A New Benzophenone C-Glucoside and Other Constituents of Pseuduvaria fragrans and Their α-Glucosidase Inhibitory Activity
Abstract
:1. Introduction
2. Results and Discussion
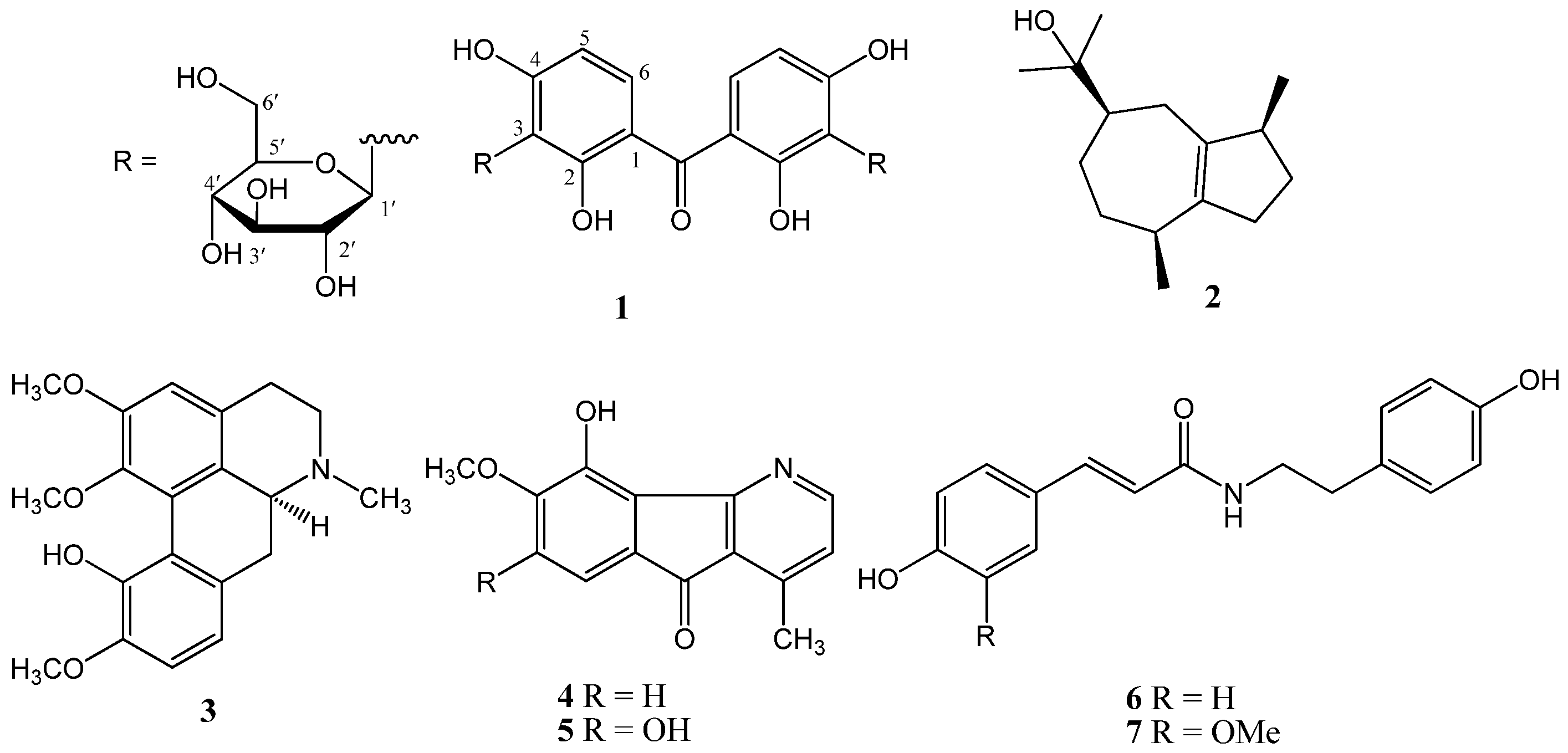
2.1. Structure Characterization
2.2. α-Glucosidase Inhibitory Activity
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Materials
3.3. Extraction, Isolation, and Purification
3.4. Assays for α-Glucosidase Inhibitory Activity
3.5. Kinetic Study of α-Glucosidase Inhibition
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sugihara, H.; Nagao, M.; Harada, T.; Nakajima, Y.; Tanimura-Inagaki, K.; Okajima, F.; Tamura, H.; Inazawa, T.; Otonari, T.; Kawakami, M.; et al. Comparison of three α-glucosidase inhibitors for glycemic control and bodyweight reduction in Japanese patients with obese type 2 diabetes. J. Diabetes Investig. 2014, 5, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhan, Q.; Liu, Y.; et al. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lian, G.; Yu, B. Naturally occurring polyphenolic glucosidase inhibitors. Isr. J. Chem. 2015, 55, 268–284. [Google Scholar] [CrossRef]
- Chatsumpun, N.; Sritularak, B.; Likhitwitayawuid, K. New Biflavonoids with α-glucosidase and pancreatic lipase inhibitory activities from Boesenbergia rotunda. Molecules 2017, 22, 1862. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.C.F.; Chaowasku, T.; Saunders, R.M.K. An extended phylogeny of Pseuduvaria (Annonaceae) with descriptions of three new species and a reassessment of the generic status of Oreomitra. Syst. Bot. 2010, 35, 30–39. [Google Scholar] [CrossRef]
- Zhao, C.G.; Yao, M.J.; Yang, J.W.; Chai, Y.L.; Sun, X.D.; Yuan, C.S. A new benzopyran derivative from Pseuduvaria indochinensis Merr. Nat. Prod. Res. 2014, 28, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.M.; Zhao, S.S.; Ning, X. Alkaloids from Pseuduvaria indochinensis. Phytochemistry 1988, 27, 4004–4005. [Google Scholar]
- Taha, H.; Arya, A.; Khan, A.K.; Shahid, N.; Noordin, M.I.B.; Mohan, S. Effect of Pseuduvaria macrophylla in attenuating hyperglycemia mediated oxidative stress and inflammatory response in STZ-nicotinamide induced diabetic rats by upregulating insulin secretion and glucose transporter-1, 2 and 4 proteins expression. J. Appl. Biomed. 2018. [Google Scholar] [CrossRef]
- Mahmood, K.; Chan, K.C.; Park, M.H.; Han, Y.N.; Han, B.H. An aporphinoid alkaloid from Pseuduvaria macrophylla. Phytochemisty 1986, 26, 1509–1510. [Google Scholar] [CrossRef]
- Taha, H.; Looi, C.Y.; Arya, A.; Wong, W.F.; Yap, L.F.; Hasanpourghadi, M.; Mohd, M.A.; Paterson, I.C.; Ali, H.M. (6E,10E) Isopolycerasoidol and (6E,10E) isopolycerasoidol methyl ester, prenylated benzopyran derivatives from Pseuduvaria monticola induce mitochondrial-mediated apoptosis in human breast adenocarcinoma cells. PLoS ONE 2015. [Google Scholar] [CrossRef] [PubMed]
- Taha, H.; Arya, A.; Paydar, M.; Looi, C.Y.; Wong, W.F.; Murthy, C.R.V.; Noordin, M.I.; Ali, H.M.; Mustafa, A.M.; Hadi, H.A. Upregulation of insulin secretion and downregulation of pro-inflammatory cytokines, oxidative stress and hyperglycemia in STZ-nicotinamide-induced type 2 diabetic rats by Pseuduvaria monticola bark extract. Food Chem. Toxicol. 2014, 66, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Uadkla, O.; Yodkeeree, S.; Buayairaksa, M.; Meepowpan, P.; Nuntasaen, N.; Limtrakul, P.; Pompimon, W. Antiproliferative effect of alkaloids via cell cycle arrest from Pseuduvaria rugosa. Pharm. Biol. 2013, 51, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Taha, H.; Haidi, A.H.A.; Nordin, N.; Najmuldeen, I.A.; Mohamad, K.; Shirota, O.; Nugroho, A.E.; Piow, W.C.; Kaneda, T.; Morita, H. Pseuduvarines A and B, two new cytotoxic dioxoaporphine alkaloids from Pseuduvaria rugosa. Chem. Pharm. Bull. 2011, 59, 896–897. [Google Scholar] [CrossRef] [PubMed]
- Wirasathien, L.; Boonarkart, C.; Pengsuparp, T.; Suttisri, R. Biological activities of alkaloids from Pseuduvaria setosa. Pharm. Biol. 2006, 44, 274–278. [Google Scholar] [CrossRef]
- Sesang, W.; Punyanitya, S.; Pitchuanchom, S.; Udomputtimekakul, P.; Nuntasaen, N.; Banjerdpongchai, R.; Wudtiwai, B.; Pompimon, W. Cytotoxic aporphine alkaloids from leaves and twigs of Pseuduvaria trimera (Craib). Molecules 2014, 19, 8762–8772. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.J.; Shi, X.C.; Jia, C.X. Telephenone D, a new benzophenone C-glycoside from Polygala telephioides. Chin. J. Nat. Med. 2009, 7, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qian, Q.; Ge, D.; Li, Y.; Wang, X.; Chen, Q.; Gao, X.; Wang, T. Identification of benzophenone C-glucosides from mango tree leaves and their inhibitory effect on triglyceride accumulation in 3T3-L1 adipocytes. J. Agric. Food Chem. 2011, 59, 11526–11533. [Google Scholar] [CrossRef] [PubMed]
- Benovit, S.C.; Silva, L.L.; Salbego, J.; Loro, V.L.; Mallmann, C.A.; Baldisserotto, B.; Flores, E.M.M.; Heinzamnn, B.M. Anesthetic activity and bio-guided fractionation of the essential oil of Aloysia gratissima (Gillies & Hook.) Tronc. in silver catfish Rhamdia quelen. Ann. Brazil. Acad. Sci. 2015, 87, 1675–1689. [Google Scholar]
- Cheng, X.; Wang, D.; Jiang, L.; Yang, D. DNA Topoisomerase I inhibitory alkaloids from Corydalis saxicola. Chem. Biodiver. 2008, 5, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Wijeratne, E.M.K.; de silva, L.B.; Kikichi, T.; Tezuka, Y.; Gunatilaka, A.A.L.; Kingston, S.G.I. Cyathocaline, an azafluorenone alkaloid from Cyathocalyx zeylanica. J. Nat. Prod. 1995, 58, 459–462. [Google Scholar] [CrossRef]
- Mueller, D.; Davis, R.A.; Duffy, S.; Avery, V.M.; Camp, D.; Quinn, R.J. Antimalarial activity of azafluorenone alkaloids from the Australian tree Mitrephora diversifolia. J. Nat. Prod. 2009, 72, 1538–1540. [Google Scholar] [CrossRef] [PubMed]
- Al-Taweel, A.M.; Perveen, S.; El-Shafae, A.M.; Fawzy, G.A.; Malik, A.; Afza, N.; Igbal, L.; Latif, M. Bioactive phenolic amides from Celtis Africana. Molecules 2012, 17, 2675–2682. [Google Scholar] [CrossRef] [PubMed]
- Sipowo, R.V.T.; Ouahouo, B.M.W.; Maza, H.L.D.; Ishikawa, H.; Nishino, H.; Mkounga, P.; Nkengfack, A.E. Triterpenes and coumaroyltyramide from Ochthocosmus africanus. J. Dis. Med. 2017, 3, 12–16. [Google Scholar]
- Pereira, F.; Madureira, A.M.; Sancha, S.; Mulhovo, S.; Luo, X.; Duarte, A.; Ferreira, M.-J.U. Cleistochlamys kirkii chemical constituents: Antibacterial activity and synergistic effects against resistant Staphylococcus aureus strains. J. Ethnopharmacol. 2016, 178, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Kanchanapoom, T.; Sommit, J.; Kasai, R.; Otsuka, H.; Yamasaki, K. Chemical constituents of Thai medicinal plant, Polyalthia cerasoides. Nat. Med. 2002, 56, 268–271. [Google Scholar]
- Chen, C.Y.; Chang, F.R.; Yen, H.F.; Wu, Y.C. Amides from stems of Annona cherimola. Phytochemistry 1998, 49, 1443–1447. [Google Scholar] [CrossRef]
- Wu, Y.C.; Chang, G.Y.; Ko, F.N.; Teng, C.M. Bioactive constituents from the stems of Annona montana. Planta Med. 1995, 61, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Efdi, M.; Itoh, T.; Akao, Y.; Nozawa, Y.; Koketsu, M.; Ishihara, H. The isolation of secondary metabolites and in vitro potent anti-cancer activity of clerodermic acid from Enicosanthum membranifolium. Bioorg. Med. Chem. 2007, 15, 3667–3671. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Huang, L.; Chen, R.; Yu, D. Chemical constituents of Uvaria kurzii. Zhongguo Zhongyao Zazhi 2009, 34, 2203–2205. [Google Scholar] [PubMed]
- Song, Y.H.; Kim, D.W.; Curtis-Long, M.J.; Park, C.; Son, M.; Kim, J.Y.; Yuk, H.J.; Lee, K.W.; Park, K.H. Cinnamic acid amides from Tribulus terrestris displaying uncompetitive α-glucosidase inhibition. Eur. J. Med. Chem. 2016, 114, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Yon-Kahn, J. Molecular and cellular enzymology; Springer-Verlag: Berlin, Germany, 2010; pp. 103–191. ISBN 978-3-642-01227-3. [Google Scholar]
Sample Availability: Samples of all the compounds are available from the authors. |

| Position | 1H * | 13C * |
|---|---|---|
| 1 | - | 133.9 |
| 2 | - | 160.6 |
| 3 | - | 105.2 |
| 4 | - | 162.6 |
| 5 | 6.65 (d, 8.1) | 115.5 |
| 6 | 7.47 (d, 8.1) | 132.5 |
| 1′ | 4.84 ** | 77.3 |
| 2′ | 3.70 (m) | 74.3 |
| 3′ | 3.38 (m) | 80.0 |
| 4′ | 3.38 (m) | 71.0 |
| 5′ | 3.30 (m) | 82.5 |
| 6′ | 3.68 (m) | 62.1 |
| Carbonyl | - | 198.6 |
| Inhibitors | Dose (µM) | Slope | Vmax ΔA405/min | Km (mM) | Ki (µM) |
|---|---|---|---|---|---|
| None | - | 37.15 | 0.0144 | 0.53 | - |
| N-trans-coumaroyltyramine (6) | 2.0 | 35.56 | 0.0018 | 0.06 | 0.20 |
| 1.0 | 34.67 | 0.0030 | 0.10 | ||
| 0.5 | 34.55 | 0.0077 | 0.27 | ||
| N-trans-feruloyltyramine (7) | 8.0 | 36.51 | 0.0027 | 0.10 | 1.83 |
| 4.0 | 37.56 | 0.0044 | 0.16 | ||
| 2.0 | 36.62 | 0.0072 | 0.26 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panidthananon, W.; Chaowasku, T.; Sritularak, B.; Likhitwitayawuid, K. A New Benzophenone C-Glucoside and Other Constituents of Pseuduvaria fragrans and Their α-Glucosidase Inhibitory Activity. Molecules 2018, 23, 1600. https://doi.org/10.3390/molecules23071600
Panidthananon W, Chaowasku T, Sritularak B, Likhitwitayawuid K. A New Benzophenone C-Glucoside and Other Constituents of Pseuduvaria fragrans and Their α-Glucosidase Inhibitory Activity. Molecules. 2018; 23(7):1600. https://doi.org/10.3390/molecules23071600
Chicago/Turabian StylePanidthananon, Wongvarit, Tanawat Chaowasku, Boonchoo Sritularak, and Kittisak Likhitwitayawuid. 2018. "A New Benzophenone C-Glucoside and Other Constituents of Pseuduvaria fragrans and Their α-Glucosidase Inhibitory Activity" Molecules 23, no. 7: 1600. https://doi.org/10.3390/molecules23071600
APA StylePanidthananon, W., Chaowasku, T., Sritularak, B., & Likhitwitayawuid, K. (2018). A New Benzophenone C-Glucoside and Other Constituents of Pseuduvaria fragrans and Their α-Glucosidase Inhibitory Activity. Molecules, 23(7), 1600. https://doi.org/10.3390/molecules23071600






