pharmacokinetic application in herbal drug interaction and the gene expression in rats. J. Pharm. Biomed. Anal. 2014, 96, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Milligan, P.; Brown, M.; Marchant, B.; Martin, S.W.; van der Graaf, P.H.; Benson, N.; Nucci, G.; Nichols, D.J.; Boyd, R.A.; Mandema, J.W.; et al. Model-based drug development: A rational approach to efficiently accelerate drug development. Clin. Pharmacol. Ther. 2013, 93, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Rajman, I. PK/PD modelling and simulations: Utility in drug development. Drug Discov. Today 2008, 13, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Costa, N.T.; Veiga Iriyoda, T.M.; Kallaur, A.P.; Delongui, F.; Alfieri, D.F.; Lozovoy, M.A.; Amin, R.B.; Delfino, V.D.; Dichi, I.; Simão, A.N. Influence of Insulin Resistance and TNF-α on the Inflammatory Process, Oxidative Stress, and Disease Activity in Patients with Rheumatoid Arthritis. Oxid. Med. Cell. Longev. 2016, 2016, 8962763. [Google Scholar] [CrossRef] [PubMed]
- Lowin, T.; Apitz, M.; Anders, S.; Straub, R.H. Anti-inflammatory effects of N-acylethanolamines in rheumatoid arthritis synovial cells are mediated by TRPV1 and TRPA1 in a COX-2 dependent manner. Arthritis Res. Ther. 2015, 17, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.J.; Li, G.; He, J.H.; Guo, Y.; Yang, L. Bioinformatics-Based Identification of MicroRNA-Regulated and Rheumatoid Arthritis-Associated Genes. PLoS ONE 2015, 10, e0137551. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Feng, X.; Ding, J.; Chang, F.; Chen, X. Nanotherapeutics relieve rheumatoid arthritis. J. Control. Release 2017, 252, 108–124. [Google Scholar] [CrossRef] [PubMed]
- Firestein, G.S.; McInnes, I.B. Immunopathogenesis of Rheumatoid Arthritis. Immunity 2016, 46, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.Y.; Jean, Y.H.; Lee, H.P.; Lin, S.C.; Pan, C.Y.; Chen, W.F.; Wu, S.F.; Su, J.H.; Tsui, K.H.; Sheu, J.H.; et al. Excavatolide B Attenuates Rheumatoid Arthritis through the Inhibition of Osteoclastogenesis. Mar. Drugs 2017, 15, 9–37. [Google Scholar] [CrossRef] [PubMed]
- Moudgil, K.D.; Kim, P.; Brahn, E. Advances in rheumatoid arthritis animal models. Curr. Rheumatol. Rep. 2011, 13, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.H.; Hu, L.F.; Lee, C.W.; Chiang, Y.C.; Lee, M.H.; How, J.M.; Wu, C.M.; Huang, C.L.; Lee, I.T. Resveratrol inhibits urban particulate matter-induced COX-2/PGE2 release in human fibroblast-like synoviocytes via the inhibition of activation of NADPH oxidase/ROS/NF-κB. Int. J. Biochem. Cell Biol. 2017, 88, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Kim, B.M.; Lee, K.A.; Lee, S.H.; Firestein, G.S.; Kim, H.R. Histamine and Histamine H4 Receptor Promotes Osteoclastogenesis in Rheumatoid Arthritis. Sci. Rep. 2017, 7, 1197. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; He, L.; Hu, N.; Luo, J.; Zhang, J.; Mo, L.F.; Wang, Y.H.; Pu, D.; Lv, X.H.; Hao, Z.M.; et al. Effect of 1,25-(OH)2D3 on Proliferation of Fibroblast-Like Synoviocytes and Expressions of Pro-Inflammatory Cytokines through Regulating MicroRNA-22 in a Rat Model of Rheumatoid Arthritis. Cell. Physiol. Biochem. 2017, 42, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Chen, Y.W.; Chi, P.L.; Lin, C.C.; Hsiao, L.D. Resveratrol inhibits BK-induced COX-2 transcription by suppressing acetylation of AP-1 and NF-κB in human rheumatoid arthritis synovial fibroblasts. Biochem. Pharmacol. 2017, 132, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Topuz, B.B.; Altinbas, B.; Ilhan, T.; Yilmaz, M.S.; Erdost, H.; Saha, S.; Savci, V.; Yalcin, M. Centrally administered CDP-choline induced cardiovascular responses are mediated by activation of the central phospholipase-prostaglandin signaling cascade. Brain Res. 2014, 1563, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Shinkai, N.; Korenaga, K.; Mizu, H.; Yamauchi, H. Intra-articular penetration of ketoprofen and analgesic effects after topical patch application in rats. J. Control. Release 2008, 131, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wu, H.; Chen, J.; Li, S.P.; Dai, L.; Zhang, Z.R.; Wang, W.Y. Anti-inflammation Effects and Mechanisms Study of Geniposide on Rats with Collagen-Induced Arthritis. Phytother. Res. 2017, 31, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Wu, H.; Li, H.; Hu, S.L.; Dai, M.M.; Chen, J. Anti-inflammatory effects and pharmacokinetic study of geniposide on rats with adjuvant arthritis. Int. Immunopharmacol. 2014, 24, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, H.; Xu, G.B.; Dai, M.M.; Hu, S.L.; Sun, L.L.; Wang, W.; Wang, R.; Li, S.P.; Li, G.Q. Determination of geniposide in adjuvant arthritis rat plasma by ultra-high performance liquid chromatography tandem mass spectrometry method and its application to oral bioavailability and plasma protein binding ability studies. J. Pharm. Biomed. Anal. 2015, 108, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, H.; Shen, C.; Chen, J.Y.; Hu, S.L.; Wu, H. Comparative pharmacokinetic Study after Oral Administration of Geniposide in Normal Rats and Adjuvant-induced Arthritis Rats by UPLC-MS/MS. Basic Clin. Pharmacol. Toxicol. 2013, 113, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Gibaldi, M.; Perrier, D. Pharmacokinetic, 2nd ed.; Marcel Dekker: New York, NY, USA, 1982. [Google Scholar]
Sample Availability: Samples of the compounds geniposide, Freund’s complete adjuvant, Tripterygium wilfordii Hook f., ulatan, methanol, acetonitrile and formic acid are available from the authors. |




| Current Speed (μL/min) | 0.5 | 0.8 | 1 | 2 |
|---|---|---|---|---|
| Incremental method Realtive recovery (RR) (%) | 56.45 ± 3.44 | 52.87 ± 4.07 | 48.05 ± 5.41 | 25.63 ± 4.30 |
| Decremental method Relative loss (RL) (%) | 53.49 ± 2.75 | 50.66 ± 5.00 | 44.42 ± 4.46 | 23.87 ± 3.82 |
| Concentration (μg/mL) | 50 | 100 | 200 |
|---|---|---|---|
| Incremental method RR (%) | 27.09 ± 0.78 | 28.98 ± 0.80 | 28.07 ± 0.37 |
| Decremental method RL (%) | 26.91 ± 1.01 | 30.41 ± 0.30 | 29.91 ± 0.66 |
| PK Parameters | Oral Administration Dose (mg/kg) | ||
|---|---|---|---|
| Low (30) | Medium (60) | High (120) | |
| t1/2 | 0.636 ± 0.057 | 0.726 ± 0.132 | 0.665 ± 0.042 |
| Tmax (h) | 2 | 2 | 2 |
| Cmax (ng/mL) | 124.479 ± 2.147 | 469.258 ± 37.999 | 805.906 ± 15.81 |
| AUC0-t (ng/mL×h) | 283.434 ± 20.924 | 1477.928 ± 80.7 | 2074.373 ± 113.885 |
| AUC0-inf (ng/mL×h) | 284.624 ± 20.052 | 1483.883 ± 81.050 | 2081.072 ± 134.014 |
| V/F (L/kg) | 96.460 ± 7.512 | 42.364 ± 7.595 | 55.461 ± 4.756 |
| CL/F (L/h/kg) | 105.803 ± 6.839 | 40.529 ± 2.081 | 57.867 ± 3.811 |
| Variable | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| IC50 | Kin | Kout | |||||||
| Dose (mg/kg) | Mean | SD | SE | Mean | SD | SE | Mean | SD | SE |
| 30 | 0.74 * | 0.23 | 0.1 | 523.75 * | 390.79 | 159.54 | 0.97 | 0.72 | 0.29 |
| 60 | 2.62 | 0.44 | 0.18 | 556.5 * | 170.94 | 69.78 | 1.08 | 0.32 | 0.13 |
| 120 | 1.81 | 0.4 | 0.17 | 272.78 | 103.67 | 42.32 | 0.53 | 0.2 | 0.08 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, R.; Wang, W.; Wu, H.; Zhang, Y.; Wang, W.; Dai, L.; Zhang, Z.; Fu, J.; Li, F. A Microdialysis in Adjuvant Arthritic Rats for Pharmacokinetics–Pharmacodynamics Modeling Study of Geniposide with Determination of Drug Concentration and Efficacy Levels in Dialysate. Molecules 2018, 23, 987. https://doi.org/10.3390/molecules23050987
Deng R, Wang W, Wu H, Zhang Y, Wang W, Dai L, Zhang Z, Fu J, Li F. A Microdialysis in Adjuvant Arthritic Rats for Pharmacokinetics–Pharmacodynamics Modeling Study of Geniposide with Determination of Drug Concentration and Efficacy Levels in Dialysate. Molecules. 2018; 23(5):987. https://doi.org/10.3390/molecules23050987
Chicago/Turabian StyleDeng, Ran, Wei Wang, Hong Wu, Yunjing Zhang, Wenyu Wang, Li Dai, Zhengrong Zhang, Jun Fu, and Feng Li. 2018. "A Microdialysis in Adjuvant Arthritic Rats for Pharmacokinetics–Pharmacodynamics Modeling Study of Geniposide with Determination of Drug Concentration and Efficacy Levels in Dialysate" Molecules 23, no. 5: 987. https://doi.org/10.3390/molecules23050987
APA StyleDeng, R., Wang, W., Wu, H., Zhang, Y., Wang, W., Dai, L., Zhang, Z., Fu, J., & Li, F. (2018). A Microdialysis in Adjuvant Arthritic Rats for Pharmacokinetics–Pharmacodynamics Modeling Study of Geniposide with Determination of Drug Concentration and Efficacy Levels in Dialysate. Molecules, 23(5), 987. https://doi.org/10.3390/molecules23050987





