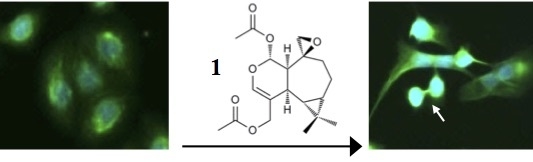
Plagiochiline A Inhibits Cytokinetic Abscission and Induces Cell Death
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Plagiochiline A
4.2. Cell Culture
4.3. Flow Cytometry
4.4. Immunofluorescence
4.5. Clonogenic Survival Assay
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aponte, J.C.; Vaisberg, A.J.; Rojas, R.; Sauvain, M.; Lewis, W.H.; Lamas, G.; Sarasara, C.; Gilman, R.H.; Hammond, G.B. A multipronged approach to the study of peruvian ethnomedicinal plants: A legacy of the ICBG-Peru Project. J. Nat. Prod. 2009, 72, 524–526. [Google Scholar] [CrossRef] [PubMed]
- Aponte, J.C.; Yang, H.; Vaisberg, A.J.; Castillo, D.; Malaga, E.; Verastegui, M.; Casson, L.K.; Stivers, N.; Bates, P.J.; Rojas, R.; et al. Cytotoxic and anti-infective sesquiterpenes present in Plagiochila disticha (Plagiochilaceae) and Ambrosia peruviana (Asteraceae). Planta Med. 2010, 76, 705–707. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, Y.; Yoyota, M.; Takemoto, T.; Kubo, I.; Nakanishi, K. Insect antifeedant secoaromadendrane-type sesquiterpenes from Plagiochila species. Phytochemistry 1980, 19, 2147–2154. [Google Scholar] [CrossRef]
- Toyota, M.; Tanimura, K.; Asakawa, Y. Cytotoxic 2,3-secoaromadendrane-type sesquiterpenoids from the liverwort Plagiochila ovalifolia. Planta Med. 1998, 64, 462–464. [Google Scholar] [CrossRef] [PubMed]
- Durán-Peña, M.J.; Ares, J.M.B.; Hanson, J.R.; Collado, I.G.; Hernández-Galán, R. Biological activity of natural Sesquiterpenoids containing a gem-Dimethylcyclopropane unit. Nat. Prod. Rep. 2015, 32, 1236–1248. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.W.; Rose, F. Plagiochila atlantica F. Rose, sp. nov.—P. ambagiosa auct. J. Bryol. 1975, 8, 417–422. [Google Scholar] [CrossRef]
- Ramírez, M.; Kamiya, N.; Popich, S.; Asakawa, Y.; Bardón, A. Insecticidal constituents from the Argentine Liverwort Plagiochila bursata. Chem. Biodivers. 2010, 7, 1855–1861. [Google Scholar] [CrossRef] [PubMed]
- Nagle, A.; Hur, W.; Gray, N.S. Antimitotic agents of natural origin. Curr. Drug Targets 2006, 7, 305–326. [Google Scholar] [CrossRef] [PubMed]
- Barr, F.A.; Gruneberg, U. Cytokinesis: Placing and making the final cut. Cell 2007, 131, 847–860. [Google Scholar] [CrossRef] [PubMed]
- Frenette, P.; Haines, E.; Loloyan, M.; Kinal, M.; Pakarian, P.; Piekny, A. An anillin-Ect2 complex stabilizes central spindle microtubules at the cortex during cytokinesis. PLoS ONE 2012, 7, e34888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fededa, J.P.; Gerlich, D.W. Molecular control of animal cell cytokinesis. Nat. Cell Biol. 2012, 14, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Gulluni, F.; Martini, M.; Hirsch, E. Cytokinetic Abscission: Phosphoinositides and ESCRTs Direct the Final Cut. J. Cell. Biochem. 2017, 118, 3561–3568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoten, C.L.; Carlton, J.G. ESCRT-dependent control of membrane remodelling during cell division. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Thoresen, S.B.; Campsteijn, C.; Vietri, M.; Schink, K.O.; Liestol, K.; Andersen, J.S.; Raiborg, C.; Stenmark, H. ANCHR mediates Aurora-B-dependent abscission checkpoint control through retention of VPS4. Nat. Cell Biol. 2014, 16, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Nähse, V.; Christ, L.; Stenmark, H.; Campsteijn, C. The abscission checkpoint: Making it to the final cut. Trends Cell Biol. 2017, 27, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cheffings, T.H.; Burroughs, N.J.; Balasubramanian, M.K. Actomyosin ring formation and tension generation in eukaryotic cytokinesis. Curr. Biol. 2016, 26, R719–R737. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Arora, P.D.; McCulloch, C.A.; Wilde, A. Cytokinesis requires localized β-actin filament production by an actin isoform specific nucleator. Nat. Commun. 2017, 8, 1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitsushima, M.; Aoki, K.; Ebisuya, M.; Matsumura, S.; Yamamoto, T.; Matsuda, M.; Toyoshima, F.; Nishida, E. Revolving movement of a dynamic cluster of actin filaments during mitosis. J. Cell Biol. 2010, 191, 453–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moulding, D.A.; Blundell, M.P.; Spiller, D.G.; White, M.R.; Cory, G.O.; Calle, Y.; Kempski, H.; Sinclair, J.; Ancliff, P.J.; Kinnon, C.; et al. Unregulated actin polymerization by WASp causes defects of mitosis and cytokinesis in X-linked neutropenia. J. Exp. Med. 2007, 204, 2213–2224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cragg, G.M.; Newman, D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngan, V.K.; Bellman, K.; Hill, B.T.; Wilson, L.; Jordan, M.A. Mechanism of mitotic block and inhibition of cell proliferation by the semisynthetic Vinca alkaloids vinorelbine and its newer derivative vinflunine. Mol. Pharmacol. 2001, 60, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.A.; Wilson, L. Microtubules and actin filaments: Dynamic targets for cancer chemotherapy. Curr. Opin. Cell Biol. 1998, 10, 123–130. [Google Scholar] [CrossRef]
- Atilla-Gokcumen, G.E.; Castoreno, A.B.; Sasse, S.; Eggert, U.S. Making the cut: The chemical biology of cytokinesis. ACS Chem. Biol. 2010, 5, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Park, J.H.; Miyamoto, T.; Yamamoto, S.; Hisada, S.; Alachkar, H.; Nakamura, Y. TOPK inhibitor induces complete tumor regression in xenograft models of human cancer through inhibition of cytokinesis. Sci. Transl. Med. 2014, 6, 259ra145. [Google Scholar] [CrossRef] [PubMed]
- Chircop, M.; Perera, S.; Mariana, A.; Lau, H.; Ma, M.P.; Gilbert, J.; Jones, N.C.; Gordon, C.P.; Young, K.A.; Morokoff, A.; et al. Inhibition of dynamin by dynole 34-2 induces cell death following cytokinesis failure in cancer cells. Mol. Cancer Ther. 2011, 10, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Perera, S.; Gilbert, J.; Smith, C.M.; Mariana, A.; Gordon, C.P.; Sakoff, J.A.; McCluskey, A.; Robinson, P.J.; Braithwaite, A.W.; et al. The dynamin inhibitors MiTMAB and OcTMAB induce cytokinesis failure and inhibit cell proliferation in human cancer cells. Mol. Cancer Ther. 2010, 9, 1995–2006. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, M.P.; Iwamori, T.; Buchold, G.M.; Matzuk, M.M. Germ cell intercellular bridges. Cold Spring Harb. Perspect. Biol. 2011, 3, a005850. [Google Scholar] [CrossRef] [PubMed]
- Lordier, L.; Jalil, A.; Aurade, F.; Larbret, F.; Larghero, J.; Debili, N.; Vainchenker, W.; Chang, Y. Megakaryocyte endomitosis is a failure of late cytokinesis related to defects in the contractile ring and Rho/Rock signaling. Blood 2008, 112, 3164–3174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujiwara, T.; Bandi, M.; Nitta, M.; Ivanova, E.V.; Bronson, R.T.; Pellman, D. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature 2005, 437, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Cortez, B.A.; Teixeira, P.R.; Redick, S.; Doxsey, S.; Machado-Santelli, G.M. Multipolar mitosis and aneuploidy after chrysotile treatment: A consequence of abscission failure and cytokinesis regression. Oncotarget 2016, 7, 8979. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Chang, M.C.; Chang, H.H.; Wang, T.M.; Tseng, W.Y.; Tai, T.F.; Yeh, H.W.; Yang, T.T.; Hahn, L.J.; Jeng, J.H. Areca nut-induced micronuclei and cytokinesis failure in Chinese hamster ovary cells is related to reactive oxygen species production and actin filament deregulation. Environ. Mol. Mutagen. 2009, 50, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Agromayor, M.; Martin-Serrano, J. Knowing when to cut and run: Mechanisms that control cytokinetic abscission. Trends Cell Biol. 2013, 23, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Carmena, M. Abscission checkpoint control: Stuck in the middle with Aurora B. Open Biol. 2012, 2, 120095. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T.; Hehnly, H.; Doxsey, S.J. Orchestrating vesicle transport, ESCRTs and kinase surveillance during abscission. Nat. Rev. Mol. Cell Biol. 2012, 13, 483–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mierzwa, B.; Gerlich, D.W. Cytokinetic abscission: Molecular mechanisms and temporal control. Dev. Cell 2014, 31, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Abreu, P.A.; Sousa, T.S.; Jimenez, P.C.; Wilke, D.V.; Rocha, D.D.; Freitas, H.P.; Pessoa, O.D.; La Clair, J.J.; Costa-Lotufo, L.V. Identification of pyrroloformamide as a cytokinesis modulator. ChemBioChem 2014, 15, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Atilla-Gokcumen, G.E.; Bedigian, A.V.; Sasse, S.; Eggert, U.S. Inhibition of glycosphingolipid biosynthesis induces cytokinesis failure. J. Am. Chem. Soc. 2011, 133, 10010–10013. [Google Scholar] [CrossRef]
- Bai, R.; Verdier-Pinard, P.; Gangwar, S.; Stessman, C.C.; McClure, K.J.; Sausville, E.A.; Pettit, G.R.; Bates, R.B.; Hamel, E. Dolastatin 11, a marine depsipeptide, arrests cells at cytokinesis and induces hyperpolymerization of purified actin. Mol. Pharmacol. 2001, 59, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Castoreno, A.B.; Smurnyy, Y.; Torres, A.D.; Vokes, M.S.; Jones, T.R.; Carpenter, A.E.; Eggert, U.S. Small molecules discovered in a pathway screen target the Rho pathway in cytokinesis. Nat. Chem. Biol. 2010, 6, 457–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, A.G.; Sider, J.R.; Verbrugghe, K.; Fenteany, G.; von Dassow, G.; Bement, W.M. Identification of small molecule inhibitors of cytokinesis and single cell wound repair. Cytoskeleton (Hoboken) 2012, 69, 1010–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grace, K.J.; Medina, M.; Jacobs, R.S.; Wilson, L. Selective inhibition of cytokinesis in sea urchin embryos by the marine natural product pseudopterolide. Mol. Pharmacol. 1992, 41, 631–638. [Google Scholar] [PubMed]
- Matesic, D.F.; Villio, K.N.; Folse, S.L.; Garcia, E.L.; Cutler, S.J.; Cutler, H.G. Inhibition of cytokinesis and akt phosphorylation by chaetoglobosin K in ras-transformed epithelial cells. Cancer Chemother. Pharmacol. 2006, 57, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Saito, Y.; Soeda, S.; Iwamoto, E.; Ogawa, S.; Yamagishi, N.; Kuga, T.; Yamaguchi, N. Genistein induces cytokinesis failure through RhoA delocalization and anaphase chromosome bridging. J. Cell. Biochem. 2014, 115, 763–771. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, E.T.; Asai, D.J.; Jacobs, R.S.; Wilson, L. Selective inhibition of cytokinesis in sea urchin embryos by low concentrations of stypoldione, a marine natural product that reacts with sulfhydryl groups. Mol. Pharmacol. 1989, 35, 635–642. [Google Scholar] [PubMed]
- Zullo, K.M.; Guo, Y.; Cooke, L.; Jirau-Serrano, X.; Mangone, M.; Scotto, L.; Amengual, J.E.; Mao, Y.; Nandakumar, R.; Cremers, S.; et al. Aurora A Kinase Inhibition Selectively Synergizes with Histone Deacetylase Inhibitor through Cytokinesis Failure in T-cell Lymphoma. Clin. Cancer Res. 2015, 21, 4097–4109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of plagiochiline A are available from the authors. |



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stivers, N.S.; Islam, A.; Reyes-Reyes, E.M.; Casson, L.K.; Aponte, J.C.; Vaisberg, A.J.; Hammond, G.B.; Bates, P.J. Plagiochiline A Inhibits Cytokinetic Abscission and Induces Cell Death. Molecules 2018, 23, 1418. https://doi.org/10.3390/molecules23061418
Stivers NS, Islam A, Reyes-Reyes EM, Casson LK, Aponte JC, Vaisberg AJ, Hammond GB, Bates PJ. Plagiochiline A Inhibits Cytokinetic Abscission and Induces Cell Death. Molecules. 2018; 23(6):1418. https://doi.org/10.3390/molecules23061418
Chicago/Turabian StyleStivers, Nicole S., Ashraful Islam, Elsa M. Reyes-Reyes, Lavona K. Casson, José C. Aponte, Abraham J. Vaisberg, Gerald B. Hammond, and Paula J. Bates. 2018. "Plagiochiline A Inhibits Cytokinetic Abscission and Induces Cell Death" Molecules 23, no. 6: 1418. https://doi.org/10.3390/molecules23061418
APA StyleStivers, N. S., Islam, A., Reyes-Reyes, E. M., Casson, L. K., Aponte, J. C., Vaisberg, A. J., Hammond, G. B., & Bates, P. J. (2018). Plagiochiline A Inhibits Cytokinetic Abscission and Induces Cell Death. Molecules, 23(6), 1418. https://doi.org/10.3390/molecules23061418