Proanthocyanidins Protect Epithelial Cells from Zearalenone-Induced Apoptosis via Inhibition of Endoplasmic Reticulum Stress-Induced Apoptosis Pathways in Mouse Small Intestines
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Effect of ZEA and PCs on Cell Viability
2.3. Effect of Different Concentrations of PCs Co-Treated with ZEA on Cell Viability
2.4. Detection of Oxidation Indexes in Intestinal Epithelial Cells
2.5. Cell Apoptosis Assay
2.6. Real-Time PCR Analysis
2.7. Western Blot Analysis
2.8. Statistical Analysis
3. Results
3.1. The Effect of ZEA and PCs on the Relative Survival of MODE-K Cells
3.2. Severity of MODE-K Cell Damage by Detection of LDH Activities
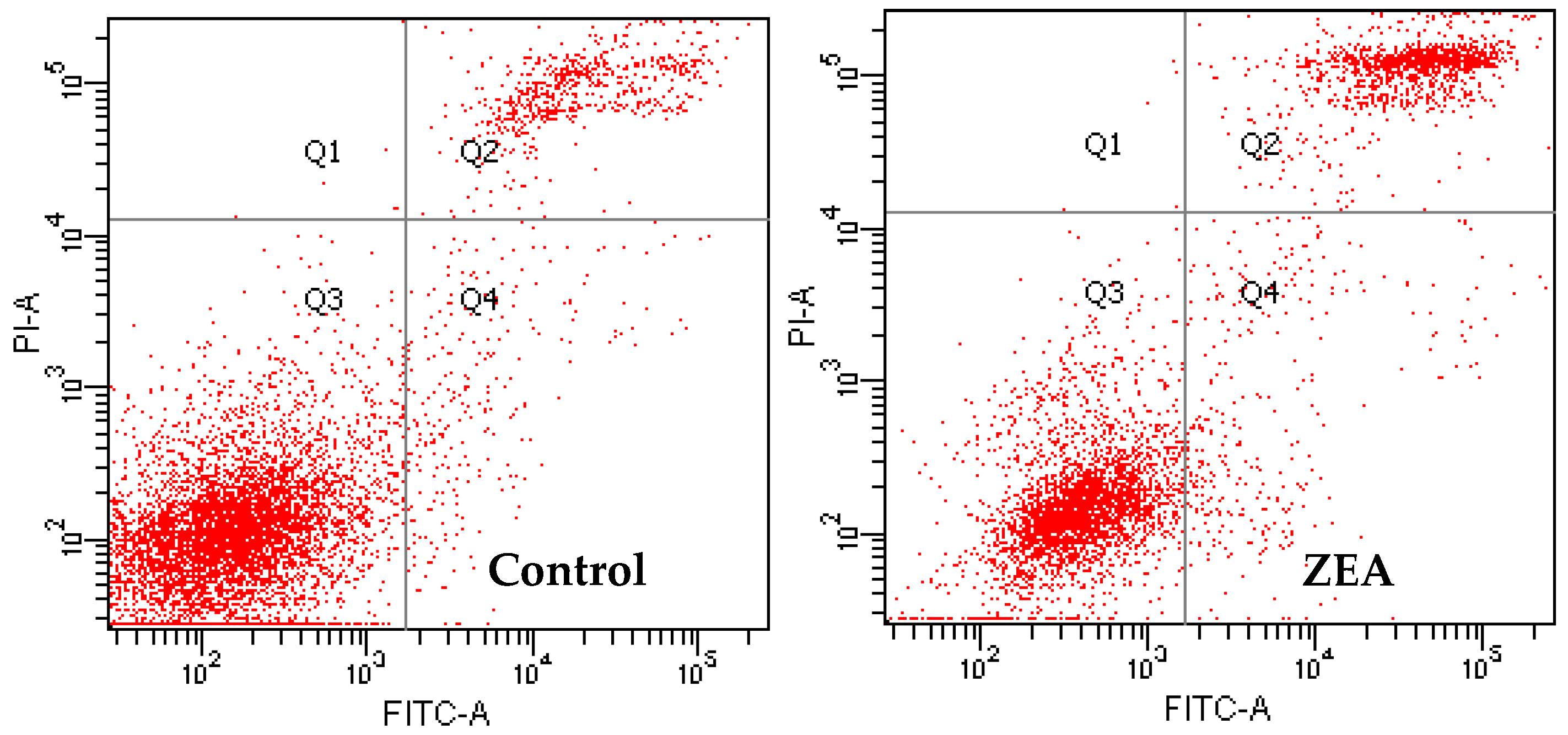
3.3. The Effects of PCs on the Apoptosis of MODE-K Cells Induced by ZEA
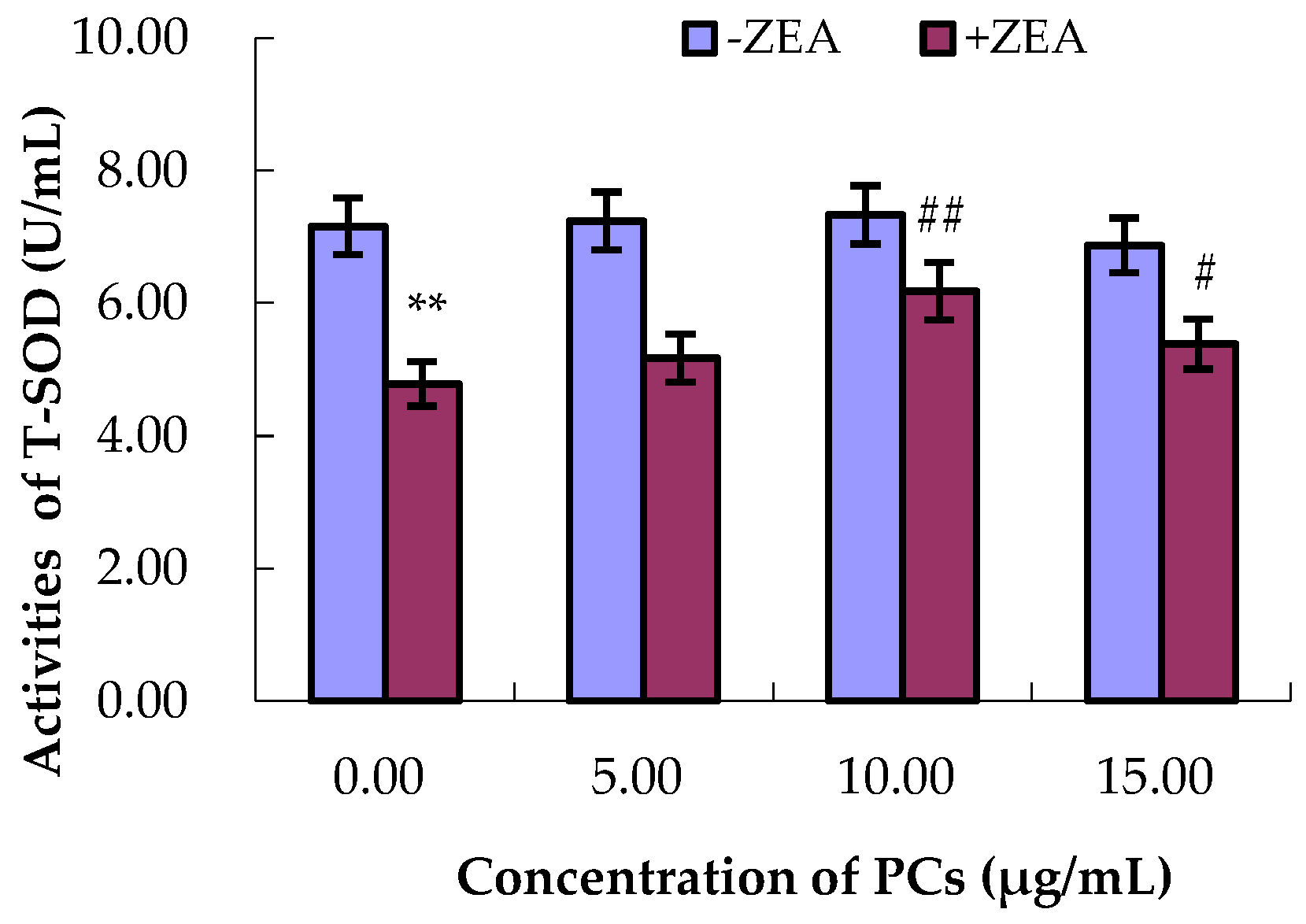
3.4. PCs Suppressed ZEA-Induced Oxidative MODE-K Cell Injury
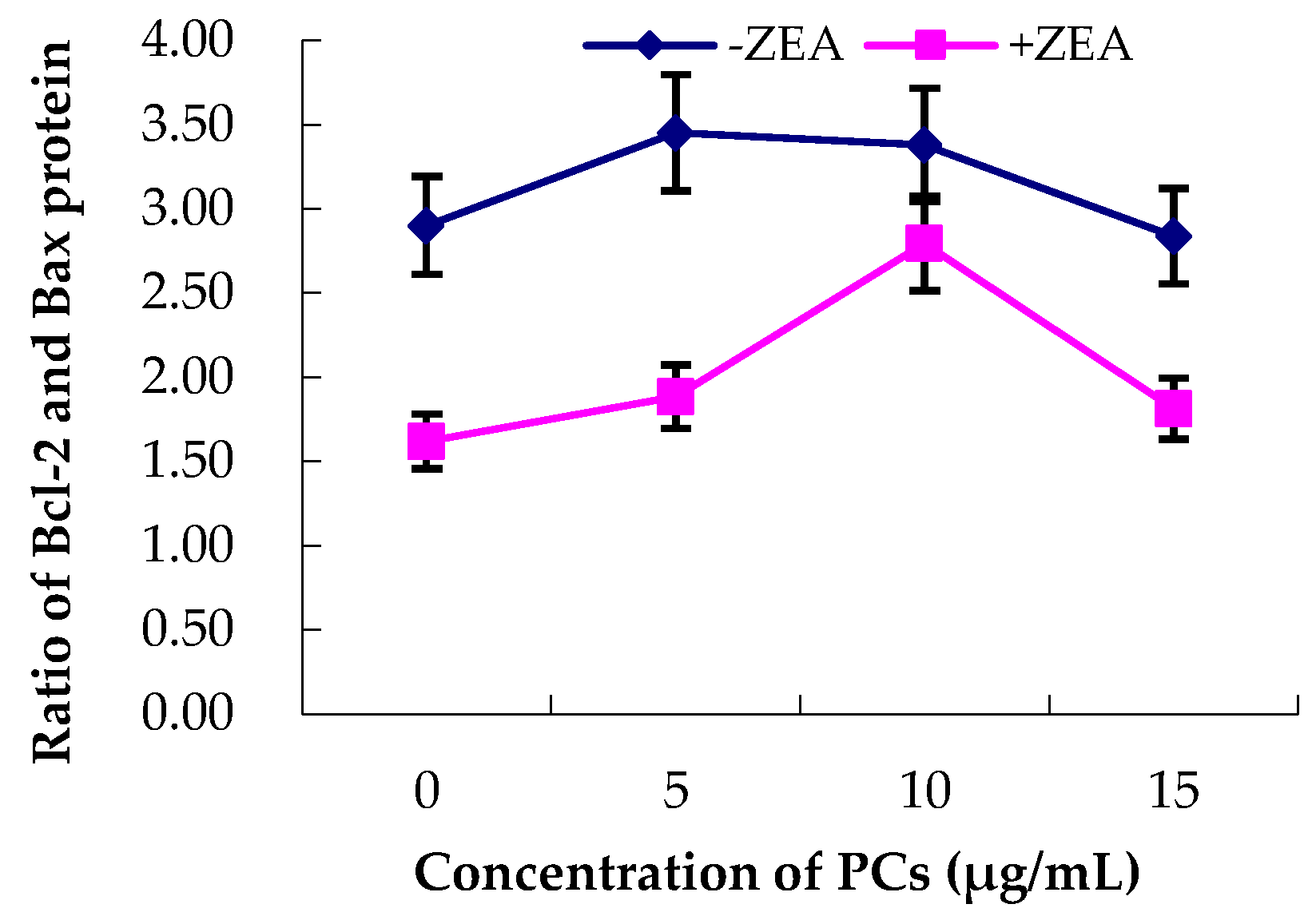
3.5. Expression of Bcl-2 and Bax Proteins in MODE-K Cells
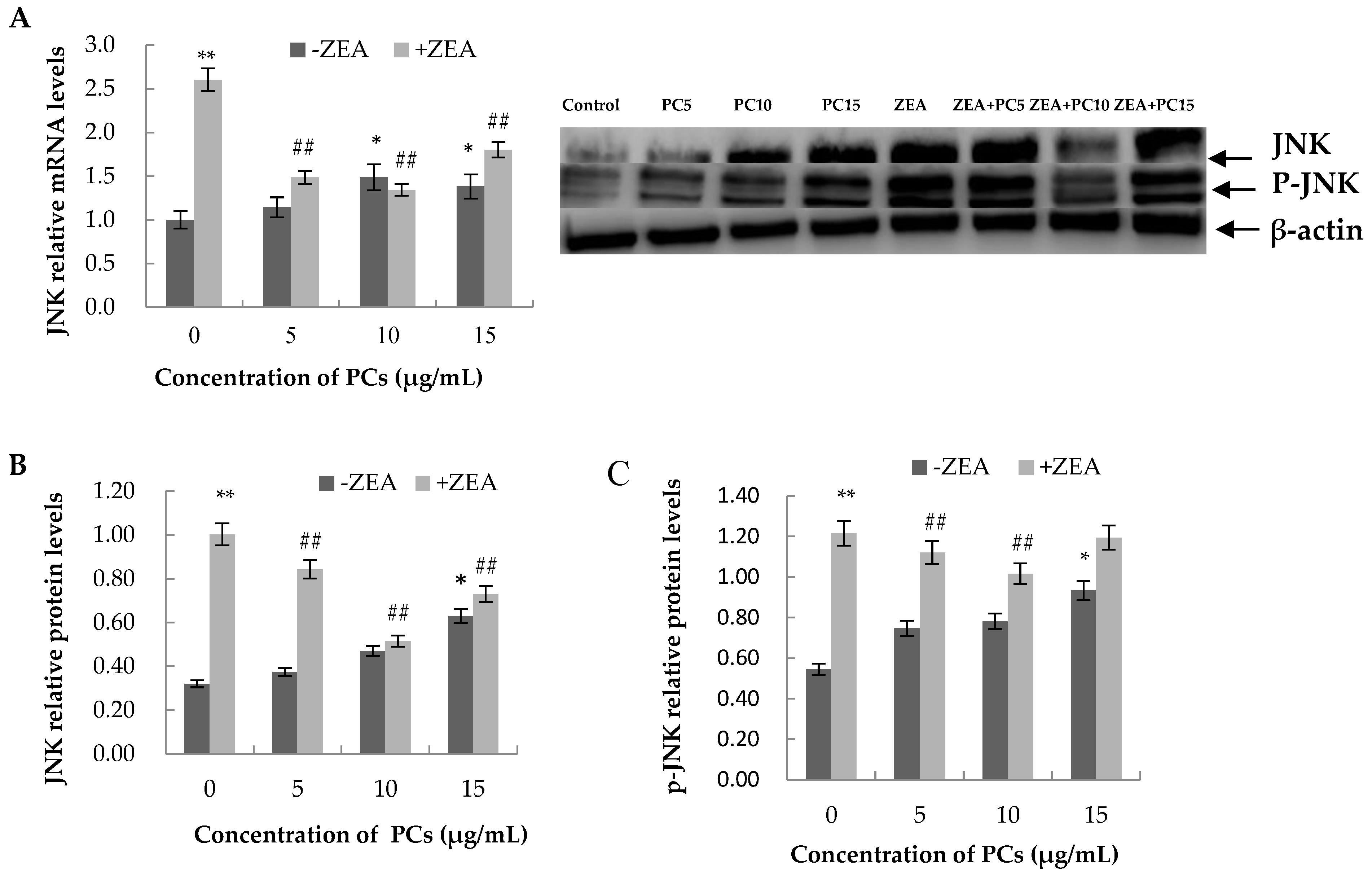
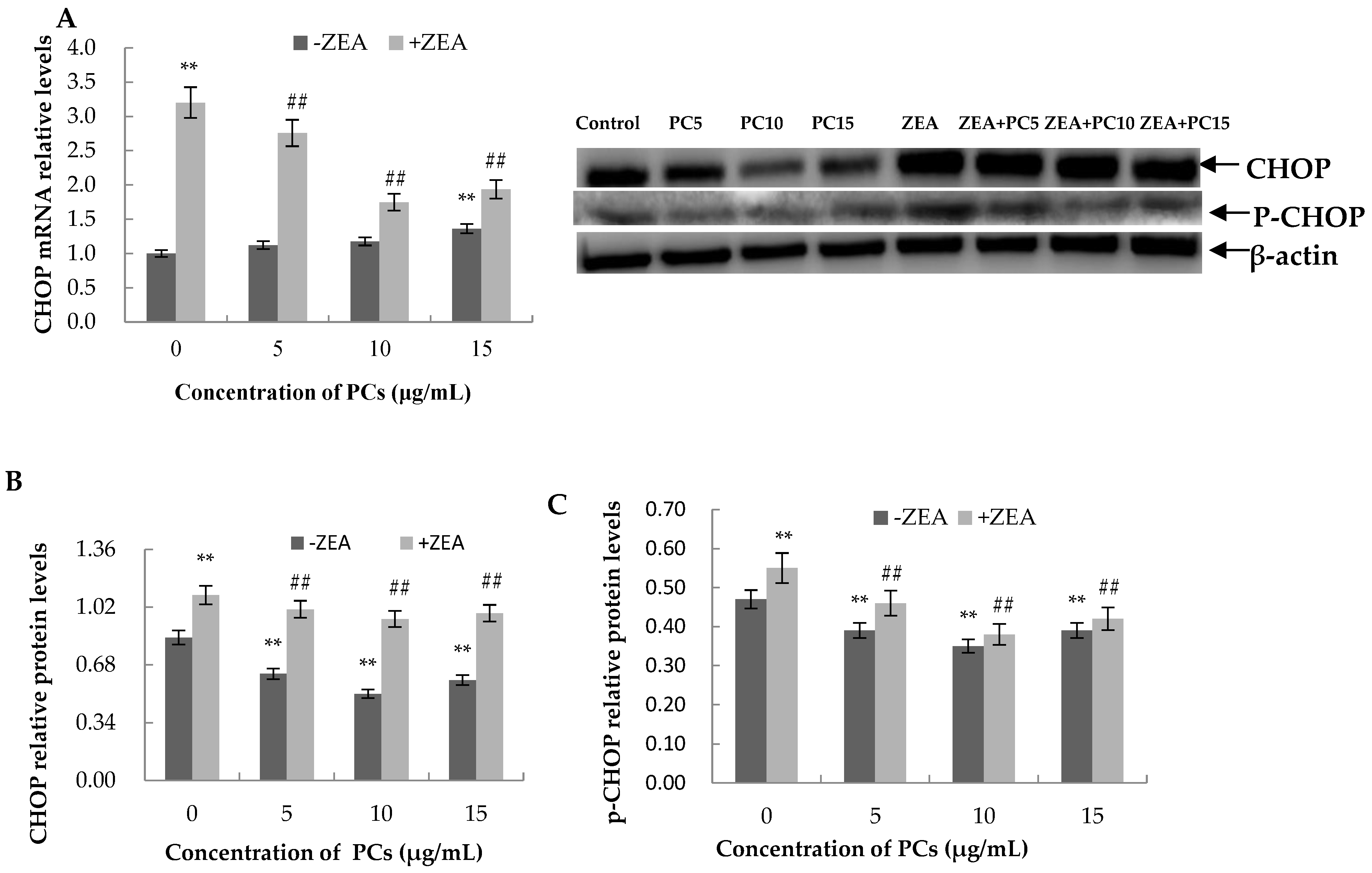
3.6. Expression of the mRNA and Protein Related to ERS-Induced Apoptosis Pathway in MODE-K Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- García-Rodríguez, A.; Vila, L.; Cortés, C.; Hernández, A.; Marcos, R. Exploring the usefulness of the complex in vitro intestinal epithelial model Caco-2/HT29/Raji-B in nanotoxicology. Food Chem. Toxicol. 2018, 113, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Grenier, B.; Applegate, T.J. Modulation of intestinal functions following mycotoxin ingestion: Meta-analysisof published experiments in animals. Toxins 2013, 5, 396–430. [Google Scholar] [CrossRef] [PubMed]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Zinedine, A.; Soriano, J.M.; Molto, J.C.; Manes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Takemura, H.; Shim, J.Y.; Sayama, K.; Tsubura, A.; Zhu, B.T.; Shimoi, K. Characterization of the estrogenic activities of zearalenone and zeranol in vivo and in vitro. J. Steroid Biochem. Mol. Biol. 2007, 103, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Salah-Abbès, J.B.; Abbès, S.; Houas, Z.; Abdel-Wahhab, M.A.; Oueslati, R. Zearalenone induces immunotoxicity in mice: Possible protective effects of radish extract (Raphanus sativus). J. Pharm. Pharmacol. 2008, 60, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Alseeni, M.; Elsawi, N.; Shaker, S.; Alamoudi, A. Investigation of the Biochemical and Histological Changes Induced by Zearalenone Mycotoxin on Liver in Male Mice and the Protective Role of Crude Venom Extracted from Jellyfish Cassiopea Andromeda. Food Nutr. Sci. 2011, 2, 314–322. [Google Scholar]
- Liang, Z.; Ren, Z.; Gao, S.; Chen, Y.; Yang, Y.; Yang, D.; Deng, J.; Zuo, Z.; Wang, Y.; Shen, L. Individual and combined effects of deoxynivalenol and zearalenone on mouse kidney. Environ. Toxicol. Pharmacol. 2015, 40, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Becci, P.J.; Voss, K.A.; Hess, F.G.; Gallo, M.A.; Parent, R.A.; Stevens, K.R.; Taylor, J.M. Long-term carcinogenicity and toxicity study of zearalenone in the rat. J. Appl. Toxicol. 1982, 2, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Ben Salem, I.; Prola, A.; Boussabbeh, M.; Guilbert, A.; Bacha, H.; Abid-Essefi, S.; Lemaire, C. Crocin and Quercetin protect HCT116 and HEK293 cells from Zearalenone-induced apoptosis by reducing endoplasmic reticulum stress. Cell Stress Chaperones 2015, 20, 927–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassen, W.; Ayed-Boussema, I.; Oscoz, A.A.; Lopez Ade, C.; Bacha, H. The role of oxidative stress in zearalenone-mediated toxicity in Hep G2 cells: Oxidative DNA damage, gluthatione depletion and stress proteins induction. Toxicology 2007, 232, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Abid-Essefi, S.; Ouanes, Z.; Hassen, W.; Baudrimont, I.; Creppy, E.; Bacha, H. Cytotoxicity, inhibition of DNA and protein syntheses and oxidative damage in cultured cells exposed to zearalenone. Toxicol. In Vitro 2004, 18, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Gerez, J.R.; Pinton, P.; Callu, P.; Grosjean, F.; Oswald, I.P.; Bracarense, A.P. Deoxynivalenol alone or in combination with nivalenol and zearalenone induce systemic histological changes in pigs. Exp. Toxicol. 2015, 67, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Marin, D.E.; Motiu, M.; Taranu, I. Food contaminant zearalenone and its metabolites affect cytokine synthesis and intestinal epithelial integrity of porcine cells. Toxins 2015, 7, 1979–1988. [Google Scholar] [CrossRef] [PubMed]
- Taranu, I.; Braicu, C.; Marin, D.E.; Pistol, G.C.; Motiu, M.; Balacescu, L.; Beridan Neagoe, I.; Burlacu, R. Exposure to zearalenone mycotoxin alters in vitro porcine intestinal epithelial cells by differential gene expression. Toxicol. Lett. 2015, 232, 310–325. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Gao, R.; Meng, Q.; Zhang, Y.; Bi, C.; Shan, A. Toxic effects of maternal zearalenone exposure on intestinal oxidative stress, barrier function, immunological and morphological changes in rats. PLoS ONE 2014, 9, e106412. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Ma, H.; Gao, F.; Zhong, L.; Ren, J. Metallothionein alleviates oxidative stress-induced endoplasmic reticulum stress and myocardial dysfunction. J. Mol. Cell. Cardiol. 2009, 47, 228–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Y.L.; Wang, Z.; Wang, H.; Zhang, C.; Zhang, Y.; Zhao, M.; Chen, Y.H.; Meng, X.H.; Xu, D.X. Ascorbic acid protects against cadmium-induced endoplasmic reticulum stress and germ cell apoptosis in testes. Reprod. Toxicol. 2012, 34, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Tatay, E.; Font, G.; Ruiz, M.J. Cytotoxic effects of zearalenone and its metabolites and antioxidant cell defense in CHO-K1 cells. Food Chem. Toxicol. 2016, 96, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Jiang, T.; Lin, P.; Chen, H.; Wang, L.; Wang, N.; Zhao, F.; Tang, K.; Zhou, D.; Wang, A.; et al. Apoptosis inducing factor gene depletion inhibits zearalenone-induced cell death in a goat leydig cell line. Reprod. Toxicol. 2017, 67, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Banjerdpongchai, R.; Kongtawelert, P.; Khantamat, O.; Srisomsap, C.; Chokchaichamnankit, D.; Subhasitanont, P.; Svasti, J. Mitochondrial and endoplasmic reticulum stress pathways cooperate in zearalenone-induced apoptosis of human leukemic cells. J. Hematol. Oncol. 2010, 3, 50. [Google Scholar] [CrossRef] [PubMed]
- Salem, I.B.; Boussabbeh, M.; Neffati, F.; Najjar, M.F.; Abid-Essefi, S.; Bacha, H. Zearalenone-induced changes in biochemical parameters, oxidative stress and apoptosis in cardiac tissue: Protective role of crocin. Hum. Exp. Toxicol. 2016, 35, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Boeira, S.P.; Funck, V.R.; Borges Filho, C.; Del’Fabbro, L.; de Gomes, M.G.; Donato, F. Lycopene protects against acute zearalenoneinduced oxidative, endocrine, inflammatory and reproductive damages in male mice. Chem. Biol. Interact. 2015, 230, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Ben Salah-Abbès, J.; Abbès, S.; Ouanes, Z.; Abdel-Wahhab, M.A.; Bacha, H.; Oueslati, R. Isothiocyanate from the Tunisian radish (Raphanus sativus) prevents genotoxicity of Zearalenone in vivo and in vitro. Mutat. Res. 2009, 677, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Abid-Essefi, S.; Zaied, C.; Bouaziz, C.; Salem, I.B.; Kaderi, R.; Bacha, H. Protective effect of aqueous extract of allium sativum against zearalenone toxicity mediated by oxidative stress. Exp. Toxicol. Pathol. 2012, 64, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Sun, Y.; Ju, D.; Chang, S.; Shi, B.; Shan, A. The detoxification effect of vitamin C on zearalenone toxicity in piglets. Ecotoxicol. Environ. Saf. 2018, 158, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Su, Y.; Chang, S.; Sun, Y.; Meng, X.; Shan, A. Vitamin C protects piglet liver against zearalenone-induced oxidative stress by modulating expression of nuclear receptors PXR and CAR and their target genes. Food Funct. 2017, 8, 3675–3687. [Google Scholar] [CrossRef] [PubMed]
- Ben Salem, I.; Prola, A.; Boussabbeh, M.; Guilbert, A.; Bacha, H.; Lemaire, C.; Abid-Essefi, S. Activation of ER stress and apoptosis by α- and β-zearalenol in HCT116 cells, protective role of Quercetin. Neurotoxicology 2016, 53, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, M.; Niu, Q.; Xu, S.; Ding, Y.; Yan, Y. Efficacy of procyanidins against in vivo cellular oxidative damage: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0139455. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lin, X.; Mi, Y.; Li, J.; Zhang, C. Grape seed proanthocyanidin extract prevents ovarian aging by inhibiting oxidative stress in the hens. Oxid. Med. Cell. Longev. 2018, 9, 9390810. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Dai, X.; Jiang, Y.; Zhang, Z.; Bao, L.; Li, Y.; Zhang, F.; Ma, X.; Cai, X.; Jing, L.; et al. Grape seed proanthocyanidin extracts alleviate oxidative stress and ER stress in skeletal muscle of low-dose streptozotocin- and high-carbohydrate/high-fat diet-induced diabetic rats. Mol. Nutr. Food Res. 2013, 57, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhu, Y.; Liu, Z.; Gao, Z.; Li, B.; Zhang, D.; Zhang, Z.; Jiang, X.; Liu, Z.; Meng, L.; et al. Grape seed proanthocyanidin extract ameliorates diabetic bladder dysfunction via the activation of the Nrf2 pathway. PLoS ONE 2015, 10, e0126457. [Google Scholar] [CrossRef] [PubMed]
- Pallarès, V.; Fernández-Iglesias, A.; Cedó, L.; Castell-Auví, A.; Pinent, M.; Ardévol, A.; Salvadó, M.J.; Garcia-Vallvé, S.; Blay, M. Grape seed procyanidin extract reduces the endotoxic effects induced by lipopolysaccharide in rats. Free Radic. Biol. Med. 2013, 60, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Liu, F.; Liu, H.; Zhang, Q.; Li, L.; Hou, X.; Zhao, J.; Li, S.; Chang, X.; Sun, Y. Grape seed procyanidins extract attenuates Cisplatin-induced oxidative stress and testosterone synthase inhibition in rat testes. Syst. Biol. Reprod. Med. 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Sönmez, M.F.; Tascioglu, S. Protective effects of grape seed extract on cadmium-induced testicular damage, apoptosis, and endothelial nitric oxide synthases expression in rats. Toxicol. Ind. Health 2016, 32, 1486–1494. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.M.; Gao, L.P.; Zhang, H.L.; Guo, J.X.; Guo, P.P. Grape seed proanthocyanidin extract prevents DDP-induced testicular toxicity in rats. Food Funct. 2014, 5, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Ulusoy, S.; Ozkan, G.; Ersoz, S.; Orem, A.; Alkanat, M.; Yucesan, F.B.; Kaynar, K.; Al, S. The effect of grape seed proanthocyanidin extract in preventing amikacin-induced nephropathy. Ren. Fail. 2012, 34, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.Z.; Ding, L.; Su, Y.L.; Guo, K.; Wang, L.; Kan, H.W.; Ou, Y.R.; Gao, S. Grape seed proanthocyanidins prevent DOCA-salt hypertension-induced renal injury and its mechanisms in rats. Food Funct. 2015, 6, 2179–2186. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Liu, Y.; Cao, Y.; Wang, N.; Dang, M.; He, J. Proanthocyanidins attenuation of chronic lead-induced liver oxidative damage in kunming Mice via the Nrf2/ARE Pathway. Nutrients 2016, 8, 656. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Shi, Z.; Bi, W.; Liu, R.; Zhang, C.; Wang, K.; Dang, X. Beneficial effect of grape seed proanthocyanidin extract in rabbits with steroid-induced osteonecrosis via protecting against oxidative stress and apoptosis. J. Orthop. Sci. 2015, 20, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Yang, S.; Zhang, Y.; Li, P.; Han, J.; Dong, S.; Chen, X.; He, J. Proanthocyanidin protects against acute zearalenone-induced testicular oxidative damage in male mice. Environ. Sci. Pollut. Res. Int. 2017, 24, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Yang, S.H.; Shi, W.; Li, P.; Guo, Y.; Guo, J.; He, J.B.; Zhang, Y. Protective effect of proanthocyanidin on mice Sertoli cell apoptosis induced by zearalenone via the Nrf2/ARE signalling pathway. Environ. Sci. Pollut. Res. Int. 2017, 24, 26724–26733. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.H.; Hsu, C.W.; Chang, W.T.; Waypa, G.B.; Li, J.; Li, D. Cytotoxicity induced by grape seed proanthocyanidins: Role of nitric oxide. Cell Biol. Toxicol. 2006, 22, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Avelar, M.M.; Gouvêa, C.M. Procyanidin b2 cytotoxicity to MCF-7 human breast adenocarcinoma cells. Indian J. Pharm. Sci. 2012, 74, 351–355. [Google Scholar] [PubMed]
- Hirata, Y.; Yamada, C.; Ito, Y.; Yamamoto, S.; Nagase, H.; Oh-Hashi, K.; Kiuchi, K.; Suzuki, H.; Sawada, M.; Furuta, K. Novel oxindole derivatives prevent oxidative stress-induced cell death in mouse hippocampal HT22 cells. Neuropharmacology 2018, 135, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.L.; Sun, X.F.; Feng, Y.Z.; Li, B.; Li, Y.P.; Yang, F. Zearalenone exposure impairs ovarian primordial follicle formation via downregulation of Lhx8 expression in vitro. Toxicol. Appl. Pharmacol. 2017, 317, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Xu, M.; Dai, Y.; Ding, X.; Xiao, C.; Ji, H. Exploration of Bcl-2 family and caspases-dependent apoptotic signaling pathway in zearalenone-treated mouse endometrial stromal cells. Biochem. Biophys. Res. Commun. 2016, 476, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.L.; Wang, B.J.; Wang, L.; Shan, Y.P.; Zou, H.; Song, R.L.; Wang, T.; Gu, J.H.; Yuan, Y.; Liu, X.Z.; et al. ROS-Mediated Cell Cycle Arrest and Apoptosis Induced by Zearalenone in Mouse Sertoli Cells via ER Stress and the ATP/AMPK Pathway. Toxins 2018, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.L.; Hu, J.; Guo, B.P.; Niu, Y.R.; Xiao, C.; Xu, Y.X. Exploration of intrinsic and extrinsic apoptotic pathways in zearalenone-treated rat Sertoli cells. Environ. Toxicol. 2016, 31, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Shen, T.; Ding, Q.; Lv, Y.; Li, L.; Huang, K.; Yan, L.; Song, S. Zearalenone induces ROS-mediated mitochondrial damage in porcine IPEC-J2 cells. J. Biochem. Mol. Toxicol. 2017, 31. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Cao, R.; Wang, X.; Zhang, Y.; Wang, P.; Gao, H.; Li, C.; Yang, F.; Zeng, R.; Wei, P.; et al. Mitochondrial PKM2 regulates oxidative stress-induced apoptosis by stabilizing Bcl2. Cell Res. 2017, 27, 329–351. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, M.; Halabian, R.; Gharehbaghian, A.; Amirizadeh, N.; Jahanian-Najafabadi, A.; Roushandeh, A.M.; Roudkenar, M.H. Nrf-2 overexpression in mesenchymal stem cells reduces oxidative stress-induced apoptosis and cytotoxicity. Cell Stress Chaperones 2012, 17, 553–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, P.; Chen, F.; Sun, J.; Zhou, J.; Wang, X.; Wang, N.; Li, X.; Zhang, Z.; Wang, A.; Jin, Y. Mycotoxin zearalenone induces apoptosis in mouse Leydig cells via an endoplasmic reticulum stress- dependent signalling pathway. Reprod. Toxicol. 2015, 52, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Mitomo, S.; Omatsu, T.; Tsuchiaka, S.; Nagai, M.; Furuya, T.; Mizutani, T. Activation of c-Jun N-terminal kinase by Akabane virus is required for apoptosis. Res. Vet. Sci. 2016, 107, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.K.; Yu, P.L.; Bai, Y.P.; Yan, S.T.; Zhao, S.P.; Zhang, G.Q. Role of PERK/eIF2α/CHOP Endoplasmic Reticulum Stress Pathway in Oxidized Low-density Lipoprotein Mediated Induction of Endothelial Apoptosis. Biomed. Environ. Sci. 2016, 29, 868–876. [Google Scholar] [PubMed]
- Yang, W.; Tiffany-Castiglioni, E.; Koh, H.C.; Son, I.H. Paraquat activates the IRE1/ASK1/JNK cascade associated with apoptosis in human neuroblastoma SH-SY5Y cells. Toxicol. Lett. 2009, 191, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, A.P.; Thandavarayan, R.A.; Palaniyandi, S.S.; Sari, F.R.; Meilei, H.; Giridharan, V.V.; Soetikno, V.; Suzuki, K.; Kodama, M.; Watanabe, K. Modulation of AT-1R/CHOP-JNK-Caspase12 pathway by olmesartan treatment attenuates ER stress-induced renal apoptosis in streptozotocin-induced diabetic mice. Eur. J. Pharm. Sci. 2011, 44, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Sano, A. Safety assessment of 4-week oral intake of proanthocyanidin-rich grape seed extract in healthy subjects. Food Chem. Toxicol. 2017, 108, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Yamakoshi, J.; Saito, M.; Kataoka, S.; Kikuchi, M. Safety evaluation of proanthocyanidin-rich extract from grape seeds. Food Chem. Toxicol. 2002, 40, 599–607. [Google Scholar] [CrossRef]
- Chen, Q.Q.; Yuan, A.H.; Yang, J. Effect of acupuncture on the endoplasmic reticulum stress IRE1-CHOP pathway and the expression levels of Bax and Bcl-2 protein as well as genes in pancreatic tissue of rats with diabetes mellitus. World J. Acupunct. Moxibustion 2017, 27, 41–46. [Google Scholar] [CrossRef]
- Lam, C.F.; Yeung, H.T.; Lam, Y.M. Reactive oxygen species activate differentiation gene transcription of acute myeloid leukemia cells via the JNK/c-JUN signaling pathway. Leuk. Res. 2018, 68, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Z.; Bao, J. Jia-Jian-Di-Huang-Yin-Zi decoction reduces apoptosis induced by both mitochondrial and endoplasmic reticulum caspase12 pathways in the mouse model of Parkinson’s disease. J. Ethnopharmacol. 2017, 203, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Li, Q.; Zhang, Z.; Lin, P.; Lei, L.; Wang, A.; Jin, Y. Endoplasmic Reticulum Stress Cooperates in Zearalenone-Induced Cell Death of RAW 264.7 Macrophages. Int. J. Mol. Sci. 2015, 16, 19780–19795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Not available. |












| Gene | Accession No. | Primer Sequence (5′–3′) | Product Length |
|---|---|---|---|
| β-actin | BC138614.1 | Forward: CTGTCCCTGTATGCCTCTG | 221 bp |
| Reverse: TTGATGTCACGCACGATT | |||
| Caspase-12 | NM_009808.4 | Forward: CTCAATAGTGGGCATCTGGGT | 151 bp |
| Reverse: GAAGGTAGGCAAGACTGGTTC | |||
| CHOP | NM_001290183.1 | Forward: TTCTCCTTCATGCGTTGCTTC | 218 bp |
| Reverse: AAAACCTTCACTACTCTTGACCCTG | |||
| JNK | NM_001310452.1 | Forward: TCCTCCAAATCCATTACCTCC | 149 bp |
| Reverse: CTCCAGCACCCATACATCAAC | |||
| GRP78 | NM_001163434.1 | Forward: CGCTGGGCATCATTGAAGTAA | 145 bp |
| Reverse: GAGGTGGGCAAACCAAGACAT |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, M.; Chen, X.; Wang, N.; Wang, M.; Pan, J.; Tong, J.; Li, P.; Yang, S.; He, J. Proanthocyanidins Protect Epithelial Cells from Zearalenone-Induced Apoptosis via Inhibition of Endoplasmic Reticulum Stress-Induced Apoptosis Pathways in Mouse Small Intestines. Molecules 2018, 23, 1508. https://doi.org/10.3390/molecules23071508
Long M, Chen X, Wang N, Wang M, Pan J, Tong J, Li P, Yang S, He J. Proanthocyanidins Protect Epithelial Cells from Zearalenone-Induced Apoptosis via Inhibition of Endoplasmic Reticulum Stress-Induced Apoptosis Pathways in Mouse Small Intestines. Molecules. 2018; 23(7):1508. https://doi.org/10.3390/molecules23071508
Chicago/Turabian StyleLong, Miao, Xinliang Chen, Nan Wang, Mingyang Wang, Jiawen Pan, Jingjing Tong, Peng Li, Shuhua Yang, and Jianbin He. 2018. "Proanthocyanidins Protect Epithelial Cells from Zearalenone-Induced Apoptosis via Inhibition of Endoplasmic Reticulum Stress-Induced Apoptosis Pathways in Mouse Small Intestines" Molecules 23, no. 7: 1508. https://doi.org/10.3390/molecules23071508
APA StyleLong, M., Chen, X., Wang, N., Wang, M., Pan, J., Tong, J., Li, P., Yang, S., & He, J. (2018). Proanthocyanidins Protect Epithelial Cells from Zearalenone-Induced Apoptosis via Inhibition of Endoplasmic Reticulum Stress-Induced Apoptosis Pathways in Mouse Small Intestines. Molecules, 23(7), 1508. https://doi.org/10.3390/molecules23071508





