Phytochemical Composition, Hepatoprotective, and Antioxidant Activities of Phyllodium pulchellum (L.) Desv
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Reagents
2.3. General Experimental Procedures
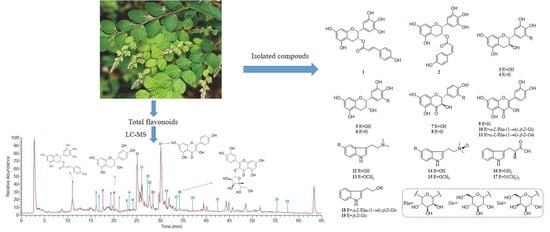
2.4. Extraction and Isolation
2.5. Hepatoprotective Activity Assay
2.6. DPPH Radical-Scavenging Activity Assay
2.7. HPLC-LTQ-Orbitrap-MS Analysis
3. Results and Discussion
3.1. Hepatoprotective and Antioxidant Activities of Organic Fractions
3.2. Structure Characterization of the Isolated Compounds from Ethyl Acetate Fraction (PPE) and n-Butanol Fraction (PPB)
3.3. Hepatoprotective Activity of the Isolated Compounds
3.4. Antioxidant Activity of the Isolated Compounds
3.5. HPLC-LTQ-Orbitrap-MS Analysis of Total Flavonoids
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Editorial Board of Flora of China. Flora of China; Science Publishing House: Beijing, China, 2010; Volume 10, p. 266. [Google Scholar]
- Chopra, R.N.; Nayar, S.L.; Chopra, I.C. Glossary of Indian Medicinal Plants; CSIR: New Delhi, India, 1956; p. 94. [Google Scholar]
- Rahman, M.K.; Barua, S.; Islam, M.F.; Islam, M.R.; Sayeed, M.A.; Parvin, M.S.; Islam, M.E. Studies on the anti-diarrheal properties of leaf extract of Desmodium puchellum. Asian Pac. J. Trop. Biomed. 2013, 3, 639–643. [Google Scholar] [CrossRef]
- Yu, S.M.; Zhong, M.; Huang, L.Y.; Zhang, Q.Q.; Yang, Z.Y. The effect of Desmodium pulchellum on the content of liver collagen protein of testing hepatic fibrosis rats. Hunan Zhongyiyao Daobao 1999, 5, 36–37. [Google Scholar]
- Wei, Y.Q.; Zhong, M.; Zhang, S.Q.; Meng, J.Q.; Li, Z.G. Effect of Desmodium pulchellum and compound Tri-herb capsule on O2−. Mod. J. Integr. Tradit. Chin. West. Med. 2003, 12, 795–796. [Google Scholar]
- Shen, C.C.; Wang, S.T.; Tsai, S.Y.; Yang, H.C.; Shieh, B.J.; Chen, C.C. Cinnamylphenols from Phyllodium pulchellum. J. Nat. Prod. 2005, 68, 791–793. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, S.; Banerjee, S.K.; Bhattacharya, S.K.; Sanyal, A.K. Chemical and pharmacological evaluation of Desmodium pulchellum. Planta Med. 1972, 21, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Rogova, L.S.; Gilev, A.P. Antiarrhythmic properties of some indolalkylamines. Bull. Exp. Biol. Med. 1968, 66, 1113–1114. [Google Scholar]
- Zhong, M.; Yang, Z.Y.; Huang, L.Y.; Yu, S.M.; Lei, S. Effects of Desmodium pulchellum (L.) Benth total alkaloids on hepatic fibrosis rats’ liver pathological and ultrastructure changes induced by chemical. Chin. J. Gastroenterol. Hepatol. 2001, 9, 168–170. [Google Scholar]
- Zhong, M.; Yu, S.M.; Nong, C.Z.; Yang, Z.Y.; Huang, L.Y.; Chen, S.F. Effects of Desmodium pulchellum (L.) benth total alkaloids on col–I and col–III gene expression of hepatic fibrosis in Rats. Chin. J. Integr. Tradit. West. Med. Liver Dis. 2003, 13, 272–274. [Google Scholar]
- Huang, J.L.; Zhong, M.; Yu, S.M. Effects of total alkaloids from Phyllodium pulchellum on proliferation of human hepatic stellate cells and collagen, cytokines related to hepatic fibrosis. Chin. J. Exp. Tradit. Med. Formulae 2013, 19, 283–286. [Google Scholar]
- Cai, L.; Wang, C.; Huo, X.K.; Dong, P.P.; Zhang, B.J.; Zhang, H.L. Effect of alkaloids isolated from Phyllodium pulchellum on monoamine levels and monoamine oxidase activity in rat brain. Evid.-Based Complement. Altern. Med 2016, 1–5. [Google Scholar] [CrossRef]
- Ghosal, S.; Mukherjee, B. Indole-3-alkylamine bases of Desmodium pulchellum. J. Org. Chem. 1966, 31, 2284–2288. [Google Scholar] [CrossRef]
- Wang, C.; Zhong, M.; Zhang, B.J.; Huo, X.K.; Huang, S.S.; Yu, S.M.; Ma, X.C. Chemical constituents against hepatic fibrosis from Phyllodium pulchellum roots. Zhongyaocai 2014, 37, 424–426. [Google Scholar] [PubMed]
- Fan, Y.C.; Guo, Z.L.; Xin, L.T.; Yue, S.J.; Bai, H.; Wang, C.Y. Chemical constituents from Phyllodium pulchellum. Zhongchengyao 2017, 39, 1195–1198. [Google Scholar]
- Zong, Y.; Zhong, M.; Li, D.M.; Zhang, B.J.; Mai, Z.P.; Huo, X.K.; Huang, S.S.; Zhang, H.L.; Wang, C.; Ma, X.C.; Yu, S.M.; Yang, D.A. Phenolic constituents from the roots of Phyllodium pulchellum. J. Asian Nat. Prod. Res. 2014, 16, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.D.; Bansal, R.K. Physcion 1-glycosyl rhamnoside from seeds of Desmodium pulchellum. Phytochemistry 1971, 10, 1921–1922. [Google Scholar] [CrossRef]
- Sinha, M.P.; Tiwari, R.D. The structure of a galactomannan from the seeds of Desmodium pulchellum. Phytochemistry 1970, 9, 1881–1883. [Google Scholar] [CrossRef]
- Zhong, M.; Zhang, B.J.; Wang, C.; Yu, S.M.; Zong, Y.; Ma, X.C.; Zhang, H.L. Quality evaluation of Phllodium pulchellum. Zhongchengyao 2016, 38, 130–133. [Google Scholar]
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A.; et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Nidhal, S.; Msaada, K.; Hamdaoui, G.; Limam, F.; Marzouk, B. Variation in phenolic composition and antioxidant activity during flower development of safflower (Carthamus tinctorius L). J. Agric. Food Chem. 2011, 59, 4455–4463. [Google Scholar]
- Nonaka, G.; Kawahara, O.; Nishioka, I. Tannins and related compounds. XV. A new class of dimeric flavan-3-ol gallates, theasinensins A and B, and proanthocyanidin gallates from green tea leaf. (1). Chem. Pharm. Bull. 1983, 31, 3906–3914. [Google Scholar] [CrossRef]
- Hashimoto, F.; Nonaka, G.I.; Nishioka, I. Tannins and related compounds. LVI isolation of four new acylated flavan-3-ols from oolong tea. (1). Chem. Pharm. Bull. 1987, 35, 611–616. [Google Scholar] [CrossRef]
- Nonaka, G.I.; Nishioka, I.; Nagasawa, T.; Oura, H. Tannins and related compounds. I. rhubarb (1). Chem. Pharm. Bull. 1981, 29, 2862–2870. [Google Scholar] [CrossRef]
- Yi, B.; Chen, T.; Feng, S.X.; Li, Q.H. Chemical constituents of Ancistrocladus tectorius. Guangxi Zhiwu 2013, 33, 564–567. [Google Scholar]
- Wang, X.W.; Mao, Y.; Wang, N.L.; Yao, X.S. A new phloroglucinol diglycoside derivative from Hypericum japonicum Thunb. Molecules 2008, 13, 2796–2803. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, X.; Zhang, P.; Li, Z.L.; Wang, Y. Antiinflammatory constituents from the roots of Smilax bockii warb. Arch. Pharm. Res. 2005, 28, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.C.; Yang, S.J.; Jin, L.H.; Hu, D.Y.; Wu, Z.B.; Yang, S. Chemical constituents of the ethyl acetate extract of Belamcanda chinensis (L.) DC roots and their antitumor activities. Molecules 2012, 17, 6156–6169. [Google Scholar] [CrossRef] [PubMed]
- Han, J.T.; Bang, M.H.; Chun, O.K.; Kim, D.O.; Lee, C.; Baek, N.I. Flavonol glycosides from the aerial parts of Aceriphyllum rossii and their antioxidant activities. Arch. Pharm. Res. 2004, 27, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Oh, I.S.; Whang, W.K.; Kim, I.H. Constituents of Crataegus pinnatifida var. psilosa leaves (II)-Flavonoids from BuOH fraction. Arch. Pharm. Res. 1994, 17, 314–317. [Google Scholar] [CrossRef]
- Somei, M.; Yamada, F.; Kurauchi, T.; Nagahama, Y.; Hasegawa, M.; Yamada, K.; Teranishi, S.; Sato, H.; Kaneko, C. The chemistry of indoles. CIII.1) simple syntheses of serotonin, N-methylserotonin, bufotenine, 5-methoxy-N-methyltryptamine, bufobutanoic acid, N-(indol-3-yl) methyl-5-methoxy-N-methyltryptamine, and lespedamine based on 1-hydroxyindole chemistry. Chem. Pharm. Bull. 2001, 49, 87–96. [Google Scholar] [CrossRef]
- Gan, N.; Yang, X.; Li, T.H.; He, P. Studies on constituents of rootsanel leaves from Desmodium blandum and their cytotoxic activity against growth of several tum or cells. Zhongguo Zhongyao Zazhi 2008, 33, 2077–2080. [Google Scholar] [PubMed]
- Zhang, P.; Cui, Z.; Liu, Y.S.; Sheng, Y. Isolation and identification of the indolealkylamines from the traditional Chinese medicine Toad Venom. J. Shenyang Pharm. Univ. 2006, 23, 216–219. [Google Scholar]
- Li, G.Q.; Deng, Z.W.; Li, J.; Fu, H.Z.; Lin, W.H. Chemical constituents from starfish Asterias rollestoni. J. Chin. Pharm. Sci. 2004, 13, 81–86. [Google Scholar]
- Sang, S.M.; Mao, S.L.; Lao, A.N.; Chen, Z.L. Studies on the chemical constituents of the seeds of Vaccariasegetalis (NECK) Garcke. III. Nat. Prod. Res. Dev. 2000, 12, 12–15. [Google Scholar]
- Wang, C.H.; Zhang, Z.X.; Wang, Y.H.; He, X.J. Cytotoxic indole alkaloids against human leukemia cell lines from the toxic plant Peganum harmala. Toxins 2015, 7, 4507–4518. [Google Scholar] [CrossRef] [PubMed]
- Yahara, S.; Shigeyama, C.; Ura, T.; Wakamatsu, K. Cyclic peptides, acyclic diterpene glycosides and other compounds from Lycium chinense MILL. Chem. Pharm. Bull. 1993, 41, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.; Ismail, S.; Ramanathan, S.; Yam, M.F. Investigation of antioxidant and hepatoprotective activity of standardized Curcuma xanthorrhiza rhizome in carbon tetrachloride-induced hepatic damaged rats. Sci. World J. 2014. [Google Scholar] [CrossRef] [PubMed]
- Babu, B.H.; Shylesh, B.S.; Padikkala, J. Antioxidant and hepatoprotective effect of Acanthus ilicifolius. Fitoterapia 2001, 72, 272–277. [Google Scholar] [CrossRef]
- Husain, S.R.; Cillard, J.; Cillard, P. Hydroxyl radical scavenging activity of flavonoids. Phytochemistry 1987, 26, 2489–2491. [Google Scholar] [CrossRef]
- Miyake, T.; Shibamoto, T. Antioxidative activities of natural compounds found in plants. J. Agric. Food Chem. 1997, 45, 1819–1822. [Google Scholar] [CrossRef]
- Ko, R.K.; Kim, G.O.; Hyun, C.G.; Jung, D.S; Lee, N.H. Compounds with tyrosinase inhibition, elastase inhibition and DPPH radical scavenging activities from the branches of Distylium racemosum Sieb. et Zucc. Phytother. Res. 2011, 25, 1451–1456. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Huang, M.Q.; Li, H.; Chen, X.W.; Zhang, Y.W.; Liu, J.; Xu, W.; Chu, K.D.; Chen, L. Chemical profiling and quantification of Gua-Lou-Gui-Zhi decoction by high performance liquid chromatography/quadrupole-time-of-flight mass spectrometry and ultra-performance liquid chromatography/triple quadrupole mass spectrometry. J. Chromatogr. B 2015, 986, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Vallverdú-Queralt, A.; Boix, N.; Piqué, E.; Gómez-Catalan, J.; Medina-Remon, A.; Sasot, G.; Mercader-Martí, M.; Llobet, J.M.; Lamuela-Raventos, R.M. Identification of phenolic compounds in red wine extract samples and zebrafish embryos by HPLC-ESI-LTQ-Orbitrap-MS. Food Chem. 2015, 181, 146–151. [Google Scholar] [CrossRef] [PubMed]
- He, L.L.; Zhang, Z.F.; Lu, L.Y.; Liu, Y.; Li, S.; Wang, J.G.; Song, Z.J.; Yan, Z.G.; Miao, J.H. Rapid identification and quantitative analysis of the chemical constituents in Scutellaria indica L. by UHPLC–QTOF–MS and UHPLC–MS/MS. J. Pharm. Biomed. Anal. 2016, 117, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Wang, B.J.; Huo, Z.P.; He, Y.; Polachi, N.; Lei, Z.D.; Liu, X.X.; Song, Z.H.; Qi, L.W. Analysis of chemical constituents in an herbal formula Jitong Ning Tablet. J. Pharm. Biomed. Anal. 2017, 140, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.T.; Lu, L.; Shao, C.L.; Yu, R.L.; Chen, F.L.; Yue, S.J.; Wang, M.; Guo, Z.L.; Fan, Y.C.; Guan, H.S.; Wang, C.Y. Discovery of DNA topoisomerase I inhibitors with low-cytotoxicity based on virtual screening from natural products. Mar. Drugs 2017, 15, 217. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–19 are available from the authors. |


| Compounds | DPPH/IC50 (μg·mL−1) | Compounds | DPPH/IC50 (μg·mL−1) |
|---|---|---|---|
| 1 | 36.1 ± 2.1 | 11 | >300 |
| 2 | 33.5 ± 1.3 | 12 | >300 |
| 3 | 3.8 ± 0.14 | 13 | >300 |
| 4 | 32.8 ± 0.85 | 14 | >300 |
| 5 | 4.0 ± 0.09 | 15 | >300 |
| 6 | 29.0 ± 1.15 | 16 | >300 |
| 7 | 47.5 ± 2.7 | 17 | >300 |
| 8 | >300 | 18 | >300 |
| 9 | 35.2 ± 2.6 | 19 | >300 |
| 10 | >300 | Vitamin C | 5.1 ± 0.09 |
| No. | Rt. (min) | Identification | Formula | Negative Ion (m/z) | Positive Ion (m/z) | ||
|---|---|---|---|---|---|---|---|
| Quasi-Molecular | MS/MS (m/z) | Quasi-Molecular | MS/MS (m/z) | ||||
| 1a | 11.02 | (−)-Gallocatechin (compound 3) | C15H14O7 | 305.0622 [M − H]− | 287 [M − H − H2O]−; 261 [M − H − H2O − O2−]−; 221 [M − H − A]−; 179 [M − H − B]−; 137 [M − H − C8H8O4]− | 307.0806 [M + H]+ | 289; 181; 139 |
| 2 | 16.13 | (−)-Epigallocatechin (compound 5) | C15H14O7 | 305.0661 [M − H]− | n.a. | 307.0825 [M + H]+ | 289; 181; 139 |
| 3 | 16.85 | 5-Hydroxyl liquiritin | C21H22O10 | 433.2018 [M − H]− | 387 [M − H − CO − H2O]−; 353 [M − H − A]−; 293; 271 [M − H − C6H11O5]− | n.a. | n.a. |
| 4a | 17.52 | (+)-Catechin (compound 4) | C15H14O6 | 289.0674 [M − H]− | 271 [M − H − H2O]− 245 [M − H − CO2]−; 205 [M − H − A]−; 179 [M − H − C6H6O2]−; | 291.0878 [M + H]+ | 273; 246; 165; 139 |
| 5 | 19.41 | Coreopsin | C21H22O10 | 433.2014 [M − H]− | 415 [M − H − H2O]−; 397 [M − H − H2O]−; 297 [M − H − C7H5O3]−; 161 [glc]− | n.a. | n.a. |
| 6a | 20.80 | (−)-Epicatechin (compound 6) | C15H14O6 | 289.0675 [M − H]− | 245 [M − H − CO2]−; 205 [M − H − A]−; 179 [M − H − C6H6O2]−; | 291.0874 [M + H]+ | n.a. |
| 7 | 21.08 | Dihydrokaempferol-7-O-β-d-glucoside | C21H22O11 | 449.1023 [M − H]− | 287 [M − H − glc]−; 267 [M − H − glc − H2O]−; 259 [M − H − glc − CO]− | n.a. | n.a. |
| 8 | 23.05 | Gossypetin 7-rhamnoside-8-glucoside | C27H30O17 | 625.1320 [M − H]− | 316 [M − H − glc − rha]−; 271 [M − H − glc − rha – OH − CO]− | 627.1585 [M + H]+ | 481; 319 |
| 9 | 23.39 | Quercetin-7-O-glucopyranoside | C21H20O12 | 463.0815 [M − H]− | 301 [M − H − glc]− | 465.1043 [M + H]+ | 303 |
| 10 | 24.23 | Viscidulin II 2′-O-glucoside | C23H26O12 | 493.1289 [M − H]− | 331 [M − H − glc]−; 313 [M − H − C6H12O6]−; 271 [M − H − glc − OCH3]− | n.a. | n.a. |
| 11a | 25.06 | Rutin (compound 10) | C27H30O16 | 609.1379 [M − H]− | 301 [M − H − C12H20O9]− | 611.1638 [M + H]+ | 303 |
| 12 | 25.54 | Morin-7-O-glucopyranoside | C21H20O12 | 463.0891 [M − H]− | 301.0454 [M − H − glc]− | 465.1047 [M + H]+ | 303 |
| 13 | 26.04 | Kaempferol 3-O-rutinoside | C27H30O15 | 593.1428 [M − H]− | 327 [M − H − C12H20O10]−; 285 [M − H − rutinoside]− | 595.1683 [M + H]+ | 449; 287 |
| 14a | 26.33 | Dihydroquercetin (compound 7) | C15H12O7 | 303.0462 [M − H]− | 285 [M − H − H2O]−; 259 [M − H − CO2]−; 177 [M − H − C8H4O6]− | 305.0673 [M + H]+ | n.a. |
| 15 | 26.55 | Luteolin 7-O-rutinoside | C27H30O15 | 593.1425 [M − H]− | 285 [M − H − rutinoside]− | 595.1456 [M + H]+ | 449; 287 |
| 16 | 26.67 | Kaempferol-7-O-glucoside | C21H20O11 | 447.0870 [M − H]− | 285 [M − H − glc]− | 449.1093 [M + H]+ | 287; 172 |
| 17a | 26.83 | Quercetin-3-O-α-l- rhamnopyranoside-(1→6)- β-d-galactopyranosyl (compound 11) | C27H30O16 | 609.1372 [M − H]− | 301 [M − H − C12H20O9]− | 611.1627 [M + H]+ | n.a. |
| 18 | 27.00 | (−)-Epigallocatechin 3-O-(E)-p-coumaroate (compound 1) | C24H20O9 | 451.0968 [M − H]− | 433 [M − H − H2O]−; 357 [M − H − C5H3O2]−; 341; 311; 217 | 453.1194 [M + H]+ | n.a. |
| 19 | 27.28 | 5,7,2-Trihydroxy-6-methoxyflavone 7-O-β-d-glucoside | C22H22O11 | 461.1028 [M − H]− | 446 [M − H − CH3]−; 299 [M − H − glc]− | 463.1251 [M + H]+ | 445; 301 |
| 20 | 27.40 | Quercetin-3,7-di-O-glucopyranoside | C27H30O17 | 625.1410 [M − H]− | 301 [M − H − glc − glc]− | 627.2453 [M + H]+ | n.a. |
| 21a | 27.51 | Dihydrokaempferol (compound 8) | C15H12O6 | 287.0521 [M − H]− | 269 [M − H − H2O]−; 243 [M − H − CO2]−; 161 [M − H − C6H6O]− | 289.0719 [M + H]+ | 272 |
| 22 | 27.73 | (−)-Epigallocatechin 3-O-(Z)-p-coumaroate (compound 2) | C24H20O9 | 451.0975 [M − H]− | 433 [M − H − H2O]−; 407 [M − H − CO2]−; 357 [M − H − C5H3O2]−; 305 [M − H − C9H6O2]−; 287 [M − H − C9H8O3]-; 269 [M − H − C9H6O4]−; 229; 163 [M − H − C9H8O4] − | 453.1199 [M + H]+ | 435; 327; 289; 247; 139 |
| 23a | 28.46 | Quercetin (compound 9) | C15H10O7 | 301.0311 [M − H]− | 273 [M − H − CO] −; 257 [M − H − OH] −; 151 [M − H − C8H7O3] − | 303.0509 [M + H]+ | n.a. |
| 24 | 30.15 | Kaempferol | C15H10O6 | 285.0361 [M − H]− | 257 [M − H − CO]−; 241 [M − H − CO2]−; 151 [M − H − C8H6O2]−; 133 [M − H − C7H4O4]−; | 287.0565 [M + H]+ | 241; 153 |
| 25 | 31.53 | Orobol | C15H10O6 | 285.0364 [M − H]− | 241 [M − H − CO2]−; 175 [M − H − B]− | 287.0558 [M + H]+ | n.a. |
| 26 | 32.36 | Demethylpraecanson B | C21H20O5 | n.a. | n.a. | 353.2312 [M + H]+ | 335 [M + H − H2O]+; 253; 235 [M + H − C8H6O]+;195 |
| 27 | 33.70 | Luteolin | C15H10O6 | 285.0363 [M − H]− | 241 [M − H − CO2]−; 175 [M − H − B]−; 133 [M − H − C7H4O4]−; | 287.0561 [M + H]+ | 269; 153; 137 |
| 28 | 34.25 | Isoquercitrin | C21H20O12 | 463.0966 [M − H]− | 445 [M − H − H2O]−; 301 [M − H − glc]−; 283 [M − H − H2O − glc]−; 253 | 465.1192 [M + H]+ | 447; 341; 286; 162 |
| 29 | 36.96 | 7,2′,4′,5′-Tetramethoxyisoflavon/ 7,2′,3′,4′-Tetramethoxyflavone | C19H18O6 | n.a. | n.a. | 343.1191 [M + H]+ | 328; 282; 253; 150 |
| 30 | 37.03 | Robinetinidol-4alpha-ol | C15H14O7 | 305.1713 [M − H]− | 287 [M − H − H2O]−; 249; 135 [M − H − C7H6O5]− | 307.2065 [M + H]+ | n.a. |
| 31 | 42.53 | Norartocarpetin/ 7,8,2′,4′-Tetrahydroxyisoflavone/ 5,7,2′,6′-Tetrahydroxyflavone/ 5,7,2′,3′-Tetrahydroxyflavone/ 5,7,2′,5′-Tetrahydroxyflavone | C15H10O6 | 285.0360 [M − H]− | 241 [M − H − CO2]−; 175 [M − H − B]− | n.a. | n.a. |
| 32 | 47.13 | Icariin | C33H40O15 | n.a. | n.a. | 677.3754 [M + H]+ | 515 |
| 33 | 55.30 | Wogonin/Oroxylin A | C16H12O5 | 283.1661 [M − H]− | 163; 107 | n.a. | n.a. |
| 34 | 57.52 | Nigrolineaxanthone N/Kanzonol M | C23H26O6 | 397.2534 [M − H]− | 329 | n.a. | n.a. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.-C.; Yue, S.-J.; Guo, Z.-L.; Xin, L.-T.; Wang, C.-Y.; Zhao, D.-L.; Guan, H.-S.; Wang, C.-Y. Phytochemical Composition, Hepatoprotective, and Antioxidant Activities of Phyllodium pulchellum (L.) Desv. Molecules 2018, 23, 1361. https://doi.org/10.3390/molecules23061361
Fan Y-C, Yue S-J, Guo Z-L, Xin L-T, Wang C-Y, Zhao D-L, Guan H-S, Wang C-Y. Phytochemical Composition, Hepatoprotective, and Antioxidant Activities of Phyllodium pulchellum (L.) Desv. Molecules. 2018; 23(6):1361. https://doi.org/10.3390/molecules23061361
Chicago/Turabian StyleFan, Ya-Chu, Shi-Jun Yue, Zhong-Long Guo, Lan-Ting Xin, Chao-Yi Wang, Dong-Lin Zhao, Hua-Shi Guan, and Chang-Yun Wang. 2018. "Phytochemical Composition, Hepatoprotective, and Antioxidant Activities of Phyllodium pulchellum (L.) Desv" Molecules 23, no. 6: 1361. https://doi.org/10.3390/molecules23061361
APA StyleFan, Y.-C., Yue, S.-J., Guo, Z.-L., Xin, L.-T., Wang, C.-Y., Zhao, D.-L., Guan, H.-S., & Wang, C.-Y. (2018). Phytochemical Composition, Hepatoprotective, and Antioxidant Activities of Phyllodium pulchellum (L.) Desv. Molecules, 23(6), 1361. https://doi.org/10.3390/molecules23061361