Interference of Quercetin on Astragalus Polysaccharide-Induced Macrophage Activation
Abstract
:1. Introduction
2. Results
2.1. Quercetin Did Not Exhibit Cytotoxicity on RAW264.7 and Peritoneal Macrophages Cells
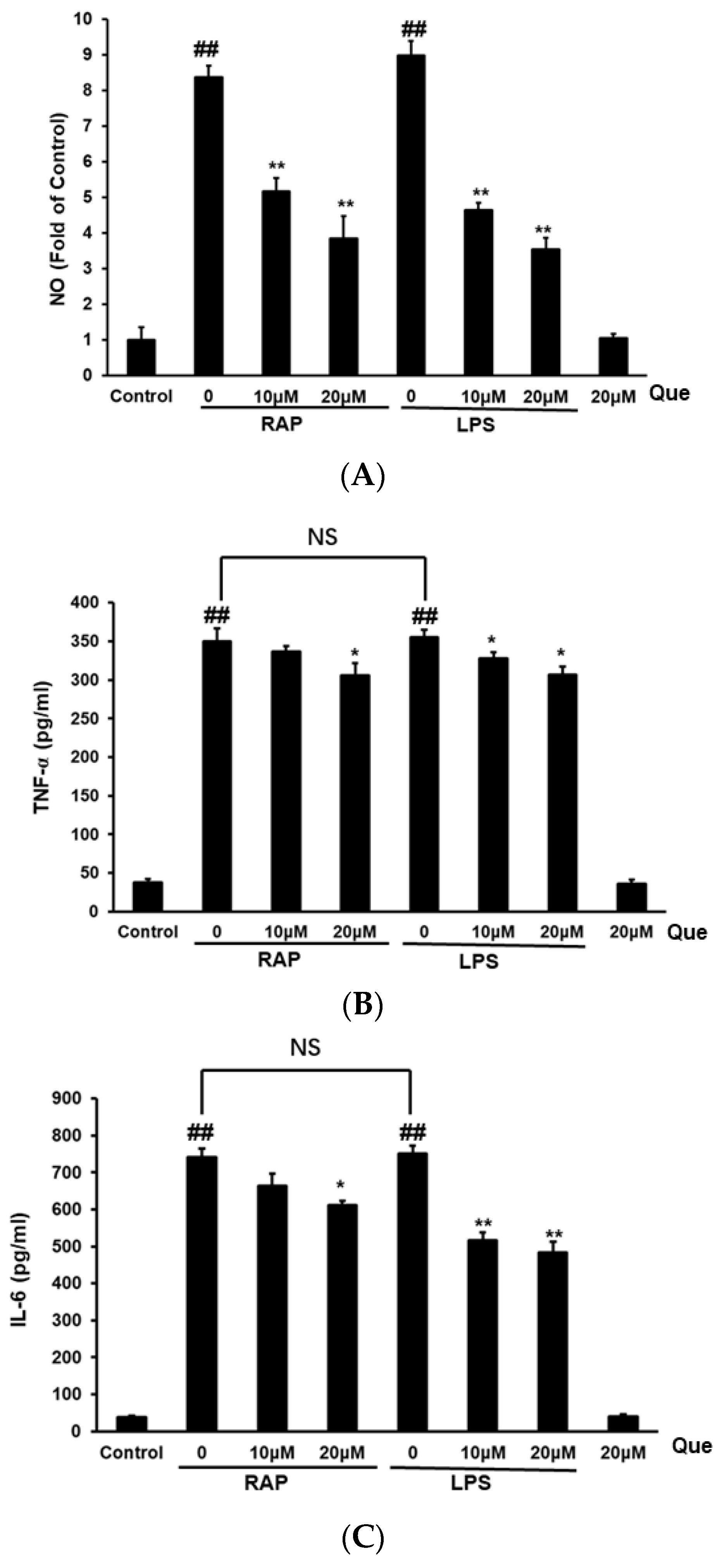
2.2. Effect of Quercetin on Production of NO, TNF-α and IL-6 in RAP and LPS Induced RAW264.7 Cells
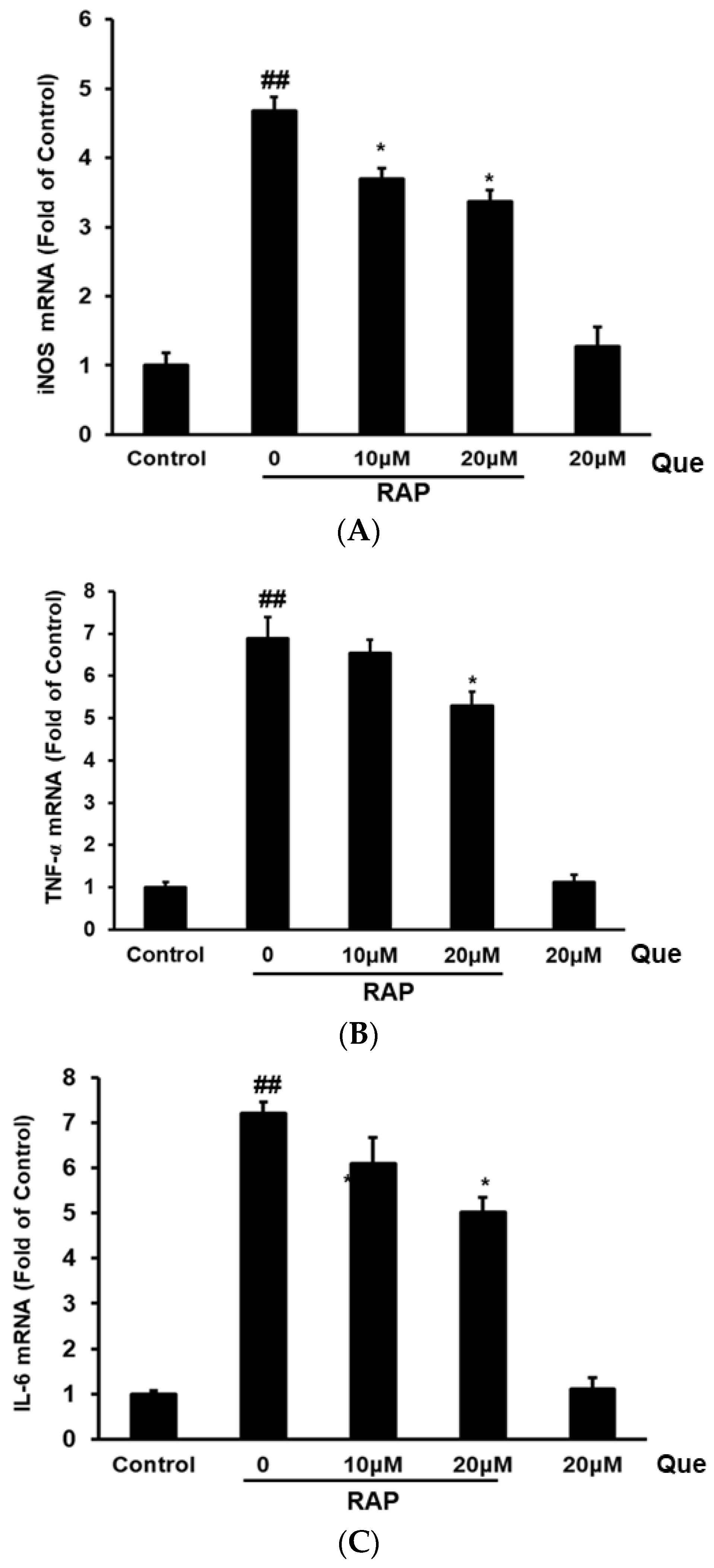
2.3. Effect of Quercetin on Gene Expression of iNOS, TNF-α, and IL-6 in RAP-Induced RAW264.7 Cells
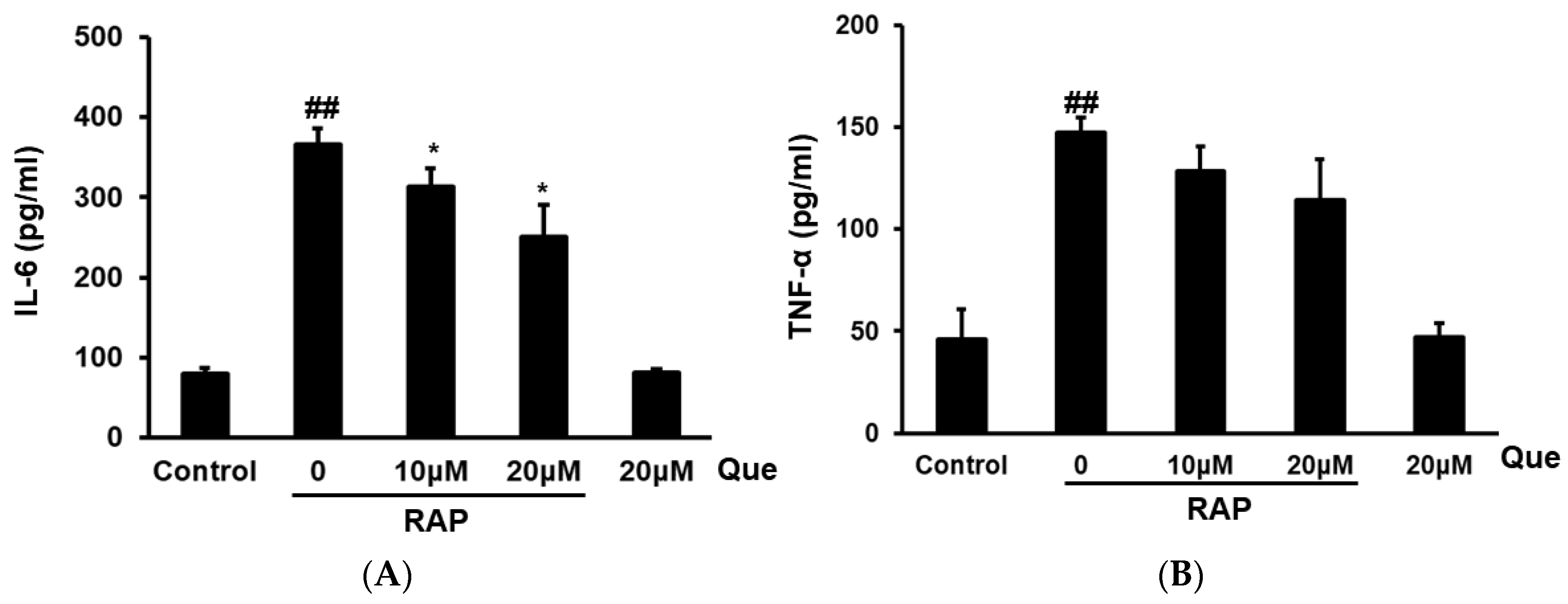
2.4. Effect of Quercetin on Production of TNF-α and IL-6 in RAP-Induced Peritoneal Macrophages Cells
2.5. Quercetin Inhibited the COX2 and iNOS in RAP-Induced RAW264.7 Cells
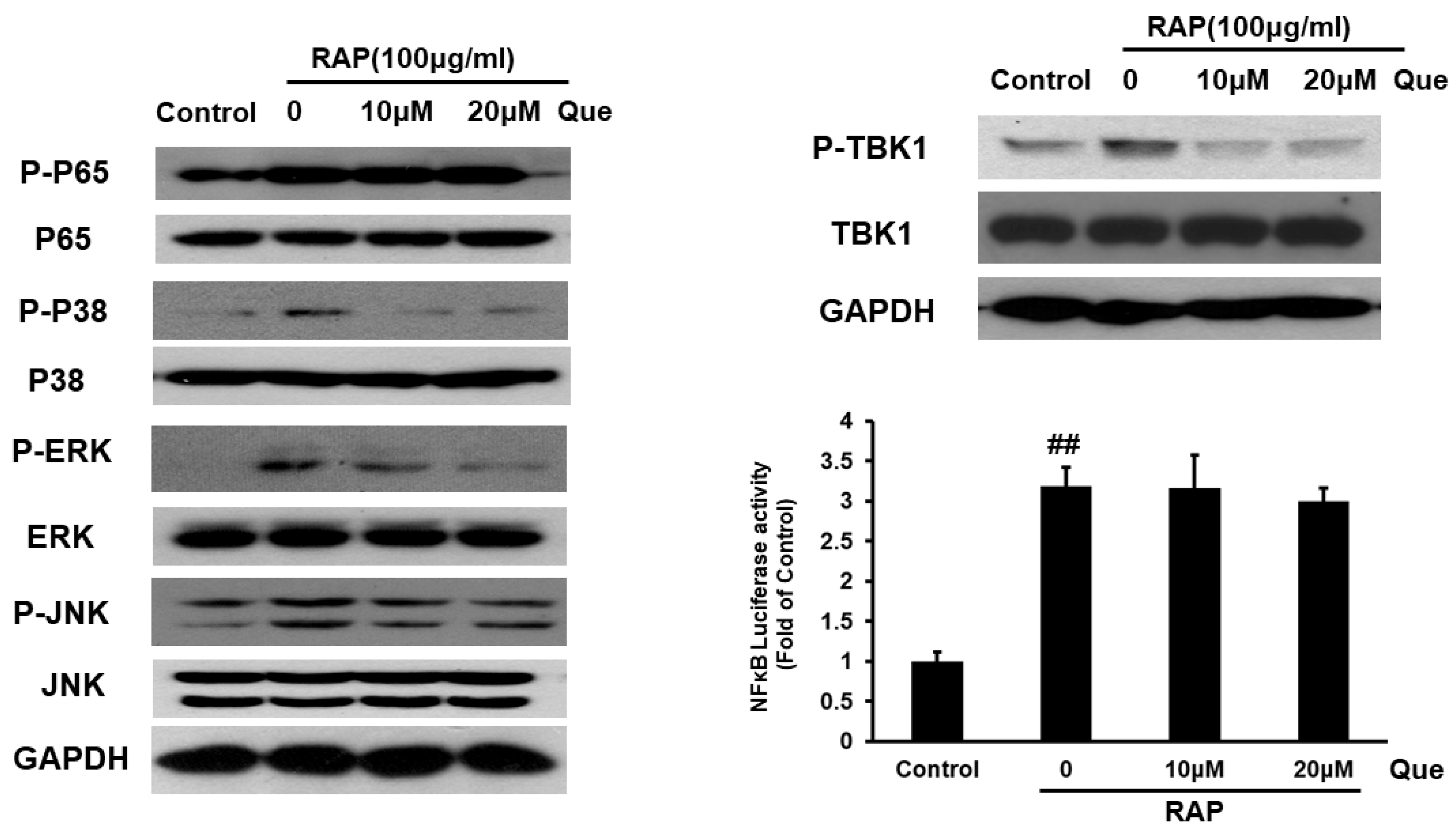
2.6. Effect of Quercetin on MyD88 Dependent and Independent Signaling Pathway in RAP-Induced RAW264.7 Cells
2.7. Quercetin Inhibited Akt and mTORC1 in RAP-Induced RAW264.7 Cells
3. Discussions
4. Materials and Methods
4.1. Materials
4.2. RAP Preparation
4.3. RAW264.7 Cells Culture
4.4. Peritoneal Macrophages
4.5. NO Level
4.6. TNF-α and IL-6 Assay
4.7. Real-Time Quantitative PCR
4.8. Western Blotting Analysis
4.9. Reporter Assays
4.10. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gerritsen, M.E.; Carley, W.W.; Ranges, G.E.; Shen, C.P.; Phan, S.A.; Ligon, G.F.; Perry, C.A. Flavonoids inhibit cytokine-induced endothelial cell adhesion protein gene expression. Am. J. Pathol. 1995, 14, 278–292. [Google Scholar]
- Kim, H.K.; Cheon, B.S.; Kim, Y.H.; Kim, S.Y.; Kim, H.P. Effect of naturally occurring flavonoid sonnitricoxide production in the macrophage cell line RAW264.7 and their structure-activity relationships. Biochem. Pharmacol. 1999, 58, 759–765. [Google Scholar] [CrossRef]
- Wadsworth, T.L.; Koop, D.R. Effects of the winepolyphenolics quercetin and resveratrolon pro-inflammatory cytokine expression in RAW264.7 macrophages. Biochem. Pharmacol. 1999, 57, 941–949. [Google Scholar] [CrossRef]
- Peterson, J.; Dwyer, J. Flavonoids: Dietary occurrence and biochemical activity. Nutr. Res. 1998, 18, 1995–2018. [Google Scholar] [CrossRef]
- Moskaug, J.O.; Carlsen, H.; Myhrstad, M.; Blomhoff, R. Molecular imaging of the biological effects of quercetin and quercetin-rich foods. Mech. Ageing Dev. 2004, 125, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Tanigawa, S.; Fujii, M.; Hou, D.X. Stabilization of p53 is involved in quercetin-induced cell cycle arrest and apoptosis in HepG2 cells. Biocsci. Biotechnol. Biochem. 2008, 72, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Formica, J.V.; Regelson, W. Review of biology of querctin and related bioflavonoids. Food Chem. Toxicol. 1995, 33, 1061–1080. [Google Scholar] [CrossRef]
- Strobel, P.; Allard, C.; Perez-Acle, T.; Calderon, R.; Aldunate, R.; Leighton, F. Myricetin, quercetin and catechin-gallate inhibit glucose uptake in isolated rat adipocytes. Biochem. J. 2005, 386, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Sikder, K.; Bhattacharjee, S.; Majumdar, S.B.; Ghosh, S.; Majumdar, S.; Dey, S. Quercetin alleviates inflammation after short-term treatment in high-fat-fed mice. Food Funct. 2013, 4, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Firdous, A.B.; Sharmila, G.; Balakrishnan, S.; RajaSingh, P.; Suganya, S.; Srinivasan, N.; Arunakaran, J. Quercetin, a natural dietary flavonoid, acts as a chemopreventive agent against prostate cancer in an in vivo model by inhibiting the EGFR signaling pathway. Food Funct. 2014, 5, 2632–2645. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Park, S.J.; Kwon, M.J.; Jeong, T.S.; Bok, S.H.; Choi, W.Y.; Jeong, W.I.; Ryu, S.Y.; Do, S.H.; Lee, C.S.; et al. Quercetin suppresses proinflammatory cytokines production through MAP kinases and NF-κB pathway in lipopolysaccharidestimulated macrophage. Mol. Cell. Biochem. 2003, 243, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Comalada, M.; Camesco, D.; Sierra, S.; Ballester, I.; Xaus, J.; Gálvez, J.; Zarzuelo, A. In vivo quercetin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through downregulation of the NF-κB pathway. Eur. J. Immunol. 2005, 35, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.Y.; Yu, Y.L.; Cheng, W.C.; OuYang, C.N.; Fu, E.; Chu, C.L. Immunosuppressive effect of quercetin on dendritic cell activation and function. J. Immunol. 2010, 184, 6815–6821. [Google Scholar] [CrossRef] [PubMed]
- Yasui, M.; Matsushima, M.; Omura, A.; Mori, K.; Ogasawara, N.; Kodera, Y.; Shiga, M.; Ito, K.; Kojima, S.; Kawabe, T. The suppressive effect of quercetin on toll-like receptor 7-mediated activation in alveolar macrophages. Pharmacology 2015, 96, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.N.; Shin, S.A.; Choo, G.S.; Kim, H.J.; Park, Y.S.; Kim, B.S.; Kim, S.K.; Cho, S.D.; Nam, J.S.; Choi, C.S.; et al. Anti-inflammatory effect of quercetin and galangin in LPS-stimulated RAW264.7 macrophages and DNCB-induced atopic dermatitis animal models. Int. J. Mol. Med. 2018, 41, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Faulkner, C.L.; Nelson-Overton, L.K.; Wiley, J.A.; Quinn, M.T. Macrophage immunomodulatory activity of polysaccharides isolated from Juniperus scopolorum. Int. Immunopharmacol. 2005, 5, 1783–1799. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Xie, G.; Kirpotina, L.N.; Klein, R.A.; Jutila, M.A.; Quinn, M.T. Macrophage immunomodulatory activity of polysaccharides isolated from Opuntia polyacantha. Int. Immunopharmacol. 2008, 8, 1455–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, W.; Xiao, H.T.; Bao, W.R.; Ma, D.L.; Leung, C.H.; Han, X.Q.; Ko, C.H.; Lau, C.B.; Wong, C.K.; Fung, K.P.; et al. TLR-4 may mediate signaling pathways of Astragalus polysaccharide RAP induced cytokine expression of RAW264.7 cells. J. Ethnopharm. 2016, 179, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, V.; Dey, M.; Dorn, R.; Raskin, I. MyD88-dependent and independent pathways of Toll-Like Receptors are engaged in biological activity of Triptolide in ligand-stimulated macrophages. BMC Chem. Biol. 2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.P.; Li, L.F.; Zhang, Q.W.; Wei, W.; Liu, H.B.; Bao, W.R.; Han, Q.B. Aktdownstream of NFκB, MAPKs and IRF3 pathway involved in macrophage activation induced by Astragalus polysaccharide RAP. J. Funct. Foods 2017, 39, 152–159. [Google Scholar] [CrossRef]
- Fang, W.; Bi, D.; Zheng, R.; Cai, N.; Xu, H.; Zhou, R.; Lu, J.; Wan, M.; Xu, X. Identification and activation of TLR4-mediated signalling pathways by alginate-derived guluronate oligosaccharide in RAW264.7 macrophages. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Chen, M.; Yan, B.; He, X.; Chen, X.; Li, D. Identification of a role for the PI3K/AKT/mTOR signaling pathway in innate immune cells. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.P.; Zhang, Q.W.; Wei, W.; Li, L.F.; Liu, H.B.; Bao, W.R.; Han, Q.B. Luteolin exerted less inhibitory effect on macrophage activation induced by Astragalus polysaccharide than by lipopolysaccharide. J. Funct. Foods 2017, 37, 618–623. [Google Scholar] [CrossRef]
- Sugiyama, T.; Kawaguchi, K.; Dobashi, H.; Miyake, R.; Kaneko, M.; Kumazawa, Y. Quercetin but not luteolin suppresses the induction of lethal shock upon infection of mice with Salmonella typhimurium. FEMS Immunol. Med. Microbiol. 2008, 53, 306–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Chen, W.C.; Wang, W.P.; Tian, W.Y.; Zhang, Y.G. Extraction, characterization of Astragalus polysaccharides and its immune modulating activities in rats with gastric cancer. Carbohydr. Polym. 2009, 78, 738–742. [Google Scholar] [CrossRef]
- Li, X.; Xu, W. TLR4-mediated activation of macrophages by the polysaccharide fraction from Polyporus umbellatus (pers.) Fries. J. Ethnopharm. 2011, 135, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Chen, W.C.; Wang, W.P.; Tian, W.Y.; Zhang, Y.G. Antioxidant activity of Astragalus polysaccharides and antitumour activity of the polysaccharides and siRNA. Carbohydr. Polym. 2010, 82, 240–244. [Google Scholar] [CrossRef]
- Li, J.; Bao, Y.; Lam, W.; Zhu, F.L.X.; Liu, J.; Wang, H. Immunoregulatory and anti-tumor effects of polysaccharopeptide and Astragalus polysaccharides on tumor-bearing mice. Immunopharm. Immunotoxicol. 2008, 30, 771–782. [Google Scholar]
- Shou, Q.Y.; Fu, R.Z.; Tan, Q.; Shen, Z.W. Geranylated flavonoids from the roots of Campylotropis hirtella and their immunosuppressive activities. J. Agric. Food Chem. 2009, 57, 6712–6719. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xuan, B.; Shou, Q.; Shen, Z. New flavonoids from Campylotropis hirtella with immunosuppressive activity. Fitoterapia 2014, 95, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.Y.; Chan, B.C.; Yu, H.; Lau, I.Y.; Han, X.Q.; Cheng, S.W.; Wong, C.K.; Lau, C.B.; Xie, M.Y.; Fung, K.P.; et al. Separation, structure char- acterization, conformation and immunomodulating effect of a hyperbranched heteroglycan from Radix Astragali. Carbohydr. Polym. 2012, 87, 667–675. [Google Scholar] [CrossRef]
- Wang, M.J.; Jeng, K.C.; Shih, P.C. Differential expression and regulation of macrophage inflammatory protein (MIP)-1alpha and MIP-2 genes by alveolar and peritoneal macrophages in LPS-hyporesponsive C3H/HeJ mice. Cell. Immunol. 2000, 204, 88–95. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.-P.; Liu, H.-B.; Zhang, Q.-W.; Li, L.-F.; Bao, W.-R.; Ma, D.-L.; Leung, C.-H.; Bian, Z.-X.; Lu, A.-P.; Han, Q.-B. Interference of Quercetin on Astragalus Polysaccharide-Induced Macrophage Activation. Molecules 2018, 23, 1563. https://doi.org/10.3390/molecules23071563
Li Z-P, Liu H-B, Zhang Q-W, Li L-F, Bao W-R, Ma D-L, Leung C-H, Bian Z-X, Lu A-P, Han Q-B. Interference of Quercetin on Astragalus Polysaccharide-Induced Macrophage Activation. Molecules. 2018; 23(7):1563. https://doi.org/10.3390/molecules23071563
Chicago/Turabian StyleLi, Zhi-Peng, Hong-Bing Liu, Quan-Wei Zhang, Li-Feng Li, Wan-Rong Bao, Dik-Lung Ma, Chung-Hang Leung, Zhao-Xiang Bian, Ai-Ping Lu, and Quan-Bin Han. 2018. "Interference of Quercetin on Astragalus Polysaccharide-Induced Macrophage Activation" Molecules 23, no. 7: 1563. https://doi.org/10.3390/molecules23071563
APA StyleLi, Z. -P., Liu, H. -B., Zhang, Q. -W., Li, L. -F., Bao, W. -R., Ma, D. -L., Leung, C. -H., Bian, Z. -X., Lu, A. -P., & Han, Q. -B. (2018). Interference of Quercetin on Astragalus Polysaccharide-Induced Macrophage Activation. Molecules, 23(7), 1563. https://doi.org/10.3390/molecules23071563







