Natural Product-Based Pesticide Discovery: Design, Synthesis and Bioactivity Studies of N-Amino-Maleimide Derivatives
Abstract
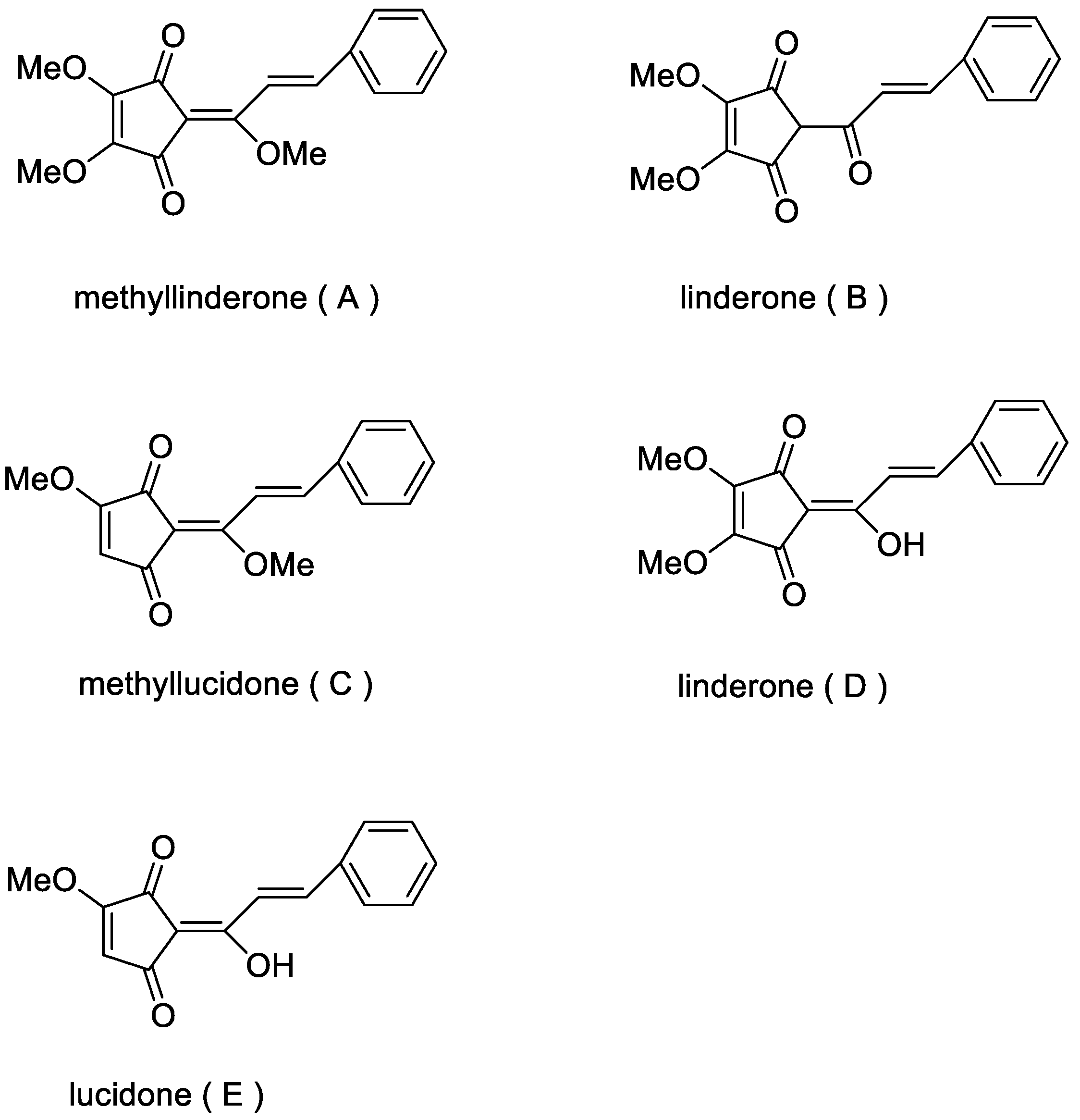
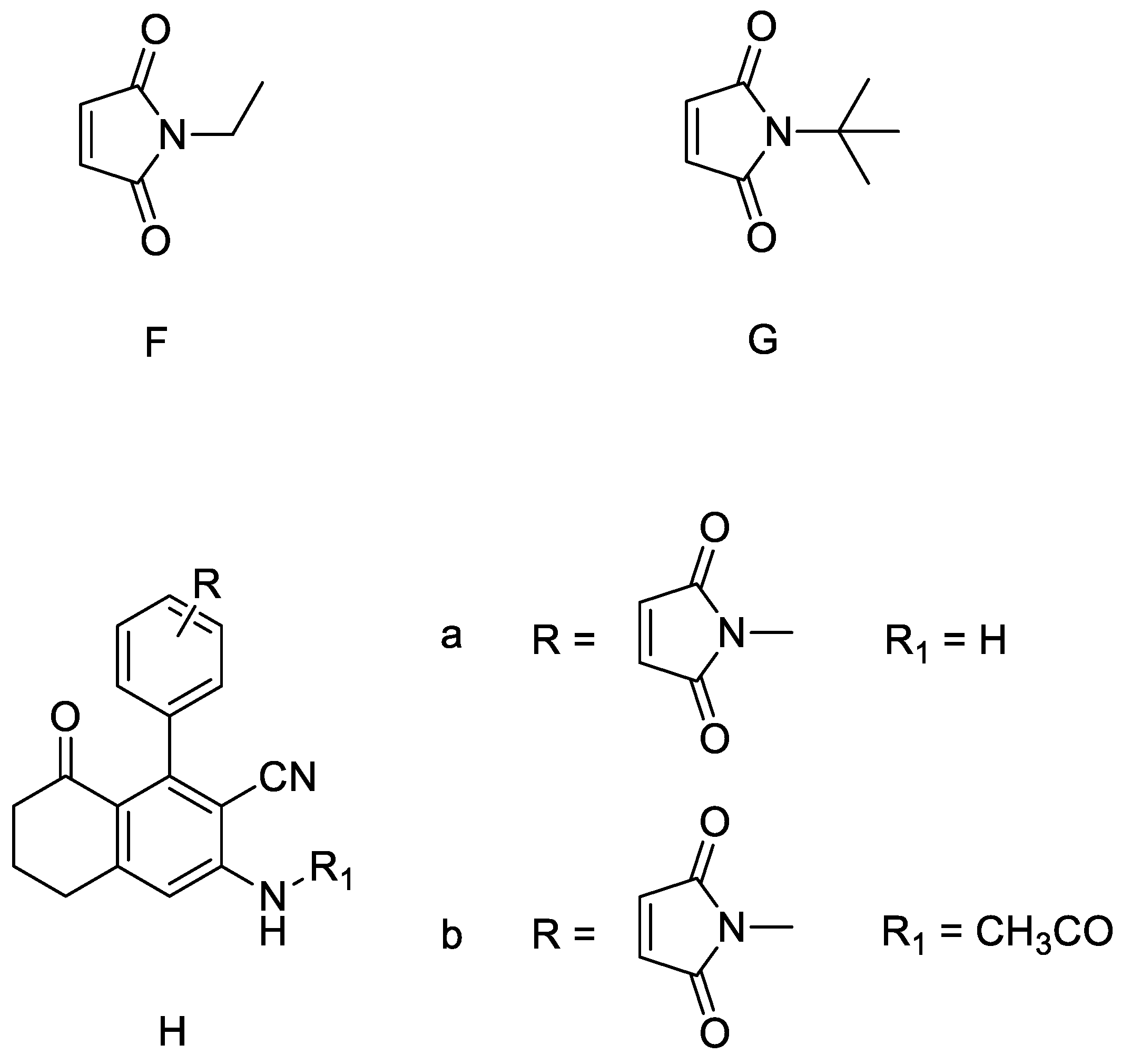
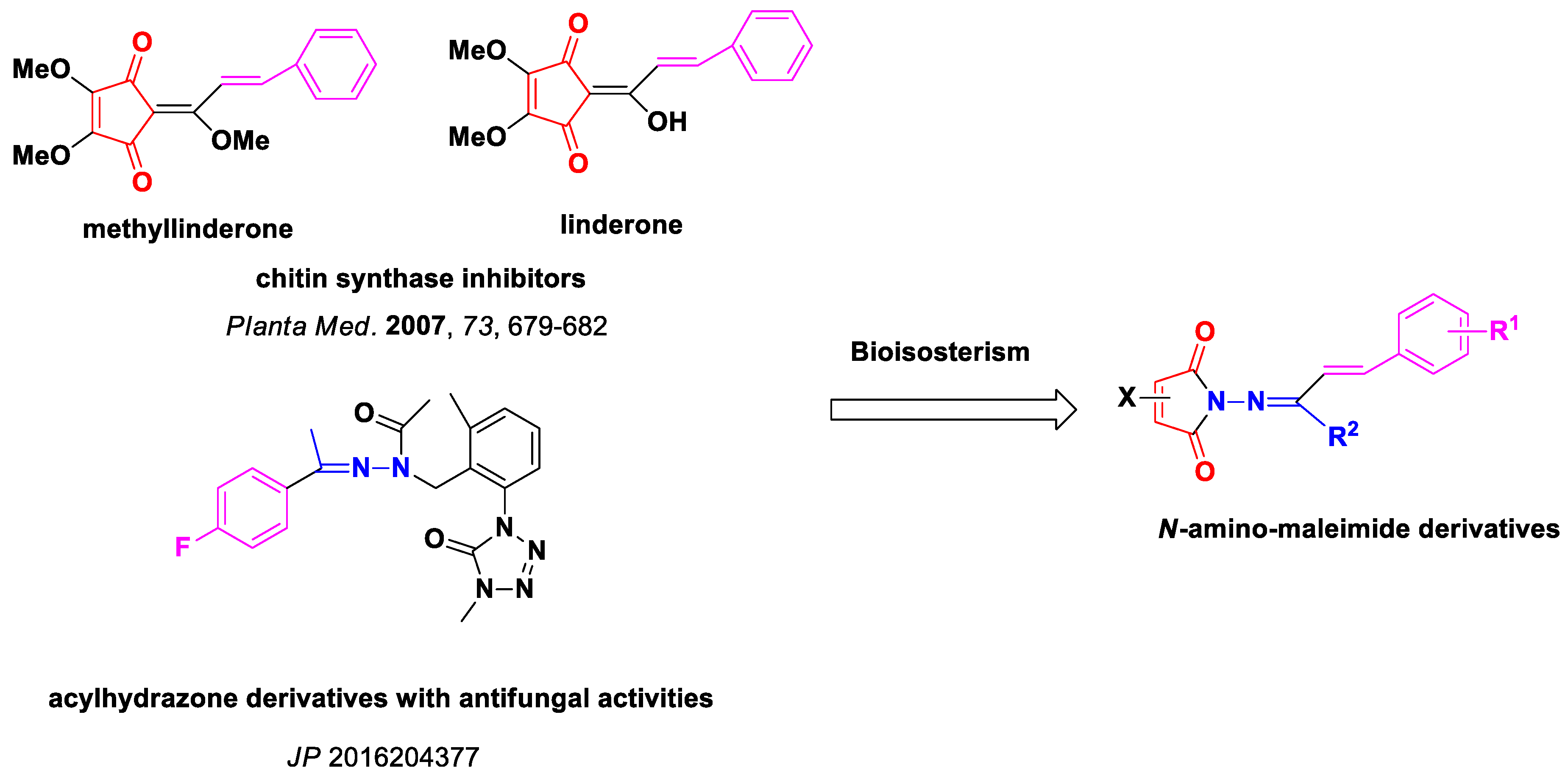
1. Introduction
2. Results
2.1. Synthesis
2.2. Bioassays
2.2.1. Stomach Toxicity against Oriental Armyworm (Mythimna separata)
2.2.2. Toxicity against Mosquito (Culex pipiens pallens)
2.2.3. In Vitro Antifungal Activity
3. Materials and Methods
3.1. Instruments
3.2. General Synthesis
3.3. General Procedure for the Synthesis of Compounds 1–16
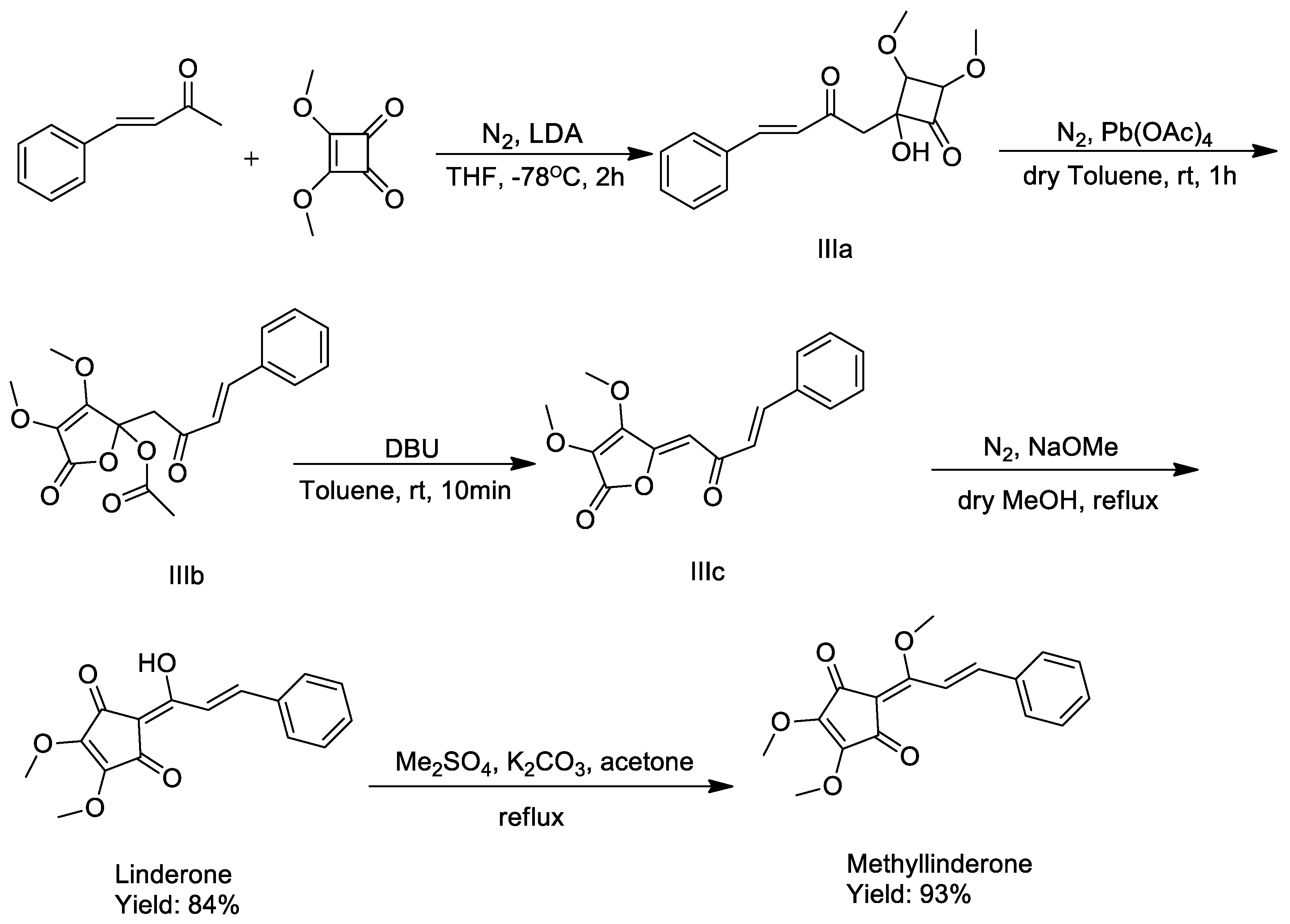
3.4. Synthesis of (E)-2-(1-hydroxy-3-phenylallylidene)-4,5-dimethoxycyclopent-4-ene-1,3-dione (Linderone)
3.5. Synthesis of (E)-4,5-dimethoxy-2-(1-methoxy-3-phenylallylidene)cyclopent-4-ene-1,3-dione (Methyllinderone)
3.6. General Procedure for the Synthesis of Copound 17
3.7. Insecticidal Biological Assay
3.7.1. Stomach Toxicity against Oriental Armyworm (Mythimna separata)
3.7.2. Toxicity against Mosquito (Culex pipiens pallens)
3.8. In Vitro Antifungal Bioassay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chen, Q.; Zhang, J.M.; Chen, L.L.; Yang, J.; Yang, X.L.; Ling, Y.; Yang, Q. Design and synthesis of chitin synthase inhibitors as potent fungicides. Chin. Chem. Lett. 2017, 28, 1232–1237. [Google Scholar] [CrossRef]
- Wei, G.; Jiang, H.; Geng, Y. Advances in studies on pharmacological activities of genus lindera. Zhonghua Zhongyiyao Xuekan 2014, 32, 61–62. [Google Scholar] [CrossRef]
- Hwang, E.I.; Lee, Y.M.; Lee, S.M.; Yeo, W.H.; Moon, J.S.; Kang, T.H.; Park, K.D.; Kim, S.U. Inhibition of chitin synthase 2 and antifungal activity of lignans from the stem bark of Lindera erythrocarp. Planta Med. 2007, 73, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Oh, H.W.; Fang, Y.; An, S.B.; Park, D.S.; Song, H.H.; Oh, S.R.; Kim, S.Y.; Kim, S.; Kim, N.; et al. Identification of plant compounds that disrupt the insect juvenile hormone receptor complex. Proc. Natl. Acad. Sci. USA 2015, 112, 1733–1738. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Lan, X.Y.; Xiao, J.H.; Yang, J.C.; Kao, Y.T.; Chang, S.T. Antiinflammatory activity of Lindera erythrocarpa fruits. Phytother. Res. 2008, 22, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Salewska, N.; Boros-Majewska, J.; Lacka, I.; Chylińska, K.; Sabisz, M.; Milewski, S.; Milewska, M.J. Chemical reactivity and antimicrobial activity of N-substituted maleimides. J. Enzyme Inhib. Med. Chem. 2012, 27, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Gholap, A.R.; Toti, K.S.; Shirazi, F.; Kumari, R.; Bhat, M.K.; Deshpande, M.V.; Srinivasan, K.V. Synthesis and evaluation of antifungal properties of a series of the novel 2-amino-5-oxo-4-phenyl-5,6,7,8-tetrahydroquinoline-3-carbonitrile and its analogues. Bioorg. Med. Chem. 2007, 15, 6705–6715. [Google Scholar] [CrossRef] [PubMed]
- Long, D.Q.; Li, D.J. Synthesis and bactericidal activity of novel triazolopyrimidine formyl hydrazones. Chem. Reag. 2008, 30, 206–208. [Google Scholar] [CrossRef]
- Zumbansen, K.; Döhring, A.; List, B. Morpholinium trifluoroacetate-catalyzed aldol condensation of acetone with both aromatic and aliphatic aldehydes. Adv. Synth. Catal. 2010, 352, 1135–1138. [Google Scholar] [CrossRef]
- Ma, X.L.; Huang, J.X.; Duan, W.G.; Mo, Q.J.; Lin, G.S.; Huang, Y. Synthesis and fungicidal activity of a-terpinene-maleimide-based acylhydrazone derivatives. Chin. J. Org. Chem. 2012, 32, 1077–1083. [Google Scholar] [CrossRef]
- Xiao, F.; Liu, W.; Wang, Y.; Zhang, Q.; Li, X.; Hu, X. Concise synthesis of linderaspirone, A. and Bi-linderone. Asian J. Org. Chem. 2013, 2, 216–219. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, Y.; Shen, Y. Natural products with maleic anhydride structure: Nonadrides, tautomycin, chaetomellic anhydride, and other compounds. Chem. Rev. 2007, 107, 1777–1830. [Google Scholar] [CrossRef] [PubMed]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Am. Mosq. Control Assoc. 1987, 3, 302–303. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.Q.; Shang, J.; Liu, Y.X.; Wang, K.Y.; Bi, F.C.; Huang, R.Q.; Wang, Q.M. Synthesis and insecticidal activities of novel N-sulfenyl-N’-tert-butyl-N,N’-diacylhydrazines. 1. N-Alkoxysulfenate derivatives. J. Agric. Food Chem. 2007, 55, 9614–9619. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.Q.; Li, Y.Q.; Xiong, L.X.; Wang, Q.M. Design, Synthesis and insecticidal activity of novel Phenylpyrazoles containing a 2,2,2-Trichloro-1-alkoxyethyl moiety. J. Agric. Food Chem. 2010, 58, 4992–4998. [Google Scholar] [CrossRef] [PubMed]
- Raymond, M.; Marquine, M. Evolution of insecticide resistance in culex pipiens populations: The corsican paradox. J. Evol. Biol. 1994, 7, 315–337. [Google Scholar] [CrossRef]
- Wang, X.; Li, P.; Li, Z.; Yin, J.; He, M.; Xue, W.; Chen, Z.; Song, B. Synthesis and bioactivity evaluation of novel arylimines containing a 3-aminoethyl-2-[(p-trifluoromethoxy)anilino]-4(3H)-quinazolinone moiety. J. Agric. Food Chem. 2013, 61, 9575–9582. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
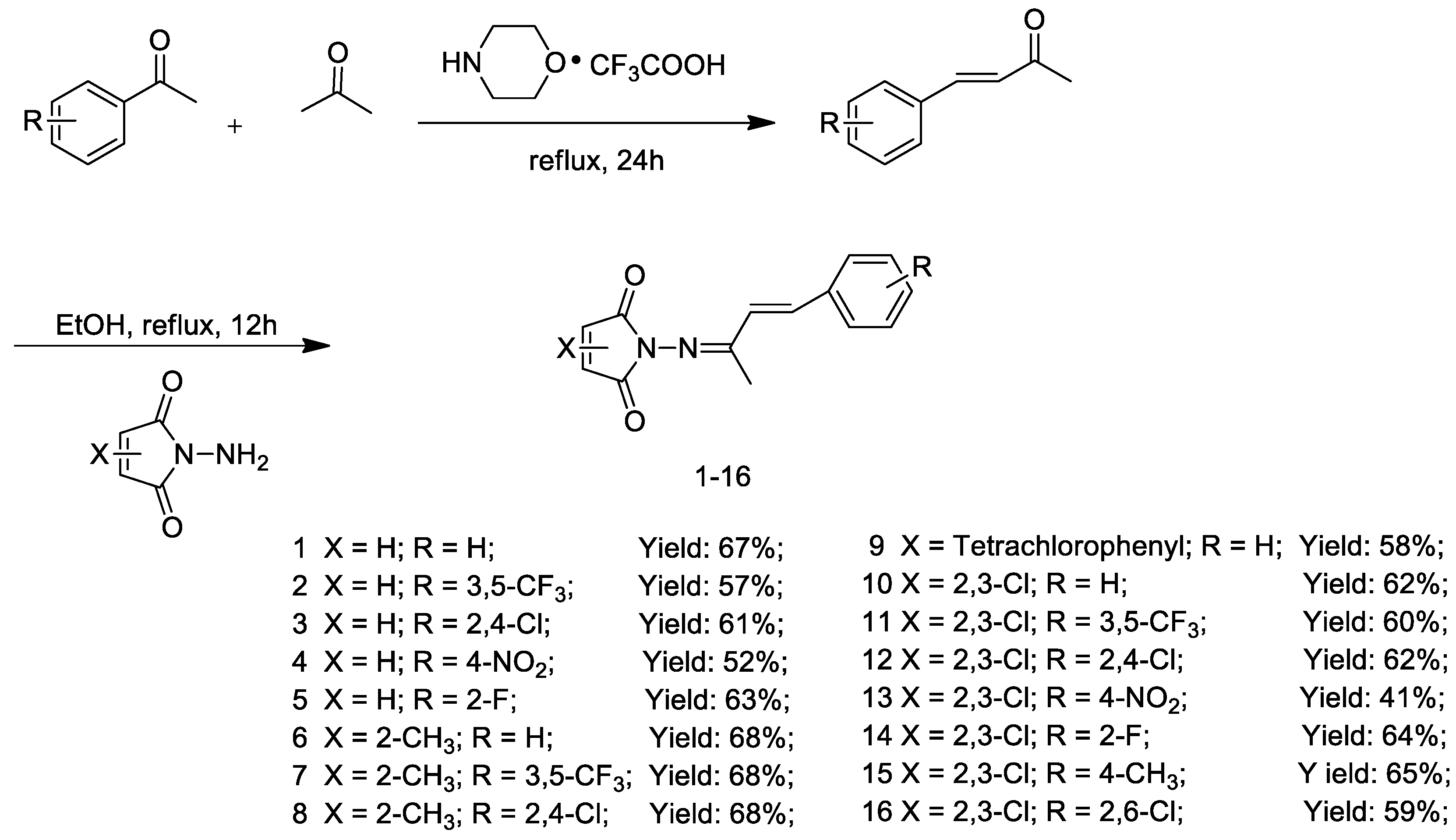

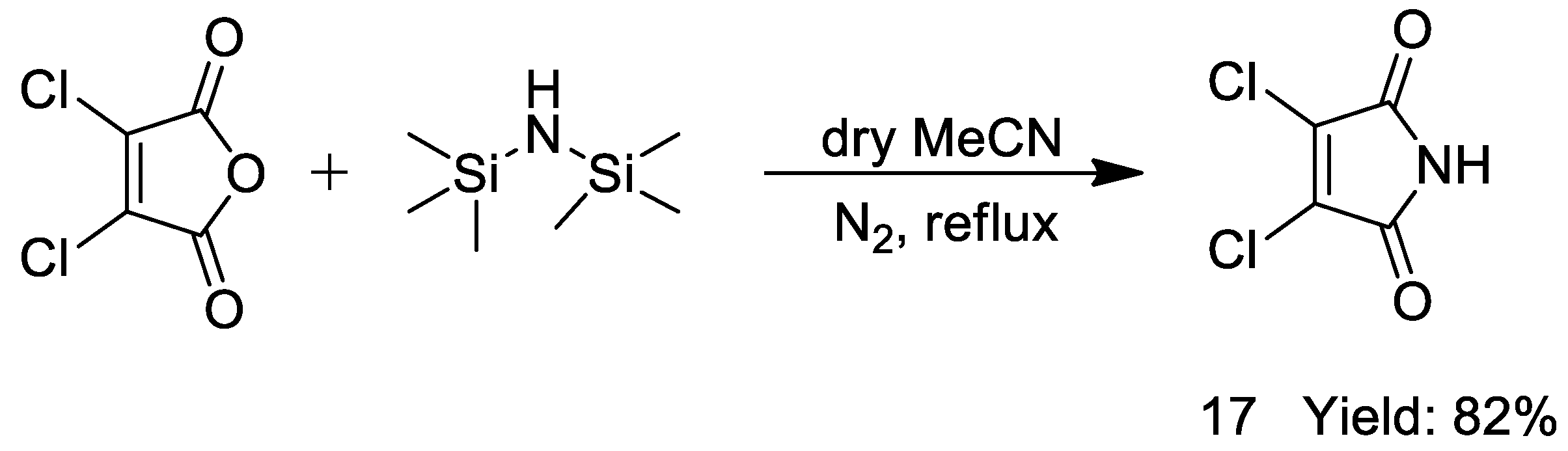
| Compd. | Larvicidal Activity (%) at Concn (µg·mL−1) | ||||||
|---|---|---|---|---|---|---|---|
| Oriental Armyworm | Mosquito | ||||||
| 600 | 10 | 5 | 2 | 1 | 0.5 | 0.25 | |
| 1 | 25 | 50 | - a | - | - | - | - |
| 2 | 60 | 100 | 100 | 100 | 100 | 100 | 60 |
| 3 | 50 | 100 | 100 | 100 | 100 | 100 | 60 |
| 4 | 10 | 40 | - | - | - | - | - |
| 5 | 25 | 30 | - | - | - | - | - |
| 6 | 65 | 50 | - | - | - | - | - |
| 7 | 40 | 100 | 20 | - | - | - | - |
| 8 | 5 | 100 | 100 | 100 | 100 | 100 | 20 |
| 10 | 20 | 100 | 100 | 40 | - | - | - |
| Compd. | Inhibition Rate (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A.Sa | G.Z | P.I | S.S | B.C | R.S | F.O | C.A | B.B | R.C | H.M | C.L | F.M | P.C | |
| 1 | 25.0 | 17.2 | 17.6 | 8.9 | 15.4 | 6.2 | 11.6 | 36.7 | 26.8 | 34.9 | 32.7 | 10.0 | 18.2 | -b |
| 2 | 33.3 | 20.7 | 23.5 | 37.5 | 34.6 | 54.3 | 23.3 | 26.7 | 66.1 | 50.0 | 32.7 | 22.5 | 30.3 | - |
| 3 | 8.3 | 24.1 | 17.6 | 17.9 | 26.9 | 37.0 | 32.6 | 46.7 | 35.7 | 57.0 | 34.7 | 32.5 | 57.6 | - |
| 4 | 25 | 37.9 | 29.4 | 26.8 | 34.6 | 18.5 | 27.9 | 46.7 | 89.3 | 29.1 | 61.2 | 27.5 | 39.4 | - |
| 5 | 8.3 | 10.3 | 17.6 | 8.9 | 7.7 | 12.3 | 14.0 | 36.7 | 25.0 | 31.4 | 30.6 | 5.0 | 15.2 | - |
| 6 | 25 | 13.8 | 11.8 | 32.1 | 19.2 | 30.9 | 16.3 | 13.3 | 62.5 | 23.3 | 20.4 | 15.0 | 30.3 | - |
| 7 | 16.7 | 10.3 | 23.5 | 21.4 | 19.2 | 53.1 | 20.9 | 36.7 | 51.8 | 58.1 | 26.5 | 22.5 | 30.3 | - |
| 8 | 16.7 | 17.2 | 17.6 | 39.3 | 23.1 | 45.7 | 25.6 | 56.7 | 48.2 | 64.0 | 34.7 | 32.5 | 27.3 | - |
| 9 | 33.3 | 44.8 | 23.5 | 5.4 | 3.8 | 14.8 | 16.3 | 30.0 | 7.1 | 40.7 | 26.5 | 17.5 | 21.2 | - |
| 10 | 41.7 | 44.8 | 58.8 | 75.0 | 73.1 | 66.7 | 39.5 | 46.7 | 75.0 | 79.1 | 44.9 | 47.5 | 48.5 | - |
| 11 | 27.8 | 39.5 | 18.2 | 63.4 | 52.4 | 43.2 | 45.7 | 50.0 | 14.3 | 89.1 | 47.2 | 40.7 | 46.2 | 33.3 |
| 12 | 44.4 | 47.4 | 50.0 | 87.5 | 71.4 | 67.9 | 65.7 | 65.0 | 42.9 | 98.2 | 63.9 | 70.4 | 61.5 | 55.6 |
| 13 | 44.4 | 36.8 | 27.3 | 26.8 | 47.6 | 42 | 51.4 | 35.0 | 34.7 | 61.8 | 27.8 | 18.5 | 38.5 | 27.8 |
| 14 | 44.4 | 39.5 | 54.5 | 60.6 | 47.6 | 67.9 | 45.7 | 25.0 | 67.3 | 100.0 | 55.6 | 44.4 | 46.2 | 69.4 |
| 15 | 44.4 | 44.7 | 63.6 | 66.2 | 52.4 | 63.0 | 45.7 | 50.0 | 36.7 | 90.9 | 52.8 | 51.9 | 46.2 | 69.4 |
| 16 | 44.4 | 42.1 | 45.5 | 63.4 | 52.4 | 61.7 | 45.7 | 55.0 | 51.0 | 89.1 | 52.8 | 37.0 | 53.8 | 63.9 |
| Linderone | 27.8 | 31.6 | 27.3 | 66.2 | 47.6 | 28.4 | 51.4 | 30.0 | 57.1 | 74.5 | 27.8 | 33.3 | 26.9 | 27.8 |
| Methyllinderon | 38.9 | 31.6 | 27.3 | 42.3 | 42.9 | 24.7 | 34.3 | 20.0 | 8.2 | 58.2 | 38.9 | 33.3 | 38.5 | 27.8 |
| carbendazim | <50 | 100 | 100 | 100 | <50 | 100 | <50 | <50 | <50 | 100 | 100 | 100 | <50 | <50 |
| chlorothalonil | 73.3 | <50 | 86.4 | <50 | 100 | 100 | 100 | 73.3 | 100 | 100 | 91.3 | 91.3 | 100 | 100 |
| Compd. | Inhibition Rate (%) | |||
|---|---|---|---|---|
| S. sclerotiorum | R. cerealis | |||
| 50 μg·mL−1 | 50 μg·mL−1 | 25 μg·mL−1 | 12.5 μg·mL−1 | |
| 12 | - a | - | 95.8 | 60.6 |
| 14 | - | - | 77.5 | 47.9 |
| 17 | 21.4 | 23.6 | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Liu, C.; Chen, P.; Zhang, H.; Sun, R. Natural Product-Based Pesticide Discovery: Design, Synthesis and Bioactivity Studies of N-Amino-Maleimide Derivatives. Molecules 2018, 23, 1521. https://doi.org/10.3390/molecules23071521
Song X, Liu C, Chen P, Zhang H, Sun R. Natural Product-Based Pesticide Discovery: Design, Synthesis and Bioactivity Studies of N-Amino-Maleimide Derivatives. Molecules. 2018; 23(7):1521. https://doi.org/10.3390/molecules23071521
Chicago/Turabian StyleSong, Xiangmin, Chunjuan Liu, Peiqi Chen, Hao Zhang, and Ranfeng Sun. 2018. "Natural Product-Based Pesticide Discovery: Design, Synthesis and Bioactivity Studies of N-Amino-Maleimide Derivatives" Molecules 23, no. 7: 1521. https://doi.org/10.3390/molecules23071521
APA StyleSong, X., Liu, C., Chen, P., Zhang, H., & Sun, R. (2018). Natural Product-Based Pesticide Discovery: Design, Synthesis and Bioactivity Studies of N-Amino-Maleimide Derivatives. Molecules, 23(7), 1521. https://doi.org/10.3390/molecules23071521





