Effects of Melatonin on Antioxidant Capacity in Naked Oat Seedlings under Drought Stress
Abstract
:1. Introduction
2. Results
2.1. Effect of Spraying 100 μM MT on Leaves of Naked Oat Seedlings under Drought Stress
2.2. Effect of MT Pretreatment on Antioxidant Enzyme Activities of Naked Oat Seedlings under Drought Stress
2.3. Effect of MT Pretreatment on Changes in ROS in Naked Oat Seedlings under Drought Stress
2.4. Effect of MT Pretreatment on MAPK Activity in Naked Oat Seedlings under Drought Stress
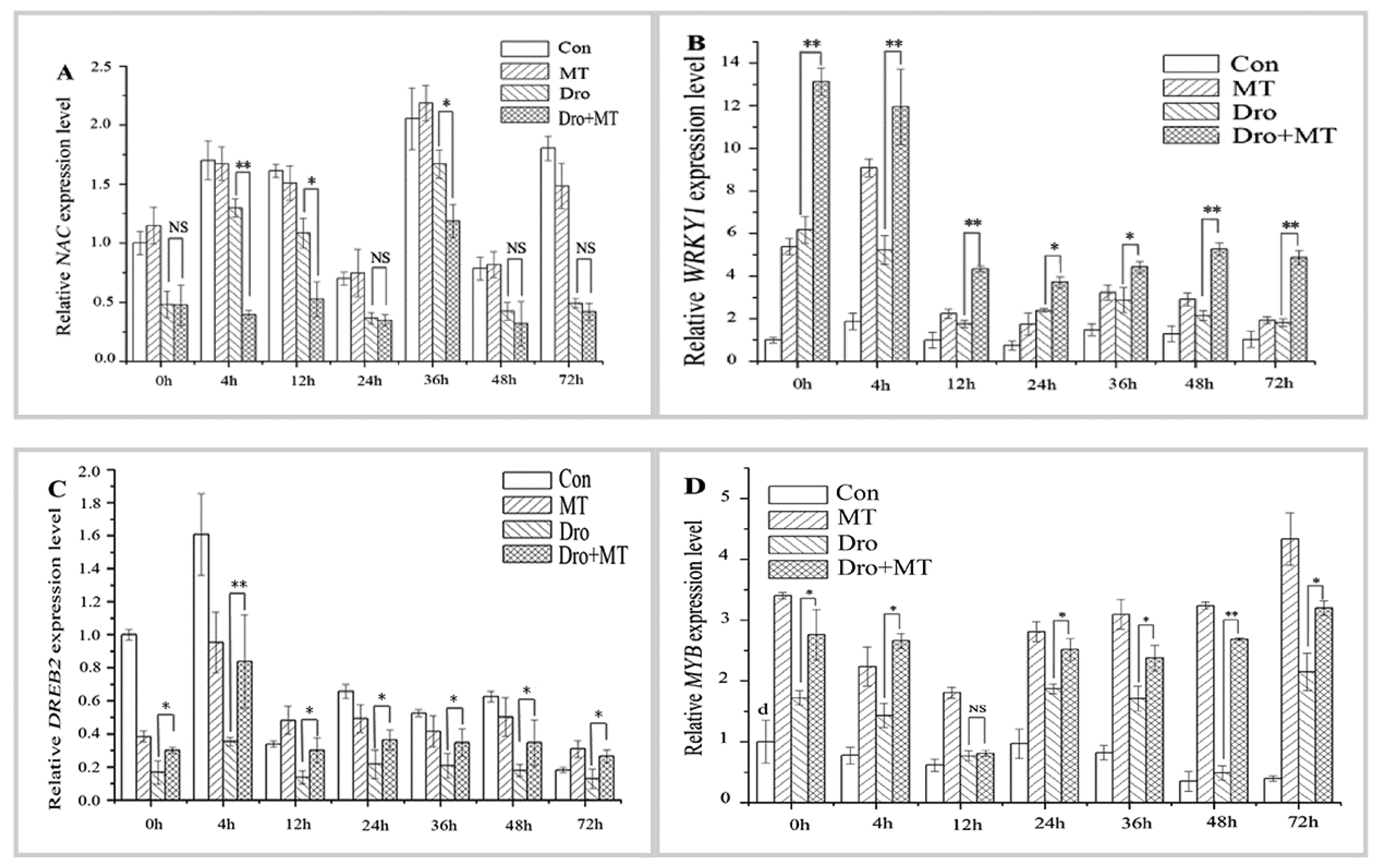
2.5. Effect of MT Pretreatment on the Expression of Antioxidant-Related TF Genes in Naked Oat Seedlings during Drought Stress
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Seed Germination Conditions
4.3. MT Pretreatment
4.4. Drought-Stress Treatment
4.5. Determination of Various Indicators
4.5.1. Calculation of Plant Height, Stem Thickness, Plant Fresh Weight, and Plant Dry Weight
4.5.2. Enzyme Extraction and Assay
4.5.3. Determination of H2O2 and O2−•
4.5.4. Quantitate Real Time-Polymerase Chain Reaction (qRT-PCR) Analysis
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| MT | Melatonin |
| H2O2 | Hydrogen peroxide |
| O2−• | Superoxide anion |
| SOD | Superoxide dismutase |
| POD | Peroxidase |
| CAT | Catalase |
| APX | Ascorbate peroxidase |
| PEG-6000 | Poly-ethylene glycol-6000 |
| MAPKs | Mitogen-activated protein kinases |
| TF | Transcription factor |
| ROS | Reactive oxygen species |
| ABA | Abscisic acid |
| BRs | Brassinosteroids |
References
- Wang, T.; Du, Y.L.; He, J.; Turner, N.C.; Wang, B.R.; Zhang, C.; Cui, T.; Li, F.M. Recently–released genotypes of naked oat (Avena nuda L.) out–yield early releases under water–limited conditions by greater reproductive allocation and desiccation tolerance. Field Crops Res. 2017, 204, 169–179. [Google Scholar] [CrossRef]
- Song, G.Y.; Huo, P.J.; Wu, B.; Zhang, Z.W. A genetic linkage map of hexaploid naked oat constructed with SSR markers. Crop J. 2015, 3, 353–357. [Google Scholar] [CrossRef]
- Lin, W.J.; Wu, G.F.; Li, C.H.; Wang, Y.; Zhou, S.M. Effects of cultivar and environment on nutritional quality of Chinese naked oats. Acta Agron. Sin. 2011, 37, 1087–1092. [Google Scholar] [CrossRef]
- Marta, B.; Katarzyna, S.; Malgorzata, M.P. Exogenous Melatonin Improves Antioxidant Defense in Cucumber Seeds (Cucumis Sativus L.) Germinated under Chilling Stress. Front. Plant Sci. 2016, 7, 575. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.S.; Song, C.P.; Wang, B.S.; Zhou, J.M.; Kangasjärvi, J.; Zhu, J.K.; Gong, Z.Z. ROS signaling and stomatalmovement in plant responses to drought stress and pathogen attack. J. Integr. Plant Biol. 2018, 1–53. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzaman, M.; Alam, M.M.; Fujita, M. Roles of exogenous glutathione in antioxidant defense system and methylglyoxal detoxification during salt stress in mung bean. Biol. Plant. 2015, 59, 745–756. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Gechev, T.S.; Van Breusegem, F.; Stone, J.M.; Denev, I.; Laloi, C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays 2006, 28, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, Q.R.; Fu, S.L.; Dong, B.L. Effects of exogenous nitric oxide on photosynthetic characteristics of poplar leaves under water stress. Chin. J. Appl. Ecol. 2005, 16, 218–222. [Google Scholar]
- Hardeand, R.; Madrid, J.A.; Tan, D.X.; Reiter, R.J. Melatonin, the circadian multioscillator system and health: The need for detailed analyses of peripheral melatonin signaling. J. Pineal Res. 2012, 34, 233–241. [Google Scholar]
- Calvo, J.R.; Gonzalez Yanes, C.; Maldonado, M.D. The role of melatonin in the cells of the innate immunity: A review. J. Pineal Res. 2013, 55, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Orendáš, P.; Kubatka, P.; Bojková, B.; Kassayová, M.; Kajo, K.; Výbohová, D.; Kružliak, P.; Péč, M.; Adamkov, M.; Kapinová, A.; et al. Melatonin potentiates the anti-tumour effect of pravastatin in rat mammary gland carcinoma model. Int. J. Exp. Pathol. 2014, 95, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Kubatka, P.; Kalická, K.; Chamilová, M.; Ahlersová, E.; Ahlers, I.; Bojková, B.; Adámeková, E. Nimesulide and melatonin in mammary carcinogenesis prevention in female Sprague Dawley rats. Neoplasma 2002, 49, 255–259. [Google Scholar] [PubMed]
- Galano, A.; Tan, D.X.; Reiter, R.J. On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J. Pineal Res. 2012, 54, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Karaaslan, C.; Suzen, S. Antioxidant properties of melatonin and its potential action in diseases. Curr. Top. Med. Chem. 2015, 15, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Suwanjang, W.; Abramov, A.Y.; Charngkaew, K.; Govitrapong, P.; Chetsawang, B. Melatonin prevents cytosolic calcium overload, mitochondrial damage and cell death due to toxically high doses of dexamethasone-induced oxidative stress in human neuroblastoma SH-SY5Y cells. Neurochem. Int. 2016, 97, 34–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubbels, R.; Reiter, R.J.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.W.; Schloot, W. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatographymass spectrometry. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Hattori, A.; Migitaka, H.; Iigo, M.; Itoh, M.; Yamamoto, K.; Ohtani Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identificaton of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar] [PubMed]
- Manchester, L.C.; Tan, D.X.; Reiter, R.J.; Park, W.; Monis, K.; Qi, W. High levels of melatonin in the seeds of edible plants: Possible function in germ tissue protection. Life Sci. 2000, 67, 3023–3029. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Giridhar, P.; Sankar, K.U.; Ravishankar, G.A. Melatonin and serotonin profiles in beans of Coffea species. J. Pineal Res. 2012, 52, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández Ruiz, J. Growth conditions influence the melatonin content of tomato plants. Food Chem. 2013, 138, 1212–1214. [Google Scholar] [CrossRef] [PubMed]
- Vitalini, S.; Gardana, C.; Simonetti, P.; Fico, G.; Iriti, M. Melatonin, melatonin isomers and stilbenes in Italian traditional grape products and their antiradical capacity. J. Pineal Res. 2013, 54, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, H.J.; Zhao, B.; Sun, Q.Q.; Cao, Y.Y.; Li, R.; Wu, X.X.; Weeda, S.; Li, L.; Ren, S.; et al. The RNA-seq approach to discriminate gene expression profiles in response to melatonin on cucumber lateral root formation. J. Pineal Res. 2014, 56, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Protective effect of melatonin against chlorophyll degradation during the senescence of barley leaves. J. Pineal Res. 2009, 46, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Hardeland, R.; Manchester, L.C.; Korkmaz, A.; Ma, S.; Rosales Corral, S.; Reiter, R.J. Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J. Exp. Bot. 2012, 63, 577–597. [Google Scholar] [CrossRef] [PubMed]
- Byeon, Y.; Park, S.; Kim, Y.S.; Park, D.H.; Lee, S.; Back, K. Light regulated melatonin biosynthesis in rice during the senescence process in detached leaves. J. Pineal Res. 2012, 53, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sun, X.; Li, C.; Wei, Z.; Liang, D.; Ma, F. Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J. Pineal Res. 2013, 54, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yin, L.; Liang, D.; Li, C.; Ma, F.; Yue, Z. Delayed senescence of apple leaves by exogenous melatonin treatment: Toward regulating the ascorbate–glutathione cycle. J. Pineal Res. 2012, 53, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, D.E.; Jang, H.; Byeon, Y.; Kim, Y.S.; Back, K. Melatonin-rich transgenic rice plants exhibit resistance to herbicide-induced oxidative stress. J. Pineal Res. 2013, 54, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Wang, P.; Li, M.; Ke, X.; Li, C.; Liang, D.; Wu, S.; Ma, X.; Zou, Y.; Ma, F. Exogenous melatonin improves malus resistance to Marssonina apple blotch. J. Pineal Res. 2013, 54, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.F.; Xu, T.F.; Wang, Z.Z.; Fang, Y.L.; Xi, Z.M.; Zhang, Z.W. The ameliorative effects of exogenous melatonin on grape cuttings under water-deficient stress: Antioxidant metabolites, leaf anatomy, and chloroplast morphology. J. Pineal Res. 2014, 57, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Zeng, L.; Cheng, Y.; Lu, G.Y.; Fu, G.P.; Ma, H.Q.; Liu, Q.Y.; Zhang, X.K. Exogenous melatonin alleviates damage from drought stress in rapeseed (Brassica napus L.) seedlings. Acta Physiol. Plant. 2018, 40. [Google Scholar] [CrossRef]
- Chen, Y.E.; Cui, J.M.; Li, G.X.; Yuan, M.; Zhang, Z.W.; Yuan, S.; Zhang, H.Y. Effect of salicylic acid on the antioxidant system and photosystem II in wheat seedlings. Biol. Plant. 2016, 60, 139–147. [Google Scholar] [CrossRef]
- Rodriguez, M.C.; Petersen, M.; Mundy, J. Mitogen-activated protein kinase signaling in plants. Annu. Rev. Plant Biol. 2010, 61, 621–649. [Google Scholar] [PubMed]
- Xuan, L.T.H.; Du, N.H.N.; Nguyen, B.A.T.; Nguyen, P.T.; Lamson, P.T. Transcription factors and their roles in signal transduction in plants under abiotic stresses. Curr. Genom. 2017, 18, 483–497. [Google Scholar]
- Wang, J.; Zhang, L.; Cao, Y.; Qi, C.; Li, S.; Liu, L.; Wang, G.; Mao, A.; Ren, S.; Guo, Y.D. CsATAF1 positively regulates drought stress tolerance by ABA-dependent pathway and promoting ROS scavenging in cucumber. Plant Cell Physiol. 2018, 59, 930–945. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Qi, W.B.; Reiter, R.J.; Wei, W.; Wang, B.M. Exogenously applied melatonin stimulates root growth and raises endogenous indoleacetic acid in roots of etiolated seedlings of Brassica juncea. J. Plant Physiol. 2009, 166, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Posmyk, M.M.; Balabusta, M.; Wieczorek, M.; Sliwinska, E.; Janas, K.M. Melatonin applied to cucumber (Cucumis sativus L.) seeds improves germination during chilling stress. J. Pineal Res. 2009, 46, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Arora, D.; Bhatla, S.C.; Janas, K.M. Melatonin and nitric oxide regulate sunflower seedling growth under salt stress accompanying differential expression of Cu/Zn SOD and MnSOD. Free Radic. Biol. Med. 2017, 106, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Helton, P.; Reiter, R.J. Phytoremediative capacity of enriched with melatonin. Plant Signal Behav. 2007, 2, 514–516. [Google Scholar] [CrossRef] [PubMed]
- Hernández, I.G.; Gomez, F.J.; Cerutti, S.; Arana, M.V.; Silva, M.F. Melatonin in Arabidopsis thaliana acts as plant growth regulator at low concentrations and preserves seed viability at high concentrations. Plant Physiol. Biochem. 2015, 94, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Poeggeler, B.; Thuermann, S.; Dose, A.; Schoenke, M.; Burkhardt, S.; Hardeland, R. Melatonin’s uniue radical scavenging properties-roles of its functional substituents as revealed by a comparison with its structural analogs. J. Pineal Res. 2002, 33, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Li, Q.T.; Chu, Y.N.; Reiter, R.J.; Yu, X.M.; Zhu, D.H.; Zhang, W.K.; Ma, B.; Lin, Q.; Zhang, J.S.; et al. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J. Exp. Bot. 2014, 66, 695–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, H.; Jiang, C.; Ye, T.; Tan, D.X.; Reiter, R.J.; Zhang, H.; Liu, R.; Chan, Z. Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in bermudagrass [Cynodon dactylon (L.) Pers.] by exogenous melatonin. J. Exp. Bot. 2014, 66, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.B.; Zhao, X.; Liu, S.; Sun, F.; Zhang, C.; Xi, Y. Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiol. Biochem. 2017, 118, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.J.; Wang, Y.J.; Zhou, Y.H.; Tao, Y.; Mao, W.H.; Shi, K.; Asami, T.; Chen, Z.X.; Yu, J.Q. Reactive oxygen species are involved in brassinosteroids-induced stress tolerance in cucumber. Plant Physiol. 2009, 150, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xi, D.; Luo, L.; Meng, F.; Li, Y.; Wu, C.A.; Guo, X. Cotton GhMPK2 is involved in multiple signaling pathways and mediates defense responses to pathogen infection and oxidative stress. FEBS J. 2011, 278, 1367–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Jiang, S.; Pan, J.; Kong, X.; Zhou, Y.; Liu, Y.; Li, D. The overexpresssion of a maize mitogen-activated protein kinase gene (ZmMPK5) confers salt stress tolerance and induces defence responses in tobacco. Plant Biol. 2014, 16, 558–570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xi, D.; Li, S.; Li, S.; Gao, Z.; Zhao, S.; Shi, J.; Wu, C.; Guo, X. A cotton group C MAP kinase gene, GhMPK2, positively regulates salt and drought tolerance in tobacco. Plant Mol. Biol. 2011, 77, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Tena, G.; Plotnikova, J.; Willmann, M.R.; Chiu, W.L.; Gomez Gomez, L.; Boller, T.; Ausubel, F.M.; Sheen, J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 2002, 415, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Danquah, A.; Zelicourt, A.D.; Colcombet, J.; Hirt, H. The role of ABA and MAPK signaling pathway in plant abiotic stress responses. Biotechnol. Adv. 2014, 32, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Y.; Lv, B.; Li, J.; Luo, L.; Lu, S.; Zhang, X.; Ma, H.; Ming, F. The NAC Family Transcription Factor OsNAP Confers Abiotic Stress Response Through the ABA Pathway. Plant Cell Physiol. 2014, 55, 604–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, Y.; Zhang, H.; Huang, L.; Li, D.; Song, F. Overexpressing of a Stress-Responsive NAC Transcription Factor Gene ONAC022 Improves Drought and Salt Tolerance in rice. Front. Plant Sci. 2016, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Dai, M.; Yao, J.; Xiao, B.; Li, X.; Zhang, Q.; Xiong, L. Overexpressing a NAM, ATAF, and CUC(NAC) transcription factor enhances drought resistance and salt resistance in rice. Proc. Natl. Acad. Sci. USA 2006, 103, 12987–12992. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Liao, K.; Du, H.; Xu, Y.; Song, H.; Li, X.; Xiong, L. A stress-responsive NAC transcription factor SNAC3 confers heat and drought tolerance through modulation of reactive oxygen species in rice. J. Exp. Bot. 2015, 66, 6803–6817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, X.; Seemann, J.R.; Neuman, D.; Shen, Q.J. A WRKY gene from creosote bush encodes an activator of the abscisic acid signaling pathway. J. Biol. Chem. 2004, 279, 55770–55779. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.P.; Yu, D.Q. Over-expression of the stress-induced OsWRKY45 enhances disease resistance and drought tolerance in Arabidopsis. Environ. Exp. Bot. 2008, 65, 35–47. [Google Scholar] [CrossRef]
- Jiang, Y.; Liang, G.; Yu, D. Activated expression of WRKY57 confers drought tolerance in Arabidopsis. Mol. Plant. 2012, 5, 1375–1388. [Google Scholar] [CrossRef] [PubMed]
- Gadjev, I.; Vanderauwera, S.; Gechev, T.S.; Laloi, C.; Minkov, I.N.; Shulaev, V.; Apel, K.; Inzé, D.; Mittler, R.; Van Breusegem, F. Transcriptomic footprints disclose specificity of reactive oxygen species signaling in Arabidopsis. Plant Physiol. 2006, 141, 436–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, H.X.; Huang, F.; Cheng, H.; Song, H.N.; Yu, D.Y. Overexpression of the GmNAC2 Gene, an NAC transcriptional factor, reduces aAbiotic stress tolerance in tobacco. Plant Mol. Biol. Rep. 2013, 31, 435–442. [Google Scholar] [CrossRef]
- Yan, H.; Jia, H.; Chen, X.; Hao, L.; An, H.; Guo, X. The cotton WRKY transcription factor GhWRKY17 functions in drought and salt stress in transgenic Nicotiana benthamiana through ABA signaling and the modulation of reactive oxygen species production. Plant Cell Physiol. 2014, 55, 2060–2076. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.A.; Jiao, Y.; Chen, C.; Shireen, F.; Zheng, Z.; Imtia, M.; Bie, Z.; Huang, Y. Melatonin pretreatment improves vanadium stress tolerance of watermelon on seedlings by reducing vanadium concentration in the leaves and regulating melatonin biosynthesis and antioxidant-related gene expression. J. Plant Physiol. 2018, 220, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurence in higher plants. Plant Physiol. 1977, 54, 309–314. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Hamurcu, M.; Sekmen, A.H.; Turkan, İ.; Gezgin, S.; Demiral, T.; Bell, R.W. Induced anti-oxidant activity in soybean alleviates oxidative stress under moderate boron toxicity. Plant Growth Regul. 2013, 70, 217–226. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplast. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Yu, C.W.; Murphy, T.M.; Lin, C.H. Hydrogen peroxideinduces chilling tolerance in mung beans mediated through ABA-independent glutathione accumulation. Funct. Plant Biol. 2003, 30, 955–963. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant system in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |






| Gene Name | Sense Primer | Anti-Sense Primer |
|---|---|---|
| NAC | GGAGTCGGAGATCGTGGACACC | TGGATGTCGTCGTAGCTGAGGTC |
| DREB2 | ATACCGTGGTGTGAGGCAG | CGAGATACGAGAAGGAGGA |
| WRKY1 | GGCGTCCTCCTTCCTCCAGTC | CCTCGTATGGCGTGCTGAAGC |
| MYB | GAACCAGCAGCCGTCTGTGAG | GCAGGAGCGGTGGATTCAGTG |
| Asmap1 | CATCCGCTCCAACCAAGAACTCTC | TACTCCGTCATCATGTCGCTCTCC |
| Aspk11 | GGTCCATACCCCCACAGA | TAGTCCAACAGCCTCATT |
| Actin | ATGTTGCCATCCAGGCTGTG | TAAGTCACGTCCAGCGAGGT |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, W.; Zhang, Y.; Feng, Z.; Bai, Q.; He, J.; Wang, Y. Effects of Melatonin on Antioxidant Capacity in Naked Oat Seedlings under Drought Stress. Molecules 2018, 23, 1580. https://doi.org/10.3390/molecules23071580
Gao W, Zhang Y, Feng Z, Bai Q, He J, Wang Y. Effects of Melatonin on Antioxidant Capacity in Naked Oat Seedlings under Drought Stress. Molecules. 2018; 23(7):1580. https://doi.org/10.3390/molecules23071580
Chicago/Turabian StyleGao, Wenying, Yujing Zhang, Zheng Feng, Qingqing Bai, Jinjin He, and Yingjuan Wang. 2018. "Effects of Melatonin on Antioxidant Capacity in Naked Oat Seedlings under Drought Stress" Molecules 23, no. 7: 1580. https://doi.org/10.3390/molecules23071580
APA StyleGao, W., Zhang, Y., Feng, Z., Bai, Q., He, J., & Wang, Y. (2018). Effects of Melatonin on Antioxidant Capacity in Naked Oat Seedlings under Drought Stress. Molecules, 23(7), 1580. https://doi.org/10.3390/molecules23071580




