Photonic Band Gap and Bactericide Performance of Amorphous Sol-Gel Titania: An Alternative to Crystalline TiO2
Abstract
:1. Introduction
1.1. Titania Structure
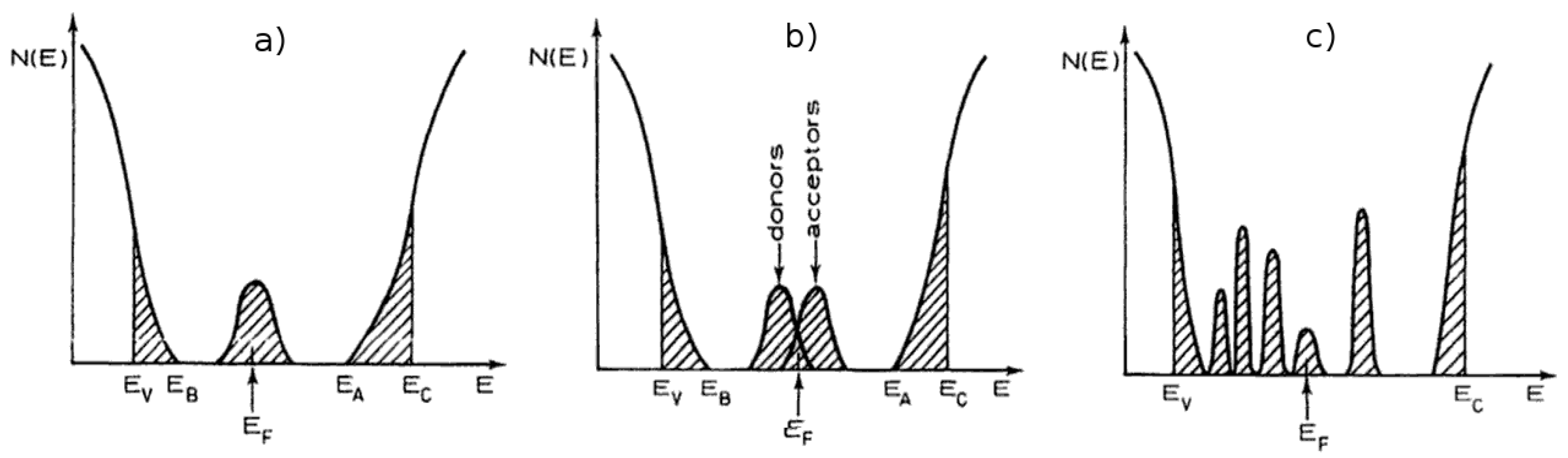
1.2. Titania Band Gap
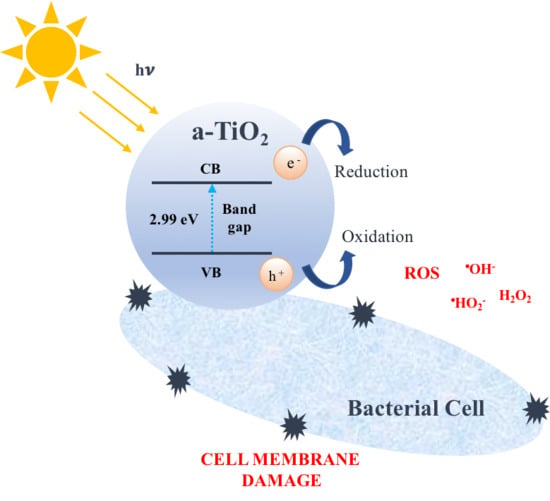
1.3. Titania Photocatalytic Activity
- (a)
- (b)
- Trapping path: responsible for about ~8% of the events, in which one or both charges migrate to defects in the crystalline network [5,6]. Trapping makes the material a somewhat weaker reductant or oxidant, although it preserves the charge separation, thus allowing for future photocatalysis reactions.
- (c)
- Reactional path: the fraction of photo-generated charges promoting chemical reactions with surface adsorbed molecules is considered to be responsible for only 1–2% of the events [5,6]. One or both charge carriers (e−, h+), after migrating to the surface, react with adsorbed molecules. As each redox reaction involves only one electron, the even number of valence electrons usually found in molecules will be upset, and radicals (with one unpaired electron) will form.
1.4. Titania Doping
1.5. Titania Case Studies
2. Results and Discussion
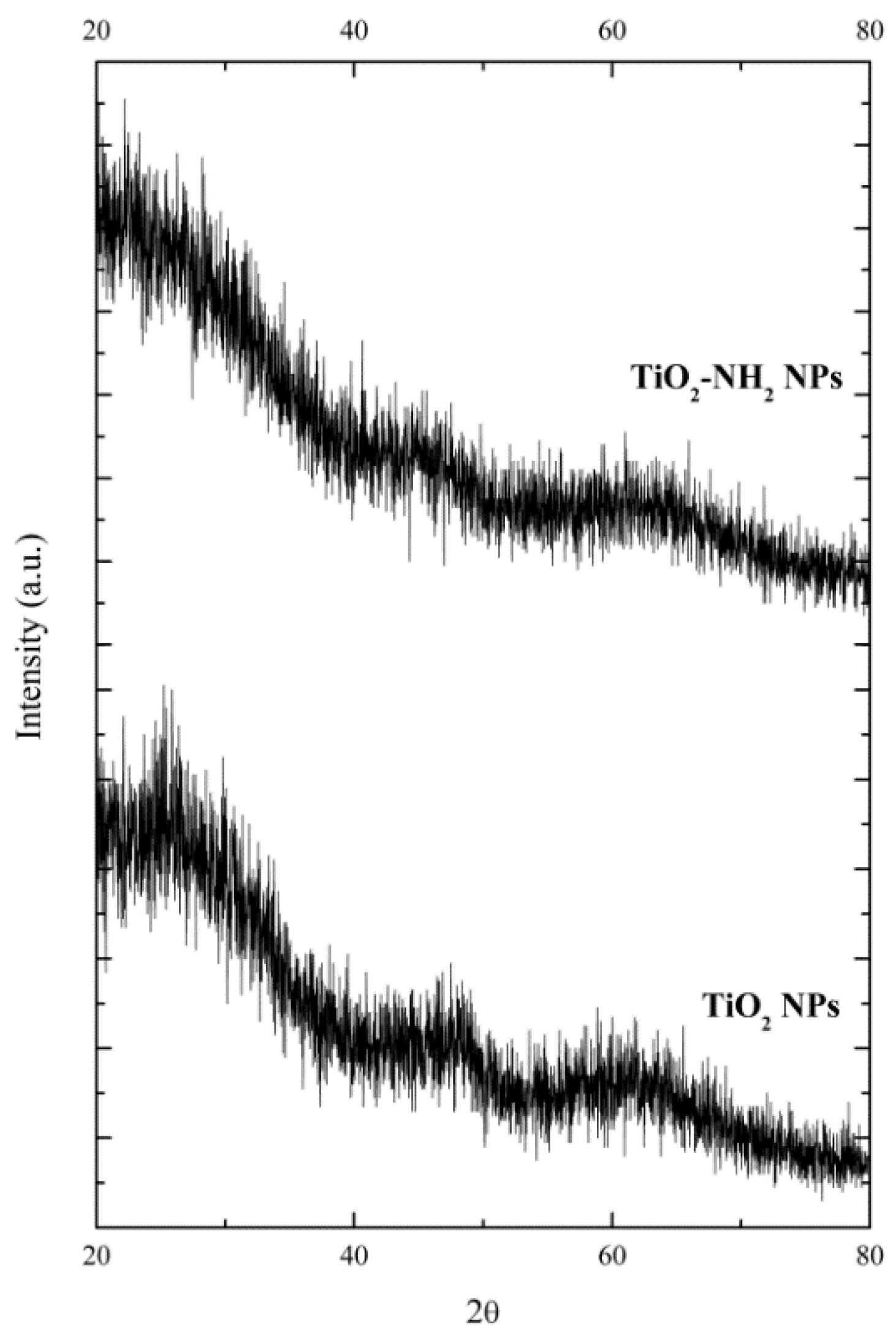
2.1. Case Study 1. Band Gap of Amorphous TiO2−xNx (x = 0; 0.75)
2.2. Case Study 2. Bactericide Performance of Amorphous TiO2-Based NPs
3. Conclusions
4. Materials and Methods
4.1. Case Study 1
4.2. Case Study 2
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catal. B 2015, 176–177, 396–428. [Google Scholar] [CrossRef]
- Yan, X.; Li, Y.; Xia, T. Black Titanium Dioxide Nanomaterials in Photocatalysis. Int. J. Photoenergy 2017, 2017. [Google Scholar] [CrossRef]
- Fagan, R.; McCormack, D.E.; Dionysiou, D.D.; Pillai, S.C. A review of solar and visible light active TiO2 photocatalysis for treating bacteria, cyanotoxins and contaminants of emerging concern. Mater. Sci. Semicond. Process. 2016, 42, 2–14. [Google Scholar] [CrossRef]
- Etacheri, V.; Valentin, C.D.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatlysts: Advances in theory and experiments. J. Photochem. Photobiol. C 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Dahl, M.; Liu, Y.; Yin, Y. Composite Titanium Dioxide Nanomaterials. Chem. Rev. 2014, 114, 9853–9889. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; Nolan, N.; Pillai, S.; Seery, M.; Falaras, P. A review on the visible light active titanium dioxide photocatalysis for environmental applications. Appl. Catal. B 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 phtocatalysis: Design and applications. J. Photochem. Photobiol. C 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Arora, H.; Doty, C.; Yuan, Y.; Boyle, J.; Petras, K.; Rabatic, B.; Paunesku, T.; Woloschak, G. Titanium Dioxide Nanocomposites. In Nanomaterials for Life Sciences; Kumar, C.S.S.R., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, German, 2010; Volume 8, ISBN 978-3-527-32168-1. [Google Scholar]
- Thirugnanasambandan, T. Review on Titania Nanopowder—Processing and Applications. Condens. Matter 2017, 17, 981. [Google Scholar]
- Zou, J.A.; Gao, J.C.; Xie, F.Y. An amorphous TiO2 sol sensitized with H2O2 with the enhancement of photocatalytic activity. J. Alloys Compd. 2010, 497, 420–427. [Google Scholar] [CrossRef]
- Randorn, C.; Irvine, J.T.S.; Robertson, P. Synthesis of Visible-Light-Activated Yellow Amorphous Photocatalyst. Int. J. Photoenergy 2008, 2008. [Google Scholar] [CrossRef]
- Han, C.H.; Lee, H.S.; Lee, K.W.; Han, S.D.; Singh, I. Synthesis of amorphous Er3+-Yb3+ co-doped TiO2 and its application as a scattering layer for dye-sensitized solar cells. Bull. Korean Chem. Soc. 2009, 30, 219–223. [Google Scholar] [CrossRef]
- Usui, H.; Miyamoto, O.; Nomiyama, T.; Horie, Y.; Miyazaki, T. Photo-rechargeability of TiO2 film electrodes prepared by pulsed laser deposition. Sol. Energy Mater. Sol. Cells 2005, 86, 123–134. [Google Scholar] [CrossRef]
- Scarel, S.; Aita, C.R.; Tanaka, H.; Hisano, K. Far-infrared spectra of amorphous titanium dioxide films. J. Non-Cryst. Solids 2002, 303, 50–53. [Google Scholar] [CrossRef]
- Jeong, H.Y.; Lee, J.Y.; Choi, S.Y. Interface-Engineered Amorphous TiO2-Based Resistive Memory Devices. Adv. Funct. Mater. 2010, 20, 3912–3917. [Google Scholar] [CrossRef]
- Gao, Y.; Masuda, Y.; Koumoto, K. Light-Excited Superhydrophilicity of Amorphous TiO2 Thin Films Deposited in an Aqueous Peroxotitanate Solution. Langmuir 2004, 20, 3188–3194. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-M.; Lee, J.-K.; Jun, H.-W.; Kim, G.-R.; Choe, Y. Preparation of super-hydrophilic amorphous titanium dioxide thin film via PECVD process and its application to dehumidifying heat exchangers. J. Ind. Eng. Chem. 2009, 15, 202–206. [Google Scholar] [CrossRef]
- Sabbah, H. Amorphous titanium dioxide ultra-thin films for self-cleaning surfaces. Mater. Express 2013, 3, 171–175. [Google Scholar] [CrossRef]
- Kanna, M.; Wongnawa, S.; Buddee, S.; Dilokkhunakul, K.; Pinpithak, P. Amorphous titanium dioxide: A recyclable dye remover for water treatment. J. Sol-Gel Sci. Technol. 2010, 53, 162–170. [Google Scholar] [CrossRef]
- Xiong, H.; Slater, M.D.; Balasubramaniam, M.; Johnson, C.S.; Rajh, T. Amorphous TiO2 Nanotube Anode for rechargeable sodium ion batteries. J. Phys. Chem. Lett. 2011, 2, 2560–2565. [Google Scholar] [CrossRef]
- Lu, H.F.; Li, F.; Liu, G.; Chen, Z.-G.; Wang, D.-W.; Fang, H.-T.; Lu, G.Q.; Jiang, Z.H.; Cheng, H.-M. Amorphous TiO2 nanotube arrays for low-temperature oxygen sensors. Nanotechnology 2008, 19, 405504. [Google Scholar] [CrossRef] [PubMed]
- Omurzak, E.; Mashimo, T.; Iwamoto, C.; Matsumoto, Y.; Sulaimankulova, S. Synthesis of blue amorphous TiO2 and Ti(n)O(2n-1) by the impulse plasma in liquid. J. Nanosci. Nanotechnol. 2009, 9, 6372–6375. [Google Scholar] [CrossRef] [PubMed]
- Vargas, M.A.; Rodriguez-Paez, J.E. Amorphous TiO2 nanoparticles: Synthesis and antibacterial capacity. J. Non-Cryst. Solids 2017, 459, 192–205. [Google Scholar] [CrossRef]
- Kulkami, M.; Mazare, A.; Gongadze, E.; Perutkova, S.; Kralj-Iglic, V.; Milosev, I.; Schmuki, P.; Iglic, A.; Mozetic, M. Titanium nanostructures for biomedical applications. Nanotechnology 2015, 26, 062002. [Google Scholar] [CrossRef] [Green Version]
- Murray, J.L.; Wriedt, H.A. The O-Ti (Oxygen-Titanium) System. J. Phase Equilib. 1987, 8, 148–165. [Google Scholar] [CrossRef]
- Asahi, R.; Taga, Y.; Mannstadt, W.; Freeman, A. Electronic and Optical Properties of Anatase TiO2. J. Phys. Rev. B 2000, 61, 7459. [Google Scholar] [CrossRef]
- Ren, R.; Yang, Z.; Shaw, L.L. Polymorphic transformation and powder characteristics of TiO2 during high energy milling. J. Mater. Sci. 2000, 35, 6015–6026. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J.F. Thermodynamic analysis of phase stability of nanocrystalline titania. J. Mater. Chem. 1998, 8, 2073–2076. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Assadi, M.H.N.; Li, S.; Yu, A.; Sorrell, C.C. Ab initio study of phase stability in doped TiO2. Comput. Mech. 2012, 50, 185–194. [Google Scholar] [CrossRef]
- Smith, S.J.; Stevens, R.; Liu, S.; Li, G.; Navrotsky, A.; Boerio-Goates, J.; Woodfield, B.F. Heat capacities and thermodynamic functions of TiO2 anatase and rutile: Analysis of phase stability. Am. Miner. 2009, 94, 236–243. [Google Scholar] [CrossRef]
- Czanderna, A.W.; Rao, C.N.R.; Honig, J.M. The Anatase-Rutile Transition. Trans. Faraday Soc. 1958, 54, 1069–1073. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J.F. Understanding Polymorphic Phase Transformation Behavior during Growth of Nanocrystalline Aggregates: Insights from TiO2. J. Phys. Chem. B 2000, 104, 3481–3487. [Google Scholar] [CrossRef]
- Ranade, M.R.; Navrotsky, A.; Zhang, H.Z.; Banfield, J.F.; Elder, S.H.; Zaban, A.; Borse, P.H.; Kulkarmi, S.K.; Doran, G.S.; Whitfield, H.J. Energetics of nanocrystalline TiO2. Proc. Natl. Acad. Sci. USA 2002, 99, 6476–6481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Z.; Chen, B.; Banfield, J.F. Atomic structure of nanometer-sized amorphous TiO2. Phys. Rev. B 2008, 78, 214106. [Google Scholar] [CrossRef]
- Hoang, V.V. Atomic mechanism of vitrification process in simple monatomic nanoparticles. Eur. Phys. J. D 2011, 61, 627–635. [Google Scholar] [CrossRef]
- Hoang, V.V.; Ganguli, D. Amorphous nanoparticles—Experiments and computer simulations. Phys. Rep. 2012, 518, 81–140. [Google Scholar] [CrossRef]
- Hoang, V.V. Structural properties of simulated liquid and amorphous TiO2. Phys. Status Solid B 2007, 244, 1280–1287. [Google Scholar] [CrossRef]
- Hoang, V.V.; Zung, H.; Trong, N.H.B. Structural properties of amorphous TiO2 nanoparticles. Eur. Phys. J. D 2007, 44, 515–524. [Google Scholar] [CrossRef]
- Kittel, C. Introduction to Solid State Physics, 8th ed.; John Wiley & Sons: New York, NY, USA, 2005; ISBN 978-0-471-41526-8. [Google Scholar]
- Christman, J.R. Fundamentals of Solid State Physics; John Wiley & Sons: New York, NY, USA, 1988; ISBN 978-0471810957. [Google Scholar]
- Hook, J.R.; Hall, H.E. Solid State Physics, 2nd ed.; John Wiley & Sons: Chichester, UK, 2001; ISBN 978-0471928058. [Google Scholar]
- Feldman, C.; Moorjani, K. Amorphous Semiconductors. Johns Hopkins APL Tech. Dig. 1968, 7, 2–9. [Google Scholar]
- Fritzche, H. Optical and Electrical energy gaps in amorphous semiconductors. J. Non-Cryst. Solids 1971, 6, 49–71. [Google Scholar] [CrossRef]
- Khor, S.F.; Talib, Z.A.; Mat Yunus, W.M. Optical properties of ternary zinc magnesium phosphate glasses. Ceram. Int. 2012, 38, 935–940. [Google Scholar] [CrossRef]
- Rothenberg, M.S. Model for amorphous semiconductors predicts energy band gaps. Phys. Today 1971, 24, 17. [Google Scholar] [CrossRef]
- Varshneya, A.K. Fundamentals of Inorganic Glasses, 1st ed.; Academic Press Inc.: London, UK, 1994; ISBN 9780127149707. [Google Scholar]
- Stachurski, Z.H. Fundamentals of Amorphous Solids: Structure and Properties; John Wiley & Sons: Weinheim, German, 2015; ISBN 978-3-527-33707-1. [Google Scholar]
- Prasai, B.; Cai, B.; Underwood, M.K.; Lewis, J.P.; Drabold, D.A. Properties of amorphous and crystalline titanium dioxide from first principles. J. Mater. Sci. 2012, 47, 7515–7521. [Google Scholar] [CrossRef]
- Hoang, V.V. The glass transition and thermodynamics of liquid and amorphous TiO2 nanoparticles. Nanotechnology 2008, 19, 105706. [Google Scholar] [CrossRef] [PubMed]
- Petkov, V.; Holzhuter, G.; Troge, U.; Gerber, Th.; Himmel, B. Atomic-scale structure of amorphous TiO2 by electron, X-ray diffraction and reverse Monte Carlo simulations. J. Non-Cryst. Solids 1998, 231, 17–30. [Google Scholar] [CrossRef]
- Brodsky, M.H. Amorphous Semiconductors, 2nd ed.; Springer: Berlin, German, 1979; pp. 357–393. ISBN 978-0387160085. [Google Scholar]
- Zallen, R. The Physics of Amorphous Solids; John Wiley & Sons: New York, NY, USA, 1983; ISBN 978-0-471-29941-7. [Google Scholar]
- Elliot, S.R. Physics of Amorphous Materials; Longman Inc.: New York, NY, USA, 1983; ISBN 0-582-44636-8. [Google Scholar]
- Myers, H.P. Introductory Solid State Physics, 1st ed.; Taylor and Francis: London, UK, 1990; ISBN 0-85066-761-5. [Google Scholar]
- Stromme, M.; Ahuja, R.; Niklasson, G.A. New Probe of the Electronic Structure of Amorphous Materials. Phys. Rev. Lett. 2004, 93, 206403. [Google Scholar] [CrossRef] [PubMed]
- Prasai, B.; Cai, B.; Drabold, D.A. Ab-initio calculation of structural and electric properties of amorphous TiO2. In Proceedings of the MS&T-11 Conference, Columbus, OH, USA, 16–20 October 2011. [Google Scholar]
- Deskins, N.A.; Du, J.; Rao, P. The structural and electronic properties of reduced amorphous titania. Phys. Chem. Chem. Phys. 2017, 19, 18671–18684. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Singh, C.V. Amorphous TiO2 as a photocatalyst for hydrogen production: A DFT study of structural and electronic properties. Energy Procedia 2012, 29, 291–299. [Google Scholar] [CrossRef]
- Afano, O.M.; Cabrera, M.I.; Cassano, A.E. Photocatalytic Reactions Involving Hydroxyl Radical Attack. J. Catal. 1997, 172, 370–379. [Google Scholar] [CrossRef]
- Fox, M.A.; Dulay, M.T. Heterogeneous photocatalysis. Chem. Rev. 1993, 93, 341–357. [Google Scholar] [CrossRef]
- Simon-Deckers, A.; Loo, S.; Mayne-L’hermite, M.; Herlin-Boime, N.; Menguy, N.; Reynaud, C.; Gouget, B.; Carrière, M. Size-composition and shape-dependent toxicological impacto of metal oxide nanoparticles and carbon nanotubes toward bacteria. Environ. Sci. Technol. 2009, 43, 8423–8429. [Google Scholar] [CrossRef] [PubMed]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen-doped Titanium dioxide as visible-light-sensitive photocatalyst: Designs, developments and prospects. Chem. Rev. 2014, 114, 9824–9852. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Banfield, J.F. Kinetics of Crystallization and Crystal Growth of Nanocrystalline Anatase in Nanometer-Sized Amorphous Titania. Chem. Mater. 2002, 14, 4145–4154. [Google Scholar] [CrossRef]
- Almeida, R.M.; Gonçalves, M.C. Sol-gel processes and products. In Encyclopedia of Glass Science, Technology, History, and Culture; Richet, P., Ed.; John Wiley and Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Gonçalves, M.C. Silica Nanoparticles: Back to basics. Design, synthesis and applications of sol-gel silica nanoparticles for medicine and pharmacy. 2018; manuscript in preparation. [Google Scholar]
- Li, H.; Hao, Y.; Lu, H.; Liang, L.; Wang, Y.; Qiu, J.; Shi, X.; Wang, Y.; Yao, J. A systematic study on visible-light N-doped TiO2 photocatalyst obtained from ethylenediamine by sol–gel method. Appl. Surf. Sci. 2015, 344, 112–118. [Google Scholar] [CrossRef]
- Tryba, B.; Wozniak, M.; Zolnierkiewicz, G.; Guskos, N.; Morawski, A.; Colbeau-Justin, C.; Wrobel, R.; Nitta, A.; Ohtani, B. Influence of an Electronic Structure of N-TiO2 on its Photocatalytic Activity towards Decomposition of Acetaldehyde under UV and Fluorescent Lamps Irradiation. Catalysts 2018, 8, 85. [Google Scholar] [CrossRef]
- Nolan, N.T.; Synnotta, D.W.; Seeryb, M.K.; Hinder, S.J.; Wassenhovend, A.V.; Pillai, S.C. Effect of N-doping on the photocatalytic activity of sol-gel TiO2. J. Hazard. Mater. 2012, 211–212, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhou, M.; Cheng, B.; Zhao, X. Preparation, characterization and photo-catalytic activity of in situ N, S-codoped TiO2 powders. J. Mol. Catal. A Chem. 2006, 246, 176–184. [Google Scholar] [CrossRef]
- Cheng, X.; Yu, X.; Xing, Z.; Yang, L. Synthesis and characterization of N-doped TiO2 and its enhanced visible-light photocatalytic activity. Arab. J. Chem. 2016, 9, S1706–S1711. [Google Scholar] [CrossRef]
- Viswanathan, B.; Krishanmurthy, K.R. Nitrogen Incorporation in TiO2: Does It Make a Visible Light Photo-Active Material. Int. J. Photoenergy 2012, 2012. [Google Scholar] [CrossRef]
- Diebold, U.; Madey, T.E. TiO2 by XPS. Surf. Sci. Spectra 1996, 4, 227. [Google Scholar] [CrossRef]
- Yao, J.; Shao, J.; He, H.; Fan, Z. Effects of annealing on laser-induced damage threshold of TiO2/SiO2 high reflectors. Appl. Surf. Sci. 2007, 253, 8911–8914. [Google Scholar] [CrossRef]
- Shei, S.C. Optical and Structural Properties of Titanium Dioxide Films from TiO2 and Ti3O5 Starting Materials Annealed at Various Temperatures. Adv. Mater. Sci. Eng. 2013, 2013, 7. [Google Scholar] [CrossRef]
- Ou, H.H.; Lo, S.L.; Liao, C.H. N-doped TiO2 prepared from microwave-assisted titanate nanotubes (NaxH2−xTi3O7): The effect of microwave irradiation during TNT synthesis on the visible light photoactivity of N-doped TiO2. J. Phys. Chem. C 2011, 115, 4000–4007. [Google Scholar] [CrossRef]
- Yang, G.D.; Jiang, Z.; Shi, H.H.; Xiao, T.C.; Yan, Z.F. Preparation of highly visible-light active N-doped TiO2 photocatalyst. J. Mater. Chem. 2010, 20, 5301–5309. [Google Scholar] [CrossRef]
- Bittencourt, C.; Rutar, M.; Umek, P.; Mrzel, A.; Vozel, K.; Arcon, D.; Henzler, K.; Krügerf, P.; Guttmann, P. Molecular nitrogen in N-doped TiO2 nanoribbons. RSC Adv. 2015, 5, 23350. [Google Scholar] [CrossRef]
- Xing, M.; Zhang, J.; Chen, F. New approaches to prepare nitrogen–doped TiO2 photocatalysts and study on their photocatalytic activities in visible light. Appl. Catal. B Environ. 2009, 89, 563–569. [Google Scholar] [CrossRef]
- Mai, W.; Wen, F.; Xie, D.; Leng, Y.; Mu, Z. Structure and composition study of carbon-doped titanium oxide film combined with first principles. J. Adv. Ceram. 2014, 3, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Matos, J.C.; Oliveira, C.; Martins, M.B.F.; Gonçalves, M.C. Amorphous titania smart textiles: A contribution to nosocomial infections control. Manuscript in preparation.
- Matos, J.C.; Avelar, I.; Martins, B.; Gonçalves, M.C. Silica vectors for Smart Textiles. Carbohydr. Polym. 2017, 156, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Zoltán, B.; Niklas, H. Effects of carbon dioxide captured from ambient air on the infrared spectra of supported amines. Vib. Spectrosc. 2016, 87, 215–221. [Google Scholar] [CrossRef]
- Seil, J.T.; Webster, T.J. Antimicrobial applications of nanotechnology: Methods and literature. Int. J. Nanomed. 2012, 7, 2767–2781. [Google Scholar] [CrossRef]
Sample Availability: Samples are available from the authors.. |










| Ti 2p | O 1s | N 1s | ||||
|---|---|---|---|---|---|---|
| Ti 2p3/2 | Ti 2p1/2 | Shoulder | Main Peak | Peak 1 | Peak 2 | |
| BE (eV) | 461.5 | 455.9 | 531.7 | 527.9 | 396.3 | 393.8 |
| Valence states/bond environment | Ti3+ Ti-N | Ti4+ Ti-O | O-H | O2− | N-O | N-Ti |
| Sample | Bacterial Reduction (%) |
|---|---|
| TiO2 NPs | ∼54.07 ± 4.49 |
| TiO2-SiO2-NH2 NPs | ∼67.28 ± 9.69 |
| Acronym | Precursors | VTiPOT:VPrec (μL) | Catalyst |
|---|---|---|---|
| TiO2 NPs | TiPOT | 500 | Ammonia solution |
| TiO2-SiO2-NH2 NPs | TiPOT:APTES 8:2 | TiPOT: 417 APTES: 83 | Ammonia solution |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, M.C.; Pereira, J.C.; Matos, J.C.; Vasconcelos, H.C. Photonic Band Gap and Bactericide Performance of Amorphous Sol-Gel Titania: An Alternative to Crystalline TiO2. Molecules 2018, 23, 1677. https://doi.org/10.3390/molecules23071677
Gonçalves MC, Pereira JC, Matos JC, Vasconcelos HC. Photonic Band Gap and Bactericide Performance of Amorphous Sol-Gel Titania: An Alternative to Crystalline TiO2. Molecules. 2018; 23(7):1677. https://doi.org/10.3390/molecules23071677
Chicago/Turabian StyleGonçalves, M. Clara, José Carlos Pereira, Joana C. Matos, and Helena Cristina Vasconcelos. 2018. "Photonic Band Gap and Bactericide Performance of Amorphous Sol-Gel Titania: An Alternative to Crystalline TiO2" Molecules 23, no. 7: 1677. https://doi.org/10.3390/molecules23071677
APA StyleGonçalves, M. C., Pereira, J. C., Matos, J. C., & Vasconcelos, H. C. (2018). Photonic Band Gap and Bactericide Performance of Amorphous Sol-Gel Titania: An Alternative to Crystalline TiO2. Molecules, 23(7), 1677. https://doi.org/10.3390/molecules23071677