Phytochemicals: Target-Based Therapeutic Strategies for Diabetic Retinopathy
Abstract
:1. Introduction
2. Results
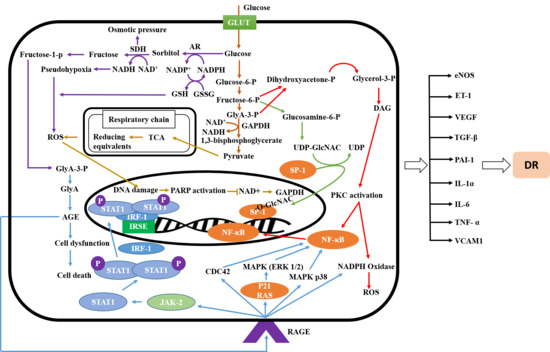
2.1. Signaling Pathway of Diabetic Retinopathy
2.2. Anti-diabetic Effects of Phenolic and Flavonoid Phytochemicals
2.2.1. Abeliophyllum distichum
2.2.2. Aegle marmelos
2.2.3. Agrimonia pilosa Ledeb
2.2.4. Aster koraiensis
2.2.5. Camellia nitidissima Chi
2.2.6. Carpobrotus edulis
2.2.7. Cochlospermum religiosum
2.2.8. Dendrobium chrysotoxum L.
2.2.9. Ginkgo biloba
2.2.10. Glycyrrhiza uralensi
2.2.11. Juglans regia L.
2.2.12. Litchi chinensis
2.2.13. Ligustrum lucidum Ait
2.2.14. Lonicerae japonicae Flos
2.2.15. Melissa officinalis
2.2.16. Moringa oleifera Lam
2.2.17. Morus alba
2.2.18. Osteomeles schwerinae C.K. Schneid
2.2.19. Perilla frutescens
2.2.20. Platycodon grandiflorum
2.2.21. Polygonatum odoratum
2.2.22. Polygonum cuspidatum
2.2.23. Polygonum multiflorum
2.2.24. Prunella vulgaris
2.2.25. Pueraria lobata
2.2.26. Salvia miltiorrhiza Bge
2.2.27. Stauntonia hexaphylla
2.2.28. Tephrosia purpurea
2.2.29. Terminalia catappa
2.2.30. Vitex negundo
2.2.31. Zea mays L.
2.3. Anti-diabetic Effects of Terpenoid and Steroid Phytochemicals
2.3.1. Alpinia zerumbet
2.3.2. Andrographis paniculata Nees
2.3.3. Astragalus membranaceous
2.3.4. Origanum majorana L.
2.3.5. Panax quinquefolius
2.3.6. Zingiber zerumbet
2.4. Anti-diabetic Effects of Alkaloid Phytochemicals
Cnidium officinale
2.5. Anti-diabetic Effect of Other Phytochemicals
2.5.1. Lycium barbarum
2.5.2. Paenonia lactiflora
2.5.3. Scutellaria barbata
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Roglic, G. WHO Global report on diabetes: A summary. Int. J. Noncommun. Dis. 2016, 1, 3–8. [Google Scholar] [CrossRef]
- Ting, D.S.W.; Cheung, G.C.M.; Wong, T.Y. Diabetic retinopathy: Global prevalence, major risk factors, screening practices and public health challenges: A review. Clin. Exp. Ophthalmol. 2016, 44, 260–277. [Google Scholar] [CrossRef] [PubMed]
- Feenstra, D.J.; Yego, E.C.; Mohr, S. Modes of retinal cell death in diabetic retinopathy. J. Clin. Exp. Ophthalmol. 2013, 4, 298. [Google Scholar] [PubMed]
- Calderon, G.D.; Juarez, O.H.; Hernandez, G.E.; Punzo, S.M.; De la Cruz, Z.D. Oxidative stress and diabetic retinopathy: Development and treatment. Eye 2017, 31, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Safi, S.Z.; Qvist, R.; Kumar, S.; Batumalaie, K.; Ismail, I.S.B. Molecular mechanisms of diabetic retinopathy, general preventive strategies, and novel therapeutic targets. BioMed res. Int. 2014, 2014, 801269. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Miao, X.; Li, F.; Wang, S.; Liu, Q.; Wang, Y.; Sun, J. Oxidative Stress-Related Mechanisms and Antioxidant Therapy in Diabetic Retinopathy. Oxidative Med. Cell. Longev. 2017, 2017, 9702820. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Koch, E. Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Curr. Drug Targets 2011, 12, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Dal, S.; Sigrist, S. The protective effect of antioxidants consumption on diabetes and vascular complications. Diseases 2016, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Parveen, A.; Jin, M.; Kim, S.Y. Bioactive phytochemicals that regulate the cellular processes involved in Diabetic nephropathy. Phytomedicine 2018, 39, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Jeetah, R.; Bhaw-Luximon, A.; Jhurry, D. Nanopharmaceutics: Phytochemical-based controlled or sustained drug-delivery systems for cancer treatment. J. Biomed. Nanotechnol. 2014, 10, 1810–1840. [Google Scholar] [CrossRef] [PubMed]
- Dewan, N.; Dasgupta, D.; Pandit, S.; Ahmed, P. Review on-Herbosomes, A new arena for drug delivery. J. Pharmacogn. Phytochem. 2016, 5, 104. [Google Scholar]
- Mishra, B.; Swaroop, A.; Kandpal, R.P. Genetic components in diabetic retinopathy. Indian J. Ophthalmol. 2016, 64, 55–61. [Google Scholar] [PubMed]
- Chuang, P.Y.; He, J.C. JAK/STAT signaling in renal diseases. Kidney Int. 2010, 78, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Marrero, M.B.; Banes-Berceli, A.K.; Stern, D.M.; Eaton, D.C. Role of the JAK/STAT signaling pathway in diabetic nephropathy. Am. J. Physiol.-Renal Physiol. 2006, 290, F762–F768. [Google Scholar] [CrossRef] [PubMed]
- Zong, H.; Ward, M.; Stitt, A.W. AGEs, RAGE, and diabetic retinopathy. Curr. Diabetes Rep. 2011, 11, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Geraldes, P.; King, G.L. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ. Res. 2010, 106, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Koya, D.; Haneda, M.; Nakagawa, H.; Isshiki, K.; Sato, H.; Maeda, S.; Sugimoto, T.; Yasuda, H.; Kashiwagi, A.; Ways, D.K. Amelioration of accelerated diabetic mesangial expansion by treatment with a PKC β inhibitor in diabetic db/db mice, a rodent model for type 2 diabetes. FASEB J. 2000, 14, 439–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldberg, H.J.; Whiteside, C.I.; Fantus, I.G. The hexosamine pathway regulates the plasminogen activator inhibitor-1 gene promoter and Sp1 transcriptional activation through protein kinase C-βI and-δ. J. Biol. Chem. 2002, 277, 33833–33841. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, M. The polyol pathway as a mechanism for diabetic retinopathy: Attractive, elusive, and resilient. J. Diabetes Res. 2007, 2007, 61038. [Google Scholar] [CrossRef] [PubMed]
- Mathebula, S.D. Polyol pathway: A possible mechanism of diabetes complications in the eye. Afr. Vis. Eye Health 2015, 74. [Google Scholar] [CrossRef] [Green Version]
- Luo, D.-W.; Zheng, Z.; Wang, H.; Fan, Y.; Chen, F.; Sun, Y.; Wang, W.-J.; Sun, T.; Xu, X. UPP mediated Diabetic Retinopathy via ROS/PARP and NF-κB inflammatory factor pathways. Curr. Mol. Med. 2015, 15, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Kowluru, R.A. Role of PARP-1 as a novel transcriptional regulator of MMP-9 in diabetic retinopathy. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- Li, H.M.; Kim, J.K.; Jang, J.M.; Cui, C.B.; Lim, S.S. Analysis of the inhibitory activity of Abeliophyllum distichum leaf constituents against aldose reductase by using high-speed counter current chromatography. Arch. Pharm. Res. 2013, 36, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Sankeshi, V.; Kumar, P.A.; Naik, R.R.; Sridhar, G.; Kumar, M.P.; Gopal, V.V.; Raju, T.N. Inhibition of aldose reductase by Aegle marmelos and its protective role in diabetic cataract. J. Ethnopharmacol. 2013, 149, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Hwang, S.H.; Suh, H.W.; Lim, S.S. Phytochemical Analysis of Agrimonia pilosa Ledeb, Its Antioxidant Activity and Aldose Reductase Inhibitory Potential. Int. J. Mol. Sci. 2017, 18, 379. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Zhu, L.; Tan, J.; Zhou, X.; Xiao, L.; Liu, X.; Wang, B. Promoting effect of triterpenoid compound from Agrimonia pilosa Ledeb on preadipocytes differentiation via up-regulation of PPARγ expression. Pharmacogn. Mag. 2015, 11, 219–225. [Google Scholar] [PubMed]
- Kim, J.; Jo, K.; Lee, I.-S.; Kim, C.-S.; Kim, J.S. The extract of aster koraiensis prevents retinal pericyte apoptosis in diabetic rats and its active compound, chlorogenic acid inhibits AGE formation and AGE/RAGE interaction. Nutrients 2016, 8, 585. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, J.; Kim, K.M.; Jung, D.H.; Choi, S.; Kim, C.-S.; Kim, J.S. Myricetin inhibits advanced glycation end product (AGE)-induced migration of retinal pericytes through phosphorylation of ERK1/2, FAK-1, and paxillin in vitro and in vivo. Biochem. Pharmacol. 2015, 93, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Hafsa, J.; Hammi, K.M.; Khedher, M.R.B.; Smach, M.A.; Charfeddine, B.; Limem, K.; Majdoub, H. Inhibition of protein glycation, antioxidant and antiproliferative activities of Carpobrotus edulis extracts. Biomed. Pharmacother. 2016, 84, 1496–1503. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Akileshwari, C.; Reddy, V.S.; Reddy, G.B. Attenuation of diabetic retinopathy in rats by ellagic acid through inhibition of AGE formation. J. Food Sci. Technol. 2017, 54, 2411–2421. [Google Scholar] [CrossRef] [PubMed]
- Stefek, M. Natural flavonoids as potential multifunctional agents in prevention of diabetic cataract. Interdiscip. Toxicol. 2011, 4, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Devi, V.G.; Rooban, B.N.; Sasikala, V.; Sahasranamam, V.; Abraham, A. Isorhamnetin-3-glucoside alleviates oxidative stress and opacification in selenite cataract in vitro. Toxicol In Vitro 2010, 24, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Kim, J.; Kim, C.-S.; Jo, K.; Yoo, N.H.; Sohn, E.; Kim, J.S. Anti-glycation and anti-angiogenic activities of 5′-methoxybiphenyl-3,4,3′-triol, a novel phytochemical component of Osteomeles schwerinae. Eur. J. Pharmacol. 2015, 760, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.-Y.; Yu, Z.-Y.; Lu, B.; Yang, L.; Sheng, Y.-C.; Fan, Y.-M.; Ji, L.-L.; Wang, Z.-T. Ethanol extract of Dendrobium chrysotoxum Lindl ameliorates diabetic retinopathy and its mechanism. Vasc. Pharmacol. 2014, 62, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Gong, C.; Lu, B.; Yang, L.; Sheng, Y.; Ji, L.; Wang, Z. Dendrobium chrysotoxum Lindl. alleviates diabetic retinopathy by preventing retinal inflammation and tight junction protein decrease. J. Diabetes Res. 2015, 2015, 518317. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.O.; Chen, D.; Fang, H.; Wu, J.; Wei, X.; Gao, X.; Li, X.; Long, Y.; Yi, Y. Investigation of synergistic mechanism and identification of interaction site of aldose reductase with the combination of gigantol and syringic acid for prevention of diabetic cataract. BMC Complement. Altern. Med. 2016, 16, 286. [Google Scholar]
- Bucolo, C.; Marrazzo, G.; Platania, C.B.M.; Drago, F.; Leggio, G.M.; Salomone, S. Fortified extract of red berry, Ginkgo biloba, and white willow bark in experimental early diabetic retinopathy. J. Diabetes Res. 2013, 2013, 432695. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-Y.; Jeng, C.; Kao, S.-C.; Yu, J.J.-H.; Liu, D.-Z. Improved haemorrheological properties by Ginkgo biloba extract (Egb 761) in type 2 diabetes mellitus complicated with retinopathy. Clin. Nutr. 2004, 23, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Spadiene, A.; Savickiene, N.; Jurgeviciene, N.; Zalinkevicius, R.; Norkus, A.; Ostrauskas, R.; Skesters, A.; Silova, A.; Rodovicius, H.; Francaite-Daugeliene, M. Effect of ginkgo extract on eye microcirculation in patients with diabetes. Cent. Eur. J. Med. 2013, 8, 736–741. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.-Y.; Yi, Q.; Ma, J.-L.; Wei, Q.-P. Clinical evaluation of Ginkgo biloba extract for diabetic retinopathy. Guoji Yanke Zazhi 2016, 16, 361–364. [Google Scholar]
- Lee, Y.S.; Kim, S.H.; Jung, S.H.; Kim, J.K.; Pan, C.-H.; Lim, S.S. Aldose reductase inhibitory compounds from Glycyrrhiza uralensis. Biol. Pharm. Bull. 2010, 33, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Nasiry, D.; Khalatbary, A.R.; Ahmadvand, H. Therapeutic potential of Juglans regia L. leaf extract against diabetic retinopathy in rat. Iran. J. Basic Med. Sci. 2017, 20, 1275–1281. [Google Scholar] [PubMed]
- Kilari, E.K.; Putta, S. Delayed progression of diabetic cataractogenesis and retinopathy by Litchi chinensis in STZ-induced diabetic rats. Cutan. Ocul. Toxicol. 2017, 36, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ke, X.; Fu, W.; Gao, X.; Zhang, H.; Wang, W.; Ma, N.; Zhao, M.; Hao, X.; Zhang, Z. Inhibition of Hypoxia-Induced Retinal Angiogenesis by Specnuezhenide, an Effective Constituent of Ligustrum lucidum Ait., through Suppression of the HIF-1α/VEGF Signaling Pathway. Molecules 2016, 21, 1756. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, T.; Lu, B.; Yu, Z.; Mei, X.; Abulizi, P.; Ji, L. Lonicerae Japonicae Flos attenuates diabetic retinopathy by inhibiting retinal angiogenesis. J. Ethnopharmacol. 2016, 189, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.Y.; Sohn, J.; Park, K.H. Chlorogenic acid decreases retinal vascular hyperpermeability in diabetic rat model. J. Korean Med. Sci. 2013, 28, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Zhou, L.; Zhang, T.; Lu, B.; Sheng, Y.; Ji, L. Chlorogenic acid attenuates diabetic retinopathy by reducing VEGF expression and inhibiting VEGF-mediated retinal neoangiogenesis. Vasc. Pharmacol. 2017, 101, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Khodsooz, S.; Moshtaghian, J.; Eivani, M. Antihyperglycemic and antihyperlipidemic effects of hydroalcoholic extract of Melissa officinalis (Lemon Balm) in alloxan-induced diabetic rats. Physiol. Pharmacol. 2016, 20, 24–30. [Google Scholar]
- Miroliaei, M.; Khazaei, S.; Moshkelgosha, S.; Shirvani, M. Inhibitory effects of Lemon balm (Melissa officinalis, L.) extract on the formation of advanced glycation end products. Food Chem. 2011, 129, 267–271. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, B.J.; Kim, J.H.; Yu, Y.S.; Kim, M.Y.; Kim, K.-W. Rosmarinic acid suppresses retinal neovascularization via cell cycle arrest with increase of p21 WAF1 expression. Eur. J. Pharmacol. 2009, 615, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Kumar Gupta, S.; Kumar, B.; Srinivasan, B.; Nag, T.C.; Srivastava, S.; Saxena, R.; Aggarwal, A. Retinoprotective effects of Moringa oleifera via antioxidant, anti-inflammatory, and anti-angiogenic mechanisms in streptozotocin-induced diabetic rats. J. Ocul. Pharmacol. Ther. 2013, 29, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Ke, M.; Hu, X.-Q.; Ouyang, J.; Dai, B.; Xu, Y. The effect of astragalin on the VEGF production of cultured Müller cells under high glucose conditions. Bio-med. Mater. Eng. 2012, 22, 113–119. [Google Scholar]
- Mahmoud, A.M.; El-Twab, S.M.A.; Abdel-Reheim, E.S. Consumption of polyphenol-rich Morus alba leaves extract attenuates early diabetic retinopathy: The underlying mechanism. Eur. J. Nutr. 2017, 56, 1671–1684. [Google Scholar] [CrossRef] [PubMed]
- Paek, J.H.; Shin, K.H.; Kang, Y.H.; Lee, J.Y.; Lim, S.S. Rapid identification of aldose reductase inhibitory compounds from Perilla frutescens. Biomed. Res. Int. 2013, 2013, 679463. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.S.; Lee, Y.M.; Jeong, I.H.; Kim, J.S. Constituents of the flowers of Platycodon grandiflorum with inhibitory activity on advanced glycation end products and rat lens aldose reductase in vitro. Arch. Pharm. Res. 2010, 33, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Ojha, S.; Balaji, V.; Sadek, B.; Rajesh, M. Beneficial effects of phytochemicals in diabetic retinopathy: Experimental and clinical evidence. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2769–2783. [Google Scholar] [PubMed]
- Dong, W.; Shi, H.B.; Ma, H.; Miao, Y.B.; Liu, T.J.; Wang, W. Homoisoflavanones from Polygonatum odoratum rhizomes inhibit advanced glycation end product formation. Arch. Pharm. Res. 2010, 33, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Sohn, E.; Kim, J.; Kim, C.-S.; Lee, Y.M.; Kim, J.S. Extract of Polygonum cuspidatum Attenuates Diabetic Retinopathy by Inhibiting the High-Mobility Group Box-1 (HMGB1) Signaling Pathway in Streptozotocin-Induced Diabetic Rats. Nutrients 2016, 8, 140. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Wu, K.; Qiu, H.; Huang, B.; Chen, R.; Xie, W.; Jiang, Q. Polydatin exhibits the hepatoprotective effects through PPAR-α/-β signaling pathway in Streptozocin-induced diabetic mice. J. Funct. Foods 2017, 36, 341–347. [Google Scholar] [CrossRef]
- Lv, L.; Shao, X.; Wang, L.; Huang, D.; Ho, C.-T.; Sang, S. Stilbene glucoside from Polygonum multiflorum Thunb.: A novel natural inhibitor of advanced glycation end product formation by trapping of methylglyoxal. J. Agric. Food Chem. 2010, 58, 2239–2245. [Google Scholar] [CrossRef] [PubMed]
- Li, H.M.; Kim, J.K.; Jang, J.M.; Kwon, S.O.; Cui, C.B.; Lim, S.S. The inhibitory effect of Prunella vulgaris L. on aldose reductase and protein glycation. BioMed Res. Int. 2012, 2012, 928159. [Google Scholar]
- Behl, T.; Kotwani, A. Chinese herbal drugs for the treatment of diabetic retinopathy. J. Pharm. Pharmacol. 2017, 69, 223–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, Y.; Cui, H.; Yang, M.; Song, H.; Zhang, Q.; Su, Y.; Zheng, J. Protective effect of puerarin on diabetic retinopathy in rats. Mol. Biol. Rep. 2009, 36, 1129. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, K.M.; Kim, C.S.; Sohn, E.; Lee, Y.M.; Jo, K.; Kim, J.S. Puerarin inhibits the retinal pericyte apoptosis induced by advanced glycation end products in vitro and in vivo by inhibiting NADPH oxidase-related oxidative stress. Free Radic. Biol. Med. 2012, 53, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dai, S.-Z.; Nie, X.-D.; Zhu, L.; Xing, F.; Wang, L.-Y. Effect of Salvia miltiorrhiza on retinopathy. Asian Pac. J. Trop. Med. 2013, 6, 145–149. [Google Scholar] [CrossRef]
- Lian, F.; Wu, L.; Tian, J.; Jin, M.; Zhou, S.; Zhao, M.; Wei, L.; Zheng, Y.; Wang, Y.; Zhang, M. The effectiveness and safety of a danshen-containing Chinese herbal medicine for diabetic retinopathy: A randomized, double-blind, placebo-controlled multicenter clinical trial. J. Ethnopharmacol. 2015, 164, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.Y.; Gao, H.Y.; Sun, L.; Huang, J.; Xu, X.M.; Wu, L.J. Constituents with alpha-glucosidase and advanced glycation end-product formation inhibitory activities from Salvia miltiorrhiza Bge. J. Nat. Med. 2011, 65, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Qiang, G.; Yang, X.; Shi, L.; Zhang, H.; Chen, B.; Zhao, Y.; Zu, M.; Zhou, D.; Guo, J.; Yang, H. Antidiabetic effect of salvianolic acid A on diabetic animal models via AMPK activation and mitochondrial regulation. Cell. Physiol. Biochem. 2015, 36, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Kwon, S.H.; Kim, S.B.; Lim, S.S. Inhibitory Activities of Stauntonia hexaphylla Leaf Constituents on Rat Lens Aldose Reductase and Formation of Advanced Glycation End Products and Antioxidant. BioMed Res. Int. 2017, 2017, 4273257. [Google Scholar] [CrossRef] [PubMed]
- Bhadada, S.V.; Vyas, V.K.; Goyal, R.K. Protective effect of Tephrosia purpurea in diabetic cataract through aldose reductase inhibitory activity. Biomed. Pharmacother. 2016, 83, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Kotwani, A. Proposed mechanisms of Terminalia catappa in hyperglycaemia and associated diabetic complications. J. Pharmacy Pharmacol. 2016. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, Y.; Xu, S.; Xu, W.; Chu, K.; Xu, W.; Zhao, H.; Lu, J. Identification and quantification of phenolic compounds in Vitex negundo L. var. cannabifolia (Siebold et Zucc.) Hand.-Mazz. using liquid chromatography combined with quadrupole time-of-flight and triple quadrupole mass spectrometers. J. Pharm. Biomed. Anal. 2015, 108, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Thiraphatthanavong, P.; Wattanathorn, J.; Muchimapura, S.; Wipawee, T.M.; Wannanon, P.; Terdthai, T.U.; Suriharn, B.; Lertrat, K. Preventive effect of Zea mays L. (purple waxy corn) on experimental diabetic cataract. Biomed. Res. Int. 2014, 2014, 507435. [Google Scholar] [CrossRef] [PubMed]
- Chompoo, J.; Upadhyay, A.; Kishimoto, W.; Makise, T.; Tawata, S. Advanced glycation end products inhibitors from Alpinia zerumbet rhizomes. Food Chem. 2011, 129, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Lu, B.; Sheng, Y.; Zhou, L.; Ji, L.; Wang, Z. Andrographolide ameliorates diabetic retinopathy by inhibiting retinal angiogenesis and inflammation. Biochim. Biophys. Acta 2015, 1850, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yuan, S.; Liu, X.; Mao, P.; Zhao, C.; Huang, Q.; Zhang, R.; Fang, Y.; Song, Q.; Yuan, D.; et al. Protective effects of astragaloside IV on db/db mice with diabetic retinopathy. PLoS ONE 2014, 9, e112207. [Google Scholar] [CrossRef] [PubMed]
- Perez Gutierrez, R.M. Inhibition of Advanced Glycation End-Product Formation by Origanum majorana L. In Vitro and in Streptozotocin-Induced Diabetic Rats. Evid. Based Complement. Altern. Med. 2012, 2012, 598638. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-H.; Hsu, C.-C.; Huang, C.-N.; Yin, M.-C. Anti-glycative effects of oleanolic acid and ursolic acid in kidney of diabetic mice. Eur. J. Pharmacol. 2010, 628, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sun, X.; Wang, F.; Liu, S. Inhibitory effects of ursolic acid on diabetic retinopathy in mice. Zhonghua Yi Xue Za Zhi 2015, 95, 2589–2593. [Google Scholar] [PubMed]
- Sen, S.; Chen, S.; Wu, Y.; Feng, B.; Lui, E.K.; Chakrabarti, S. Preventive effects of North American ginseng (Panax quinquefolius) on diabetic retinopathy and cardiomyopathy. Phytother. Res. 2013, 27, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Godoy, D.L.; Betts-Obregon, B.S.; Tsin, A.T. Ginsenoside-Rb1 inhibition of VEGF release–structure and activity relations (sar) perspective. Med. Hypothesis Discov. Innov. Ophthalmol. 2014, 3, 38–39. [Google Scholar] [PubMed]
- Sun, H.-Q.; Zhou, Z.-Y. Effect of ginsenoside-Rg3 on the expression of VEGF and TNF-α in retina with diabetic rats. Int. J. Ophthalmol. 2010, 3, 220–223. [Google Scholar] [PubMed]
- Liu, W.Y.; Tzeng, T.F.; Liu, I.M. Zerumbone, a Bioactive Sesquiterpene, Ameliorates Diabetes-Induced Retinal Microvascular Damage through Inhibition of Phospho-p38 Mitogen-Activated Protein Kinase and Nuclear Factor-kappaB Pathways. Molecules 2016, 21, 1708. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-S.; Sohn, E.; Kim, J.S.; Kim, J.; Jo, K.; Lee, Y.-R.; Lee, Y.M. Cnidium officinale extract and butylidenephthalide inhibits retinal neovascularization in vitro and in vivo. BMC Complement. Altern. Med. 2016, 16, 231. [Google Scholar]
- Song, M.; Salam, N.K.; Roufogalis, B.D.; Huang, T.H.-W. Lycium barbarum (Goji Berry) extracts and its taurine component inhibit PPAR-γ-dependent gene transcription in human retinal pigment epithelial cells: Possible implications for diabetic retinopathy treatment. Biochem. Pharmacol. 2011, 82, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Xu, Z.; Mi, M.; Xu, H.; Zhu, J.; Wei, N.; Chen, K.; Zhang, Q.; Zeng, K.; Wang, J. Dietary taurine supplementation ameliorates diabetic retinopathy via anti-excitotoxicity of glutamate in streptozotocin-induced Sprague-Dawley rats. Neurochem. Res. 2008, 33, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.T.-H.; Yeh, S.-M.; Chen, Y.-C.; Lin, S.-L.; Tseng, J.-K. Investigation of the protective effects of taurine against alloxan-induced diabetic retinal changes via electroretinogram and retinal histology with New Zealand white rabbits. Int. J. Endocrinol. 2014, 2014, 631549. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.H.; Liu, B.Q.; Hao, M.J.; Fan, Y.X.; Qian, C.; Teng, P.; Zhou, X.W.; Hu, L.; Liu, W.T.; Yuan, Z.L.; et al. Paeoniflorin Suppressed High Glucose-Induced Retinal Microglia MMP-9 Expression and Inflammatory Response via Inhibition of TLR4/NF-κB Pathway through Upregulation of SOCS3 in Diabetic Retinopathy. Inflammation 2017, 40, 1475–1486. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, C.-S.; Kim, Y.S.; Lee, I.S.; Kim, J.S. Jakyakgamcho-tang and Its Major Component, Paeonia Lactiflora, Exhibit Potent Anti-glycation Properties. J. Exerc. Nutr. Biochem. 2016, 20, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.-Y.; Zhou, L.-Y.; Zhang, T.-Y.; Lu, B.; Ji, L.-L. Scutellaria barbata attenuates diabetic retinopathy by preventing retinal inflammation and the decreased expression of tight junction protein. Int. J. Ophthalmol. 2017, 10, 870–877. [Google Scholar] [PubMed]
- Pedro, R.-A.; Ramon, S.-A.; Marc, B.-B.; Juan, F.-B.; Isabel, M.-M. Prevalence and relationship between diabetic retinopathy and nephropathy, and its risk factors in the North-East of Spain, a population-based study. Ophthalmic Epidemiol. 2010, 17, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Sobrin, L.; Lee, M.J.; Kang, M.H.; Seong, M.; Cho, H. The relationship between diabetic retinopathy and diabetic nephropathy in a population-based study in Korea (KNHANES V-2, 3). Investig. Ophthalmol. Vis. Sci. 2014, 55, 6547–6553. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.W. Treatment of diabetic retinopathy: Recent advances and unresolved challenges. World J. Diabetes 2016, 7, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Duh, E.J.; Sun, J.K.; Stitt, A.W. Diabetic retinopathy: Current understanding, mechanisms, and treatment strategies. JCI Insight 2017, 2, e93751. [Google Scholar] [CrossRef] [PubMed]
- Osaadon, P.; Fagan, X.; Lifshitz, T.; Levy, J. A review of anti-VEGF agents for proliferative diabetic retinopathy. Eye 2014, 28, 510–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulki, L.; Foster, C. Difluprednate for inflammatory eye disorders. Drugs Today (Barc.) 2011, 47, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-J.; Kim, H.; Brian, H.; Choi, K.H.; Lee, B.-Y. Traditional Knowledge of Wild Edible Plants on Jeju Island, Korea; NISCAIR-CSIR: New Delhi, India, 2013. [Google Scholar]
- Ahmad, I.; Aqil, F.; Owais, M. Modern Phytomedicine: Turning Medicinal Plants into Drugs; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Dogan, Y.; Baslar, S.; Ay, G.; Mert, H.H. The use of wild edible plants in western and central Anatolia (Turkey). Econ. Bot. 2004, 58, 684–690. [Google Scholar] [CrossRef]




| Sr. No | Plant Name | Active Constituent | Traditional Use | Family | Class | Pharmacological Target | Pharmacological Activity | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | Abeliophyllum distichum | Acteoside | Oleaceae | Phenolic | Aldose reductase | Anti-hypertensive Anti-diabetic Anti-inflammatory Anti-cancer | [23] | |
| 2 | Aegle marmelos | Cinnamic acid | Diabetes mellitus Ulcer Beriberi Cholera Ophthalmia | Rutaceae | Phenolic acid | Aldose reductase | Anti-inflammatory Analgesic Anti-pyretic Anti-hyperglycemic Anti-diarrheal Anti-microfilarial | [24] |
| 3 | Agrimonia pilosa ledeb | Agrimoniin | Abdominal pain Heat stroke Headache Sore throat | Rosaceae | Phenolic | Aldose reductase PPAR-γ Glucose transporter type 4 | Anti-cancer Anti-oxidant Anti-allergy Anti-inflammatory Anti-nociception | [26] |
| 4 | Alpinia zerumbet | Labdadiene | Anti-inflammatory Ati-fungal Anti-bacterial | Zingiberaceae | Triterpenoid | AGE | Anti-hypertensive Anti-ulcerogenic Diuretic Sedative | [74] |
| 5 | Andrographis paniculata | Andrographolide | Cooling effect Detoxification Sore throat Respiratory tract infection | Acanthaceae | Diterpenoid | TNF-α IL-1β IL-6 NF-κB PAI-1 EGR1 | Hepatoprotective Anti-oxidant Anti-hyperglycemic Anti-cancer Anti-platelet aggregation | [75] |
| 6 | Aster Koraiensis | Chlorogenic acid 3,5,di-caffeoylquinic acid | Diabetes Pertussis Chronic bronchitis Pneumonia | Asteraceae | Phenolic acid | AGE NF-κB | Anti-diabetic Anti-apoptosis | [27] |
| 7 | Astragalus membranaceous | Astragaloside IV | Stomach ulcer Diabetes Diuretics Tonic Fever | Leguminosae | Triterpenoid | NF-κB VEGF TNF-α IL-1 IL-4 IL-5 IL-10 IL-6 MMPs IL-1β iNOS TGF-β | Immunomodulatory Anti-apoptosis Anti-inflammatory | [76] |
| 8 | Camellia nitidissima | Cancer Diarrhea Sore throat High blood pressure Irregular menstruation | Theaceae | Flavonoids | AGE OS | Anti-oxidant | [33] | |
| 9 | Carpobrotus edulis | Ellagic acid | Throat infection Tuberculosis Diarrhea Dysentery Mouth ulcer Stomach ailments | Aizoaceae | Phenolic acid | AGE OS GAP VEGF Bax HIF-1α | Anti-bacterial Anti-oxidant Anti-proteus Anti-microbial | [30] |
| 10 | Cnidium officinale | Butylidenephthalide | Inflammation High blood pressure Menstrual pain | Umbelliferae | Alkaloid | ERK1/2 AGE/RAGE VEGF DLL4 | Larvicidal Acaricidal Anti-hyperglycemic Anti-angiogenic Anti-inflammatory | [84] |
| 11 | Cochlospermum religiosum | Isorhamnetin | Sedative Jaundice Gonorrhea Syphilis Stomach ailments | Cochlospermaceae | Flavonoid | RAGE-Src-ERK1/2-FAK-1 paxillin signaling pathway | Anti-oxidant Anti-microbial Immunomodulatory | [28] |
| 12 | Dendrobium chrysotoxum | Gigantol Syringic acid | Moisten and nourish skin Longevity Tuberculosis Anorexia Eye sight | Orichidaceae | Phenolic | VEGF MMP2/9 IL-1β, IL-3, IL-6, IL-10, IL-12 IκB ICAM-1 | Anti-angiogenesis Anti-inflammatory Anti-oxidant Anti-hyperglycemic Immunomodulatory | [36] |
| 13 | Ginkgo biloba | Blood disorders | Ginkgoaceae | Flavonoid | TNF-α VEGF | Anti-angiogenesis Anti-oxidant Anti-inflammatory Neuroprotective Hepatoprotective Anti-stress | [40] | |
| 14 | Glycyrrhiza uralensi | Hepatitis C Peptic ulcer Diabetes Skin diseases Pulmonary diseases | Fabaceae | Flavonoid | PPAR-γ | Anti-depressant Anti-oxidant Immunomodulatory Hepatoprotective Anti-viral Anti-inflammatory Hepatoprotective Anti-cancer | [41] | |
| 15 | Juglans regia | Diabetes Inflammation Infection | Flavonoid Phenolic | PARP OS COX-2 Caspase-3 | Anti-oxidant Anti-microbial Sedative Anti-hyperglycemic | [42] | ||
| 16 | Litchi chinenesis | Diabetes Obesity Epigastric pain Herniae-like conditions Neuralgic apin | Sapindaceae | Polyphenol | AGE OS | Anti-inflammatory Ant-ioxidant Hepatoprotective | [43] | |
| 17 | Ligustrum lucidum | Specnuezhenide | Eyesight Dizziness Fever Insomnia Cancer | Oleaceae | Phenolic | HIF-1α VEGF | Anti-angiogenesis Hepatoprotective Anti-diabetic | [44] |
| 18 | Lonicerae japonicae | Chlorogenic acid | Inflammation Headache Acute fever Eye sight Heat stroke | Caprifoliaceae | Phenolic | VEGF | anti-angiogenesis anti-nociceptive anti-inflammatory analgesic anti-bacterial | [47] |
| 19 | Lycium barbarum | Taurine | Blurry vision Abdominal pain Infertility Dry cough Fatigue Dizziness Headache | Solanceae | Amino acid | Bcl-2 Bax Caspase-3 PPAR VEGF | Anti-apoptosis Anti-angiogenesis Immunomodulator Anti-aging Neuroprotective | [87] |
| 20 | Melissa officinalis | Rosmarinic acid | Indigestion Cardiac failure Anemia | Lamiaceae | Phenolic acid | AGE | Anti-angiogenesis Anti-cancer Anti-oxidant Neurotropic Anti-microbial Anti-bacterial | [48] |
| 21 | Moringa oleifera | Culinary use Malnutrition | Mornigaceae | Polyphenolic | TNF-α IL-1β VEGF PKC-β | Hypolipidemic Anti-atherosclerosis Hypocholesterolemic Anti-angiogenesis Anti-inflammatory Anti-oxidant | [51] | |
| 22 | Morus albla | Feedstock for silkworms Constipation Diabetes | Moraceae | Phenolic | PKC OS Caspase-3 VEGF Bax Bcl-2 | Anti-apoptosis Anti-angiogenesis Antioxidant Anti-microbial Hypoglycemic hepatoprotective Anti-inflammatory | [53] | |
| 23 | Origanum majorana L. | Ursolic acid Oleanolic acid | Disinfectant Headache Indigestion Rheumatism Insomnia Diabetes Asthma Cataract Nervousness | Lamiaceae | Triterpenoid | AGE COX-2 MMP-2 OS | Anti-oxidant Anti-hyperglycemic Anti-microbial Anti-proliferative Anti-cholinesterase | [79] |
| 24 | Osteomeles schwerinae | 5′-methoxybiphenyl-3,4,3′-triol | Diarrhea Sore throat Arthritis Dysentery | Rosaceae | Phenolic | Aldose reductase VEGF FGF-2 IGFBPS PAI-1 | Anti-diabetic Anti-angiogenesis Anti-oxidant | [33] |
| 25 | Paenonia lactiflora | Aminoguanidine | Rheumatoid arthritis Systemic lupus erythematous Fever Spasm Muscles cramping Dysmenorrhea | Paeoniaceae | TLR4 MMP-9 NF-κB | Anti-inflammatory Anti-oxidant Anti-thrombosis | [88] | |
| 26 | Panax quinquefolius | Ginsenoside-Rb1 | Aphrodisiac Restorative Nootropic Antiaging Tonic | Araliaceae | Steroid glycoside | VEGF TNF-α | Antioxidant Anti-angiogenesis Antidiabetic Anti-coagulant | [81] |
| 27 | Perilla frutescens | Rosmarinic acid | Cough Bacterial infection Fungal infection Allergy Tumor Intestinal disorder | Lamiaceae | Phenolic acid | p21WAF1 | Anti-angiogenesis Anticancer Anti-inflammatory Anti-allergy Anti-depressant Anti-allergy | [54] |
| 28 | Platycodon grandiflorum | Luteolin | Cough Inflammation Fever | Cmpanulaceae | Flavonoid | Aldose reductase | Anti-oxidant Anti-cancer Hepatoprotective Anti-hyperlipidemia | [56] |
| 29 | Polygonatum odoratum | Diabetes | Liliaceae | Flavonoid | AGE | Antihyperglycemic Antioxidant | [57] | |
| 30 | Polygonum cuspidatum | Polydatin Resveratrol Emodin glucopyranoside Emodin | Allergy Diabetes | Polygonaceae | Phenol | HMGB1 AGE NF-κB IL-4 PPAR-α/-β | Anti-AGE formation Anti-inflammatory Anti-oxidant Anti-bacterial Anti-apoptosis | [57] |
| 31 | Polygonum multiflorum | Anti-aging Tonic | Polygonaceae | Phenolic Flavonoid | AGE | Neuroprotective Anti-oxidant Myocardial protective Anti-inflammatory | [60] | |
| 32 | Prunella vulgaris | Headache Goiter Cancer High blood pressure Lymphatic system disorder | Lamiaceae | Phenolic Flavonoid | Aldose reductase AGE OS | Anti-oxidant Immunostimulatory Anti-HIV Anti-allergy Anti-inflammatory | [61] | |
| 33 | Pueraria lobata | Puerarin | Neuro-protective Hepato-protective Analgesic inflammation Fever | Fabaceae | Flavone | iNOS IL-1β ICAM HIF-1α VEGF Bax Caspase-3 TNF-α ERK p38 MAPK | Anti-apoptosis Anti-angiogenesis Antioxidant Vasodilatory Neuroprotective Hepatoprotective Anti-pyretic Analgesic Anti-inflammatory | [64] |
| 34 | Salvia miltiorrhiza | Coronary heart disease Cerebrovascular | Labiatae | Phenolic | Lp-PLA2 IL-1 IL-6 | Antihyperglycemic Anti-arrhythmic Anti-pulmonary fibrosis Anti-inflammatory | [68] | |
| 35 | Scutellaria barbata | Toxicity Heat relief Blood circulation promoter Pain and swelling | Lamiaceae | TNF-α IL-1β ICAM NF-κB | Anti-cancer Anti-leukemic | [90] | ||
| 36 | Stauntonia hexaphylla | Sedative Analgesic Diuretic | Lardizabalaceae | Flavonoid and phenolic | Aldose reductase | Anti-inflammatory Anti-HIV | [69] | |
| 37 | Tephrosia purpurea | Ulcer Asthma Leprosy Cancer | Fabaceae | Flavonoid Phenolic | Aldose reductase | Anti-ulcer Anticarcinogenic Anti-lipidperoxidative Immunomodulator Anti-cancer | [70] | |
| 38 | Terminalia catappa | Dermatitis Hepatitis Pyresis Diarrhea | Combretaceae | Tannin | LDL HDL | Anti-oxidant Anti-angiogenesis Anti-inflammatory Hepatoprotective Anti-diabetic Anti-bacterial Analgesic | [71] | |
| 39 | Vitex negundo | Luteolin-7-glucoside | Eczema Ringworm Liver disorder Rheumatic pain Gout vermicide | Verbenaceae | Flavonoid | OS | Anti-oxidant Anti-inflammatory Analgesic Anti-microfilarial | [72] |
| 40 | Zea mays | Quercetin Coumaric acid | Diuretic Dysuria vasodilator Menorrhagia Nose bleeds | Poaceae | Flavonoid Phenolic | Aldose reductase OS | Anti-oxidant | [73] |
| 41 | Zingiber zerumbet | Zerumbone | Inflammation Toothache Fever Ingestion Diarrhea | Zingiberaceae | Sesquiterpene | NF-κB AGE/RAGE p38 MAPK | Anti-microbial Anti-nociceptive Anti-hyperglycemic Anti-inflammatory Anti-cancer Anti-allergy | [83] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parveen, A.; Kim, J.H.; Oh, B.G.; Subedi, L.; Khan, Z.; Kim, S.Y. Phytochemicals: Target-Based Therapeutic Strategies for Diabetic Retinopathy. Molecules 2018, 23, 1519. https://doi.org/10.3390/molecules23071519
Parveen A, Kim JH, Oh BG, Subedi L, Khan Z, Kim SY. Phytochemicals: Target-Based Therapeutic Strategies for Diabetic Retinopathy. Molecules. 2018; 23(7):1519. https://doi.org/10.3390/molecules23071519
Chicago/Turabian StyleParveen, Amna, Jin Hyun Kim, Byeong Gyu Oh, Lalita Subedi, Zahra Khan, and Sun Yeou Kim. 2018. "Phytochemicals: Target-Based Therapeutic Strategies for Diabetic Retinopathy" Molecules 23, no. 7: 1519. https://doi.org/10.3390/molecules23071519