The Current Status of Heterogeneous Palladium Catalysed Heck and Suzuki Cross-Coupling Reactions
Abstract
:1. Introduction
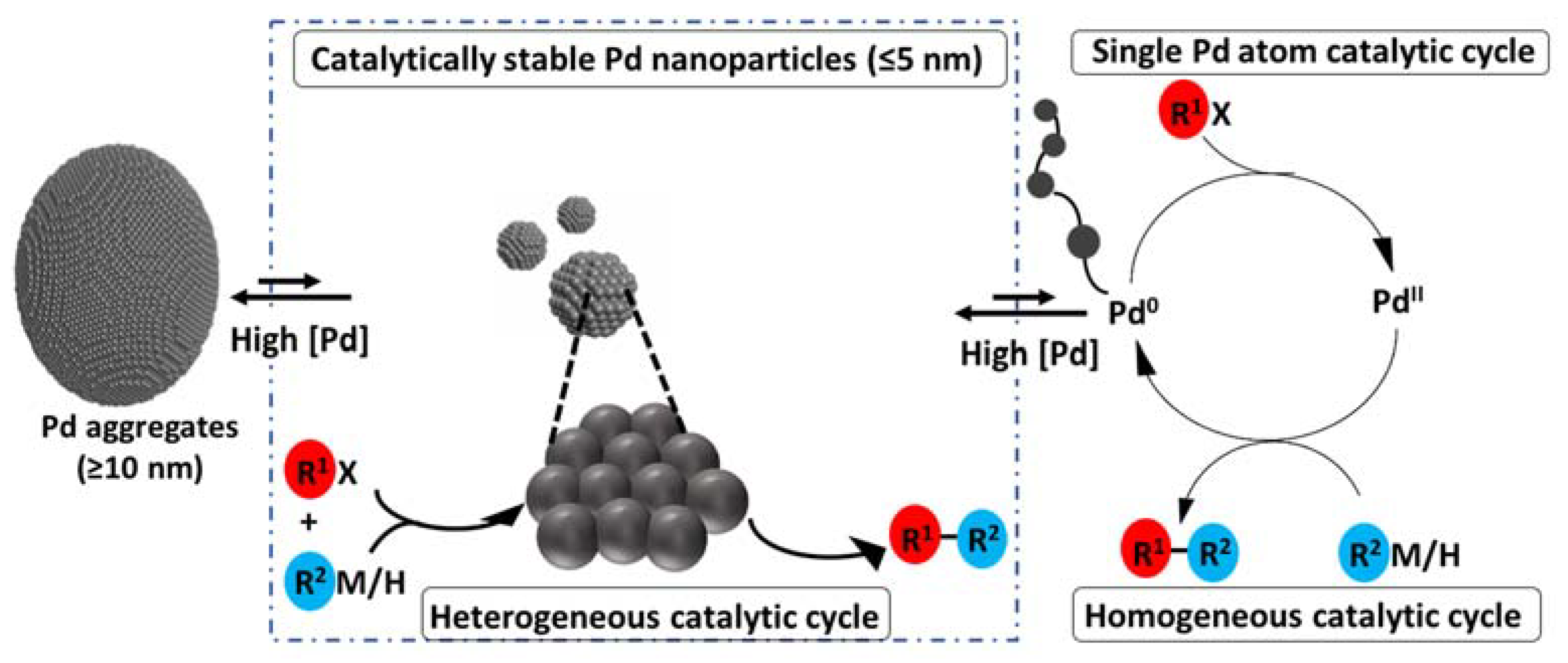
2. “Naked” Palladium Nanoparticles as Pre-Catalysts
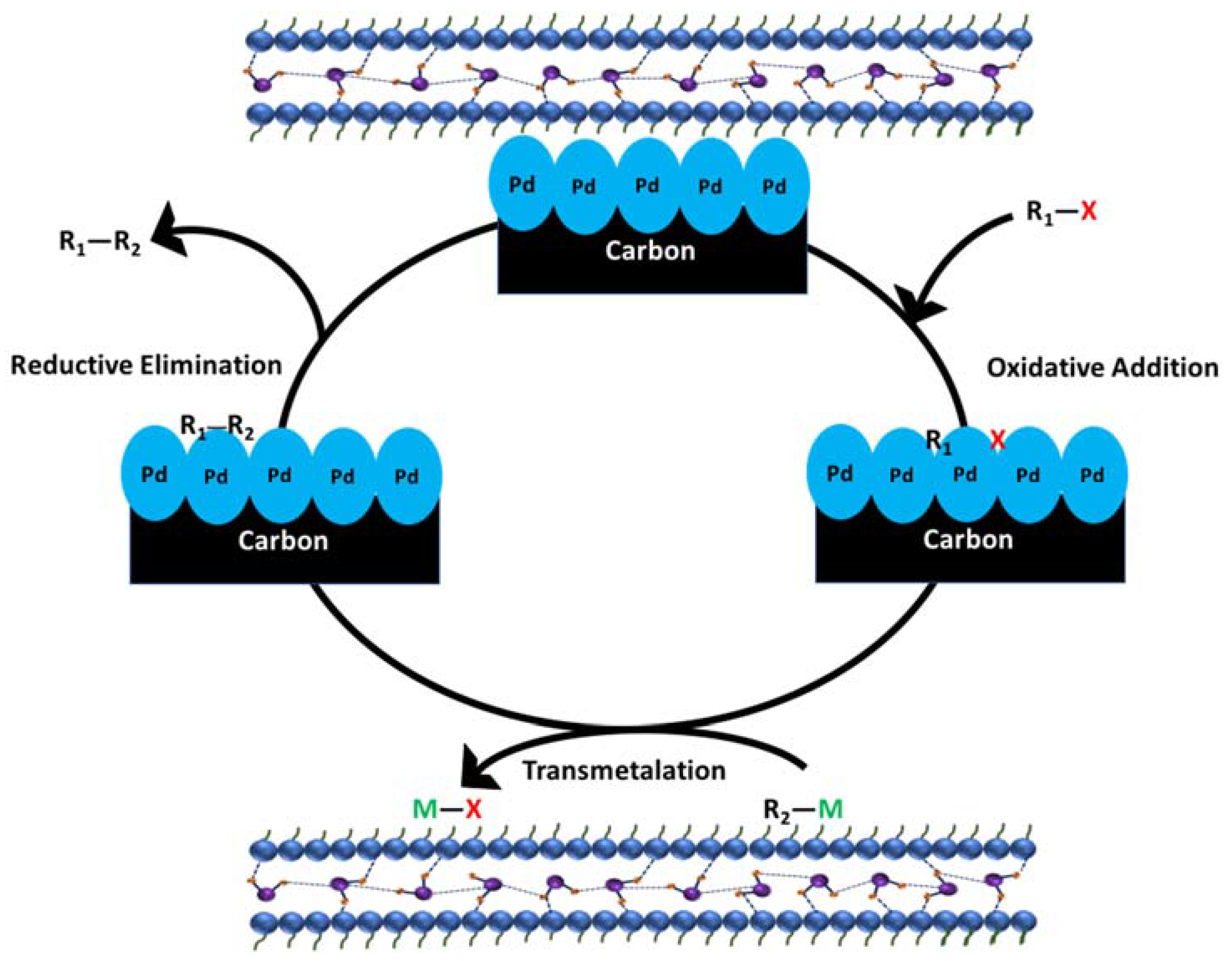
3. Palladium Nanoparticles on Carbonaceous Supports
4. Palladium Nanoparticles on Metal Oxide Supports
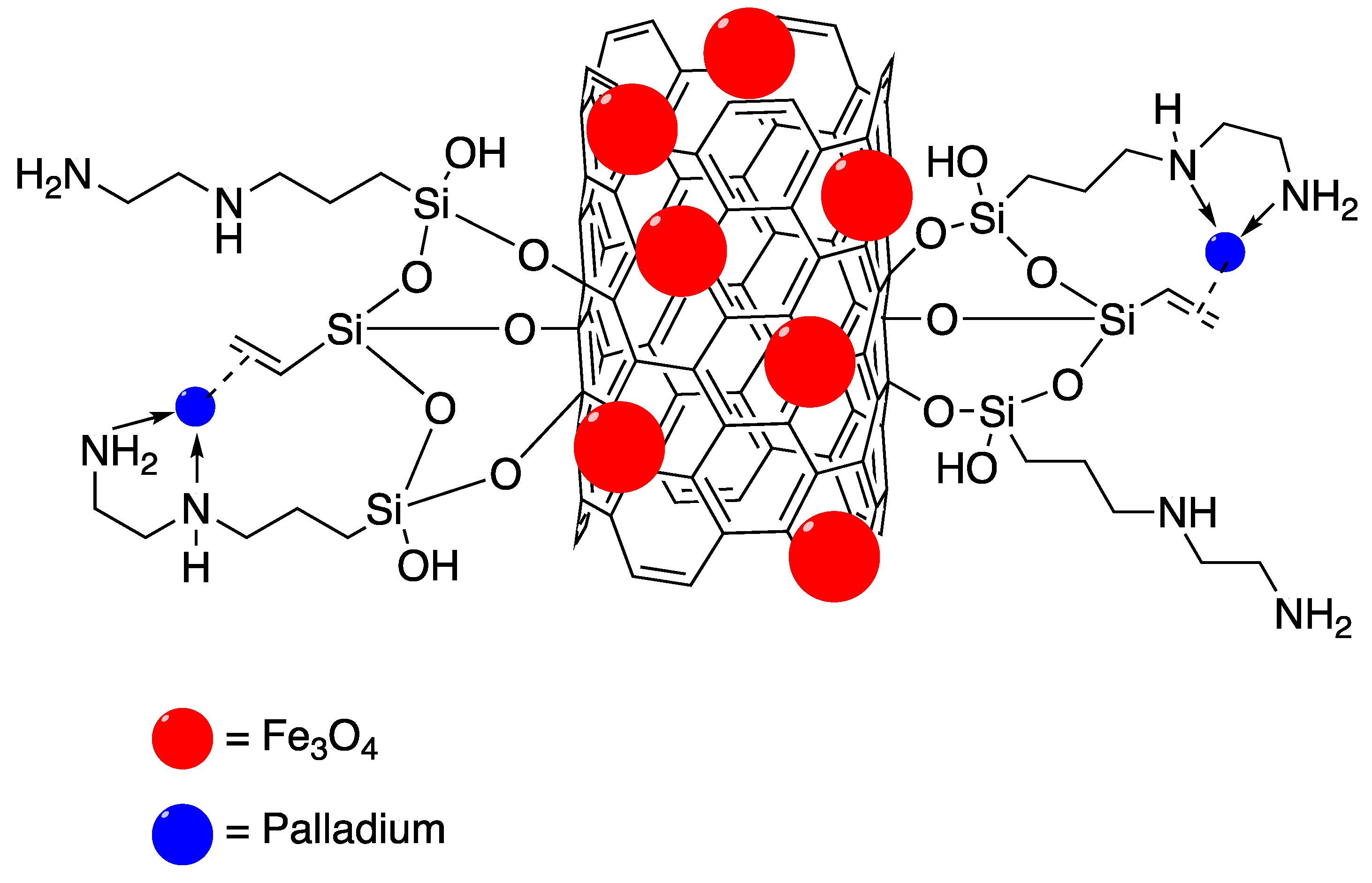
5. Palladium Nanoparticle Immobilised on Magnetic Supports
6. Polymer Supported Palladium Nanoparticles
7. Palladium Nanoparticles Immobilized on Hybrid Inorganic-Organic Material
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martinez, A.V.; Garcia, J.I.; Mayoral, J.A. An expedient synthesis of resveratrol through a highly recoverable palladium catalyst. Tetrahedron 2017, 73, 5581–5584. [Google Scholar] [CrossRef]
- Biajoli, A.F.P.; Schwalm, C.S.; Limberger, J.; Claudino, T.S.; Monteiro, A.L. Recent progress in the use of Pd-catalyzed C-C cross-coupling reactions in the synthesis of pharmaceutical compounds. J. Braz. Chem. Soc. 2014, 25, 2186–2214. [Google Scholar] [CrossRef]
- Barnard, B.C. Palladium-catalysed C-C coupling: Then and now. Platin. Met. Rev. 2008, 52, 38–45. [Google Scholar] [CrossRef]
- Pagliaro, M.; Pandarus, V.; Ciriminna, R.; Béland, F.; Demma Carà, P. Heterogeneous versus homogeneous palladium catalysts for cross-coupling reactions. ChemCatChem 2012, 4, 432–445. [Google Scholar] [CrossRef]
- Veerakumar, P.; Thanasekaran, P.; Lu, K.L.; Lin, K.C.; Rajagopal, S. Computational studies of versatile heterogeneous palladium-catalyzed Suzuki, Heck and Sonogashira coupling reactions. ACS Sustain. Chem. Eng. 2017, 5, 8475–8490. [Google Scholar] [CrossRef]
- Yin, L.; Liebscher, J. Carbon−carbon coupling reactions catalyzed by heterogeneous palladium catalysts. Chem. Rev. 2007, 107, 133–173. [Google Scholar] [CrossRef] [PubMed]
- Chinchilla, R.; Nájera, C. The Sonogashira Reaction: A Booming Methodology in Synthetic Organic Chemistry. Chem. Rev. 2007, 107, 874–922. [Google Scholar] [CrossRef] [PubMed]
- Phan, N.T.S.; Van Der Sluys, M.; Jones, C.W. On the nature of the active species in palladium catalyzed Mizoroki–Heck and Suzuki–Miyaura couplings—Homogeneous or heterogeneous catalysis, a critical review. Adv. Synth. Catal. 2006, 348, 609–679. [Google Scholar] [CrossRef]
- Ananikov, V.P.; Beletskaya, I.P. Toward the ideal catalyst: From atomic centers to a “cocktail” of catalysts. Organometallics 2012, 31, 1595–1604. [Google Scholar] [CrossRef]
- Amoroso, F.; Colussi, S.; Del Zotto, A.; Llorca, J.; Trovarelli, A. Room-temperature Suzuki–Miyaura reaction catalyzed by Pd supported on rare earth oxides: Influence of the point of zero charge on the catalytic activity. Catal. Lett. 2013, 143, 547–554. [Google Scholar] [CrossRef]
- Sanjaykumar, S.R.; Mukri, B.D.; Patil, S.; Madras, G.; Hegde, M.S. Ce0.98Pd0.02O2-δ: Recyclable, ligand free palladium(II) catalyst for Heck reaction. J. Chem. Sci. 2011, 123, 47–54. [Google Scholar] [CrossRef]
- Khalili, D.; Banazadeh, A.R.; Etemadi-Davan, E. Palladium stabilized by amino-vinyl silica functionalized magnetic carbon nanotube: Application in Suzuki-Miyaura and Heck-Mizoroki coupling reactions. Catal. Lett. 2017, 147, 2674–2687. [Google Scholar] [CrossRef]
- Veerakumar, P.; Thanasekaran, P.; Lu, K.L.; Liu, S.B.; Rajagopal, S. Functionalized silica matrices and palladium: A versatile heterogeneous catalyst for Suzuki, Heck, and Sonogashira reactions. ACS Sustain. Chem. Eng. 2017, 5, 6357–6376. [Google Scholar] [CrossRef]
- Chorkendorff, I.B.; Niemantsverdriet, J.W. Introduction to Catalysis in Concepts of Modern Catalysis and Kinetics; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003; pp. 1–22. [Google Scholar]
- Astakhov, A.V.; Khazipov, O.V.; Chernenko, A.Y.; Pasyukov, D.V.; Kashin, A.S.; Gordeev, E.G.; Khrustalev, V.N.; Chernyshev, V.M.; Ananikov, V.P. A new mode of operation of Pd-NHC systems studied in a catalytic Mizoroki–Heck reaction. Organometallics 2017, 36, 1981–1992. [Google Scholar] [CrossRef]
- Felpin, F.X.; Ayad, T.; Mitra, S. Pd/C: An old catalyst for new applications—Its use for the Suzuki-Miyaura reaction. Eur. J. Org. Chem. 2006, 2006, 2679–2690. [Google Scholar] [CrossRef]
- Felpin, F.X. Ten years of adventures with Pd/C catalysts: From reductive processes to coupling reactions. Synlett 2014, 25, 1055–1067. [Google Scholar] [CrossRef]
- Cini, E.; Petricci, E.; Taddei, M. Pd/C catalysis under microwave dielectric heating. Catalysts 2017, 7, 89. [Google Scholar] [CrossRef]
- Wang, Y.; Mao, Y.; Lin, Q.; Yang, J. Preparation of nano Pd/C catalyst and catalysis for Heck reaction. Huagong Xinxing Cailiao 2014, 42, 132–135. [Google Scholar]
- Zhou, X.Y.; Chen, X.; Wang, L.G. Recycled Pd/C catalyzed Heck reaction of 2-iodoanilines under ligand-free conditions. Synthesis 2017, 49, 5364–5370. [Google Scholar] [CrossRef]
- Seki, M. Practical synthesis of multifunctional compounds through Pd/C-catalyzed coupling reactions. Yuki Gosei Kagaku Kyokaishi 2006, 64, 853–866. [Google Scholar] [CrossRef]
- Schils, D.; Stappers, F.; Solberghe, G.; Van Heck, R.; Coppens, M.; Van den Heuvel, D.; Van der Donck, P.; Callewaert, T.; Meeussen, F.; De Bie, E.; et al. Ligandless Heck coupling between a halogenated aniline and acrylonitrile catalyzed by Pd/C: Development and optimization of an industrial-scale heck process for the production of a pharmaceutical intermediate. Org. Process Res. Dev. 2008, 12, 530–536. [Google Scholar] [CrossRef]
- Dighe, M.G.; Lonkar, S.L.; Degani, M.S. Mechanistic insights into palladium leaching in novel Pd/C-catalyzed boron-Heck reaction of arylboronic acid. Synlett 2013, 24, 347–350. [Google Scholar]
- Freundlich, J.S.; Landis, H.E. An expeditious aqueous Suzuki–Miyaura method for the arylation of bromophenols. Tetrahedron Lett. 2006, 47, 4275–4279. [Google Scholar] [CrossRef]
- Schmidt, B.; Riemer, M. Suzuki–Miyaura coupling of halophenols and phenol boronic acids: Systematic investigation of positional isomer effects and conclusions for the synthesis of phytoalexins from pyrinae. J. Org. Chem. 2014, 79, 4104–4118. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.Q.; Guo, S.R. Remarkably facile Heck reactions in aqueous two-phase system catalyzed by reusable Pd/C under ligand-free conditions. Synth. Commun. 2012, 42, 1059–1069. [Google Scholar] [CrossRef]
- Horikoshi, S.; Serpone, N. Role of microwaves in heterogeneous catalytic systems. Catal. Sci. Technol. 2014, 4, 1197–1210. [Google Scholar] [CrossRef]
- Hattori, T.; Tsubone, A.; Sawama, Y.; Monguchi, Y.; Sajiki, H. Palladium on carbon-catalyzed Suzuki-Miyaura coupling reaction using an efficient and continuous flow system. Catalysts 2015, 5, 18–25. [Google Scholar] [CrossRef]
- García-Suárez, E.J.; Lara, P.; García, A.B.; Ojeda, M.; Luque, R.; Philippot, K. Efficient and recyclable carbon-supported Pd nanocatalysts for the Suzuki–Miyaura reaction in aqueous-based media: Microwave vs conventional heating. Appl. Catal. A 2013, 468, 59–67. [Google Scholar] [CrossRef]
- Giacalone, F.; Campisciano, V.; Calabrese, C.; La Parola, V.; Liotta, L.F.; Aprile, C.; Gruttadauria, M. Supported C60-IL-PdNPs as extremely active nanocatalysts for C-C cross-coupling reactions. J. Mater. Chem. 2016, 4, 17193–17206. [Google Scholar] [CrossRef]
- Bowman, M.D.; Schmink, J.R.; McGowan, C.M.; Kormos, C.M.; Leadbeater, N.E. Scale-up of microwave-promoted reactions to the multigram level using a sealed-vessel microwave apparatus. Org. Process Res. Dev. 2008, 12, 1078–1088. [Google Scholar] [CrossRef]
- Lakshminarayana, B.; Mahendar, L.; Ghosal, P.; Satyanarayana, G.; Subrahmanyam, C. Nano-sized recyclable PdO supported carbon nanostructures for Heck reaction: Influence of carbon materials. ChemistrySelect 2017, 2, 2700–2707. [Google Scholar] [CrossRef]
- Jadhav, S.N.; Kumbhar, A.S.; Rode, C.V.; Salunkhe, R.S. Ligand-free Pd catalyzed cross-coupling reactions in an aqueous hydrotropic medium. Green Chem. 2016, 18, 1898–1911. [Google Scholar] [CrossRef]
- Labulo, A.H.; Martincigh, B.S.; Omondi, B.; Nyamori, V.O. Advances in carbon nanotubes as efficacious supports for palladium-catalysed carbon–carbon cross-coupling reactions. J. Mater. Sci. 2017, 52, 9225–9248. [Google Scholar] [CrossRef]
- Yang, L.; Cheng, G.; Xing, L.; Cheng, C.; Xia, L. Method for Activating Carbon Nanotube-Supported Palladium Nanoparticle Catalyst and Application Thereof. Patent CN106902817A, 30 June 2017. [Google Scholar]
- Hosseini-Sarvari, M.; Razmi, Z.; Doroodmand, M.M. Palladium supported on zinc oxide nanoparticles: Synthesis, characterization, and application as heterogeneous catalyst for Mizoroki–Heck and Sonogashira reactions under ligand-free and air atmosphere conditions. Appl. Catal. A 2014, 475, 477–486. [Google Scholar] [CrossRef]
- Del Zotto, A.; Colussi, S.; Trovarelli, A. Pd/REOs catalysts applied to the Suzuki-Miyaura coupling. A comparison of their catalytic performance and reusability. Inorg. Chim. Acta 2018, 470, 275–283. [Google Scholar] [CrossRef] [Green Version]
- Köhler, K.; Heidenreich, R.G.; Soomro, S.S.; Pröckl, S.S. Supported palladium catalysts for Suzuki reactions: Structure-property relationships, optimized reaction protocol and control of palladium leaching. Adv. Synth. Catal. 2008, 350, 2930–2936. [Google Scholar] [CrossRef]
- Gruttadauria, M.; Giacalone, F.; Noto, R. "Release and catch" catalytic systems. Green Chem. 2013, 15, 2608–2618. [Google Scholar] [CrossRef]
- Adair, J.H.; Suvaci, E.; Sindel, J. Surface and Colloid Chemistry in Encyclopedia of Materials: Science and Technology, 2nd ed.; Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Veyssière, P., Eds.; Elsevier: Oxford, UK, 2001; pp. 1–10. [Google Scholar]
- Kosmulski, M. Compilation of PZCs/IEPs. In Surface Charging and Point of Zero Charge; Hubbard, A.T., Ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2009; pp. 101–524. [Google Scholar]
- Del Zotto, A.; Zuccaccia, D. Metallic palladium, PdO, and palladium supported on metal oxides for the Suzuki-Miyaura cross-coupling reaction: A unified view of the process of formation of the catalytically active species in solution. Catal. Sci. Technol. 2017, 7, 3934–3951. [Google Scholar] [CrossRef]
- Bera, P.; Hegde, M.S. Noble metal ions in CeO2 and TiO2: Synthesis, structure and catalytic properties. RSC Adv. 2015, 5, 94949–94979. [Google Scholar] [CrossRef]
- Cwele, T.; Mahadevaiah, N.; Singh, S.; Friedrich, H.B. Effect of Cu additives on the performance of a cobalt substituted ceria (Ce0.90Co0.10O2-δ) catalyst in total and preferential CO oxidation. Appl. Catal. B 2016, 182, 1–14. [Google Scholar] [CrossRef]
- Cwele, T.; Mahadevaiah, N.; Singh, S.; Friedrich, H.B.; Yadav, A.K.; Jha, S.N.; Bhattacharyya, D.; Sahoo, N.K. CO oxidation activity enhancement of Ce0.95Cu0.05O2-δ induced by Pd co-substitution. Catal. Sci. Technol. 2016, 6, 8104–8116. [Google Scholar] [CrossRef]
- Gupta, A.; Waghmare, U.V.; Hegde, M.S. Correlation of oxygen storage capacity and structural distortion in transition-metal, noble-metal, and rare-earth-ion-substituted CeO2 from first principles calculation. Chem. Mater. 2010, 22, 5184–5198. [Google Scholar] [CrossRef]
- Deshpande, P.A.; Hegde, M.S.; Madras, G. A mechanistic model for the water-gas shift reaction over noble metal substituted ceria. AIChE J. 2010, 56, 1315–1324. [Google Scholar] [CrossRef]
- Hegde, M.S.; Madras, G.; Patil, K.C. Noble metal ionic catalysts. Acc. Chem. Res. 2009, 42, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Mpungose, P.P.; Sehloko, N.I.; Dasireddy, V.D.B.C.; Mahadevaiah, N.; Maguire, G.E.; Friedrich, H.B. Pd0.09Ce0.91O2-δ: A sustainable ionic solid-solution precatalyst for heterogeneous, ligand free Heck coupling reactions. Mol. Catal. 2017, 443, 60–68. [Google Scholar] [CrossRef]
- Mpungose, P.P.; Sehloko, N.I.; Maguire, G.E.M.; Friedrich, H.B. PdCuCeO-TPAB: A new catalytic system for quasi-heterogeneous Suzuki-Miyaura cross-coupling reactions under ligand-free conditions in water. New J. Chem. 2017, 41, 13560–13566. [Google Scholar] [CrossRef]
- Mpungose, P.P.; Sehloko, N.I.; Cwele, T.; Maguire, G.E.M.; Friedrich, H.B. Pd0.02Ce0.98O2-δ: A copper- and ligand-free quasi-heterogeneous catalyst for aquacatalytic Sonogashira cross-coupling reaction. J. South. Afr. Inst. Min. Metall. 2017, 117, 955–962. [Google Scholar] [CrossRef]
- Burange, A.S.; Shukla, R.; Tyagi, A.K.; Gopinath, C.S. Palladium supported on fluorite structured redox CeZrO4-δ for heterogeneous suzuki coupling in water: A green protocol. ChemistrySelect 2016, 1, 2673–2681. [Google Scholar] [CrossRef]
- Lichtenegger, G.J.; Maier, M.; Hackl, M.; Khinast, J.G.; Gössler, W.; Griesser, T.; Kumar, V.S.P.; Gruber-Woelfler, H.; Deshpande, P.A. Suzuki-Miyaura coupling reactions using novel metal oxide supported ionic palladium catalysts. J. Mol. Catal. A Chem. 2017, 426, 39–51. [Google Scholar] [CrossRef]
- Karimi, B.; Mansouri, F.; Mirzaei, H.M. Recent applications of magnetically recoverable nanocatalysts in C-C and C-X coupling reactions. ChemCatChem 2015, 7, 1736–1789. [Google Scholar] [CrossRef]
- Feng, C.; Liu, J.; Gui, J.; Liu, L. Application of magnetic nanoparticles supported Pd catalysts in C-C bond formation reactions. Yingyong Huaxue 2015, 32, 19–26. [Google Scholar]
- Cheng, T.; Zhang, D.; Li, H.; Liu, G. Magnetically recoverable nanoparticles as efficient catalysts for organic transformations in aqueous medium. Green Chem. 2014, 16, 3401–3427. [Google Scholar] [CrossRef]
- Baig, R.B.N.; Varma, R.S. Magnetically retrievable catalysts for organic synthesis. Chem. Commun. 2013, 49, 752–770. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.M.; Garcia, M.A.S.; Vono, L.L.R. Recent advances in the development of magnetically recoverable metal nanoparticle catalysts. J. Braz. Chem. Soc. 2012, 23, 1959–1971. [Google Scholar] [CrossRef]
- Xiao, C.; Yan, N.; Kou, Y. Quasi-homogeneous catalysis: Towards green and efficiency. Cuihua Xuebao 2009, 30, 753–764. [Google Scholar]
- Mulahmetovic, E.; Hargaden, G.C. Recent advances in the development of magnetic catalysts for the Suzuki reaction. Rev. J. Chem. 2017, 7, 373–398. [Google Scholar] [CrossRef]
- Elhampour, A.; Nemati, F. Palladium nanoparticles supported on modified hollow-Fe3O4@TiO2: Catalytic activity in Heck and Sonogashira cross coupling reactions. Org. Prep. Proced. Int. 2017, 49, 443–458. [Google Scholar] [CrossRef]
- Hudson, R.; Feng, Y.; Varma, R.S.; Moores, A. Bare magnetic nanoparticles: Sustainable synthesis and applications in catalytic organic transformations. Green Chem. 2014, 16, 4493–4505. [Google Scholar] [CrossRef]
- Rossi, L.M.; Costa, N.J.S.; Silva, F.P.; Wojcieszak, R. Magnetic nanomaterials in catalysis: Advanced catalysts for magnetic separation and beyond. Green Chem. 2014, 16, 2906–2933. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Mohammad Sajadi, S.; Rostami-Vartooni, A.; Khalaj, M. Green synthesis of Pd/Fe3O4 nanoparticles using Euphorbia condylocarpa M. bieb root extract and their catalytic applications as magnetically recoverable and stable recyclable catalysts for the phosphine-free Sonogashira and Suzuki coupling reactions. J. Mol. Catal. A Chem. 2015, 396, 31–39. [Google Scholar] [CrossRef]
- Xie, C.X.; Liu, Y.; Yu, S.T. Research progress of magnetic catalysts. Qingdao Keji Daxue Xuebao Ziran Kexueban 2015, 36, 237–244. [Google Scholar]
- Kumar, B.S.; Anbarasan, R.; Amali, A.J.; Pitchumani, K. Isolable C@Fe3O4 nanospheres supported cubical Pd nanoparticles as reusable catalysts for Stille and Mizoroki-Heck coupling reactions. Tetrahedron Lett. 2017, 58, 3276–3282. [Google Scholar] [CrossRef]
- Heidari, F.; Hekmati, M.; Veisi, H. Magnetically separable and recyclable Fe3O4@SiO2/isoniazide/Pd nanocatalyst for highly efficient synthesis of biaryls by Suzuki coupling reactions. J. Colloid Interface Sci. 2017, 501, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Collinson, J.M.; Wilton-Ely, J.D.E.T.; Diez-Gonzalez, S. Functionalised [(NHC)Pd(allyl)Cl] complexes: Synthesis, immobilisation and application in cross-coupling and dehalogenation reactions. Catal. Commun. 2016, 87, 78–81. [Google Scholar] [CrossRef]
- Fareghi-Alamdari, R.; Saeedi, M.S.; Panahi, F. New bis(N-heterocyclic carbene) palladium complex immobilized on magnetic nanoparticles: As a magnetic reusable catalyst in Suzuki-Miyaura cross coupling reaction. Appl. Organomet. Chem. 2017, 31, e3870. [Google Scholar] [CrossRef]
- Fan, H.; Qi, Z.; Sui, D.; Mao, F.; Chen, R.; Huang, J. Palladium nanoparticles in cross-linked polyaniline as highly efficient catalysts for Suzuki-Miyaura reactions. Chin. J. Catal. 2017, 38, 589–596. [Google Scholar] [CrossRef]
- Taher, A.; Choudhary, M.; Nandi, D.; Siwal, S.; Mallick, K. Polymer-supported palladium: A hybrid system for multifunctional catalytic application. Appl. Organomet. Chem. 2017, 32, e3898. [Google Scholar] [CrossRef] [Green Version]
- Ashouri, F.; Zare, M.; Bagherzadeh, M. The effect of framework functionality on the catalytic activation of supported Pd nanoparticles in the Mizoroki-Heck coupling reaction. C. R. Chim. 2017, 20, 107–115. [Google Scholar] [CrossRef]
- Wang, C.A.; Li, Y.W.; Hou, X.M.; Han, Y.F.; Nie, K.; Zhang, J.P. N-Heterocyclic carbene-based microporous organic polymer supported palladium catalyst for carbon-carbon coupling reaction. ChemistrySelect 2016, 1, 1371–1376. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, X.; Lu, M. Novel polymer supported iminopyridylphosphine palladium (II) complexes: An efficient catalyst for Suzuki-Miyaura and Heck cross-coupling reactions. J. Organomet. Chem. 2014, 768, 23–27. [Google Scholar] [CrossRef]
- Kodicherla, B.; Perumgani, C.P.; Keesara, S.; Mandapati, M.R. Polystyrene-supported palladium(II) N,N-dimethylethylenediamine complex: A recyclable catalyst for Suzuki-Miyaura cross-coupling reactions in water. Inorg. Chim. Acta 2014, 423, 95–100. [Google Scholar] [CrossRef]
- Aravinda Reddy, P.; Babul Reddy, A.; Ramachandra Reddy, G.; Subbarami Reddy, N. Suzuki-Miyaura cross-coupling reaction of naphthyl triflate with indole boronic acids catalyzed by a recyclable polymer-supported n-heterocyclic carbene-palladium complex catalyst: Synthesis of naphthalene-linked bis-heterocycles. J. Heterocycl. Chem. 2013, 50, 1451–1456. [Google Scholar] [CrossRef]
- Tamami, B.; Dodeji, F.N. Synthesis and application of modified polystyrene-supported palladium nanoparticles as a new heterogeneous catalyst for Heck and Suzuki cross-coupling reactions. J. Iran. Chem. Soc. 2012, 9, 841–850. [Google Scholar] [CrossRef]
- Nemygina, N.A.; Nikoshvili, L.Z.; Bykov, A.V.; Sidorov, A.I.; Molchanov, V.P.; Sulman, M.G.; Tiamina, I.Y.; Stein, B.D.; Matveeva, V.G.; Sulman, E.M.; et al. Catalysts of Suzuki cross-coupling based on functionalized hyper-cross-linked polystyrene: Influence of precursor nature. Org. Process Res. Dev. 2016, 20, 1453–1460. [Google Scholar] [CrossRef]
- Li, Y.; Xu, L.; Xu, B.; Mao, Z.; Xu, H.; Zhong, Y.; Zhang, L.; Wang, B.; Sui, X. Cellulose sponge supported palladium nanoparticles as recyclable cross-coupling catalysts. ACS Appl. Mater. Interfaces 2017, 9, 17155–17162. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.N.R.; Cheetham, A.K.; Thirumurugan, A. Hybrid inorganic–organic materials: A new family in condensed matter physics. J. Phys. Condens. Matter. 2008, 20, 1–21. [Google Scholar]
- Wang, Y.; Dou, L.; Zhang, H. Nanosheet array-like palladium-catalysts Pdx/rGO@CoAl-LDH via lattice atomic-confined in situ reduction for highly efficient heck coupling reaction. ACS Appl. Mater. Interfaces 2017, 9, 38784–38795. [Google Scholar] [CrossRef] [PubMed]
- Arsalani, N.; Akbari, A.; Amini, M.; Jabbari, E.; Gautam, S.; Chae, K.H. POSS-Based Covalent networks: Supporting and stabilizing pd for heck reaction in aqueous media. Catal. Lett. 2017, 147, 1086–1094. [Google Scholar] [CrossRef]
- Woo, H.; Lee, K.; Park, K.H. Optimized dispersion and stability of hybrid Fe3O4/Pd catalysts in water for Suzuki coupling reactions. Impact of organic capping agents. ChemCatChem 2014, 6, 1635–1640. [Google Scholar] [CrossRef]
- Jing, X.; Sun, F.; Ren, H.; Tian, Y.; Guo, M.; Li, L.; Zhu, G. Targeted synthesis of micro-mesoporous hybrid material derived from octaphenylsilsesquioxane building units. Microporous Mesoporous Mater. 2013, 165, 92–98. [Google Scholar] [CrossRef]
- Qu, K.; Wu, L.; Ren, J.; Qu, X. Natural DNA-modified graphene/Pd nanoparticles as highly active catalyst for formic acid electro-oxidation and for the Suzuki reaction. ACS Appl. Mater. Interfaces 2012, 4, 5001–5009. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ma, Z.; Qing, Y.; Xie, G.; Gao, J.; Zhang, L.; Gao, J.; Du, L. A periodic mesoporous hybrid material with a built-in palladium complex: An efficient catalyst for the Suzuki coupling and alcohol oxidation. Appl. Catal. A 2010, 382, 312–321. [Google Scholar] [CrossRef]
- Wang, B.; Gu, Y.L.; Yang, L.M.; Suo, J.S. Organic/inorganic hybrid materials-supported metal complex catalysts: New applications of sol-gel technology. Fenzi Cuihua 2003, 17, 468–480. [Google Scholar]
- Shaikh, M.N.; Abdul Aziz, M.; Helal, A.; Kalanthoden, A.N.; Yamani, Z.H. PdNPs@ZIF-8 micro-nanostructured catalyst of regioselective Mizoriki-Heck olefination. ChemistrySelect 2017, 2, 9052–9057. [Google Scholar] [CrossRef]
- Kozell, V.; Giannoni, T.; Nocchetti, M.; Vivani, R.; Piermatti, O.; Vaccaro, L. Immobilized palladium nanoparticles on zirconium carboxy-aminophosphonates nanosheets as an efficient recoverable heterogeneous catalyst for suzuki–miyaura and heck coupling. Catalysts 2017, 7, 186. [Google Scholar] [CrossRef]
- Omar, S.; Abu-Reziq, R. Palladium nanoparticles supported on magnetic organic-silica hybrid nanoparticles. J. Phys. Chem. C 2014, 118, 30045–30056. [Google Scholar] [CrossRef]
- Lin, B.; Liu, X.; Zhang, Z.; Chen, Y.; Liao, X.; Li, Y. Pd(0)-CMC@Ce(OH)4 organic/inorganic hybrid as highly active catalyst for the Suzuki-Miyaura reaction. J. Colloid Interface Sci. 2017, 497, 134–143. [Google Scholar] [CrossRef] [PubMed]






















| Metal | Concentration (ppm) | |
|---|---|---|
| Oral | Parenteral | |
| Pd, Pt, Ir, Rh, Ru, Os | 5 | 0.5 |
| Mo, V, Ni, Cr | 10 | 1.0 |
| Cu, Mn | 15 | 1.5 |
| Zn, Fe | 20 | 2.0 |
 | |||
|---|---|---|---|
| Catalyst a | Reaction Time (min) | Yield (%) b | TOF (h−1) |
| PdO/SWCNT | 20 | 72 | 939 |
| PdO/MWCNT | 15 | 63 | 1080 |
| PdO/CNF | 40 | 38 | 274 |
| PdO/GO | 5 | 80 | 4144 |
| PdO/RGO | 90 | 68 | 194 |
 | ||||||
|---|---|---|---|---|---|---|
| Compound (Pd/MxOy) a | Pd Loading (wt %) | Surface Area (m2/g) | Pore Volume (cm3/g) | PZC (pH) | Time (min) | TOF (h−1) |
| Pd/La2O3 | 1.76 | 16.6 | 0.24 | 8.8 | 13 | 9130 |
| Pd/CeO2 | 1.93 | 31.5 | 0.18 | 6.7 | 260 | 450 |
| Pd/Pr6O11 | 1.93 | 22.4 | 0.36 | 7.8 | 17 | 6980 |
| Pd/Sm2O3 | 1.84 | 11.9 | 0.21 | 7.4 | 21 | 5650 |
| Pd/Gd2O3 | 1.99 | 10.2 | 0.09 | 7.5 | 20 | 5940 |
| Reaction Conditions | |||
|---|---|---|---|
| Solvent | Base | Temperature (°C) | |
| Collinson et al. [68] a | Isopropanol | NaOH | 60 |
| Fareghi-Alamdari et al. [69] b | DMF/H2O | K2CO3 | 80 |
 | ||
|---|---|---|
| Entry a | Yield (%) b | Pd Leaching (ppm) |
| Run 1 | 98 | 5 |
| Run 2 | 98 | 3 |
| Run 3 | 98 | 3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mpungose, P.P.; Vundla, Z.P.; Maguire, G.E.M.; Friedrich, H.B. The Current Status of Heterogeneous Palladium Catalysed Heck and Suzuki Cross-Coupling Reactions. Molecules 2018, 23, 1676. https://doi.org/10.3390/molecules23071676
Mpungose PP, Vundla ZP, Maguire GEM, Friedrich HB. The Current Status of Heterogeneous Palladium Catalysed Heck and Suzuki Cross-Coupling Reactions. Molecules. 2018; 23(7):1676. https://doi.org/10.3390/molecules23071676
Chicago/Turabian StyleMpungose, Philani P., Zanele P. Vundla, Glenn E. M. Maguire, and Holger B. Friedrich. 2018. "The Current Status of Heterogeneous Palladium Catalysed Heck and Suzuki Cross-Coupling Reactions" Molecules 23, no. 7: 1676. https://doi.org/10.3390/molecules23071676
APA StyleMpungose, P. P., Vundla, Z. P., Maguire, G. E. M., & Friedrich, H. B. (2018). The Current Status of Heterogeneous Palladium Catalysed Heck and Suzuki Cross-Coupling Reactions. Molecules, 23(7), 1676. https://doi.org/10.3390/molecules23071676





