Phytotoxic Activity of Metabolites Isolated from Rutstroemia sp.n., the Causal Agent of Bleach Blonde Syndrome on Cheatgrass (Bromus tectorum)
Abstract
1. Introduction
2. Material and Methods
2.1. General Experimental Procedures
2.2. Fungal Strain
2.3. Extraction and Purification of R. capillus-albis Secondary Metabolites
2.4. Compound Characterization
2.5. Computational Section
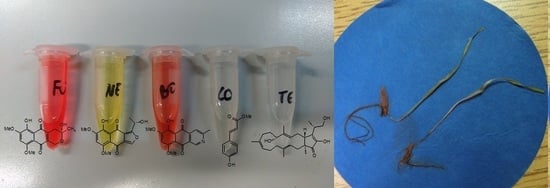
2.6. Bioassays
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brooks, M.L.; D’Antonio, C.M.; Richardson, D.M.; Grace, J.B.; Keeley, J.E.; DiTomaso, J.M.; Hobbs, R.J.; Pellant, M.; Pyke, D. Effects of invasive alien plants on fire regimes. BioScience 2004, 54, 677–688. [Google Scholar] [CrossRef]
- Chambers, J.C.; Bradley, B.A.; Brown, C.S.; D’Antonio, C.; Germino, M.J.; Grace, J.B.; Hardegree, S.P.; Miller, R.F.; Pyke, D.A. Resilience to stress and disturbance, and resistance to Bromus tectorum L. invasion in cold desert shrublands of western North America. Ecosystems 2014, 17, 360–375. [Google Scholar] [CrossRef]
- Meyer, S.E.; Beckstead, J.; Pearce, J. Community ecology of fungal pathogens on Bromus tectorum, Chapter 7. In Exotic Brome-Grasses in Arid and Semiarid Ecosystems of the Western US; Springer International Publishing: New York, NY, USA, 2016; pp. 193–223. [Google Scholar]
- Baughman, O.W.; Meyer, S.E. Is Pyrenophora semeniperda the cause of downy brome (Bromus tectorum) die-offs? Invasive Plant Sci. Manag. 2013, 6, 105–111. [Google Scholar] [CrossRef]
- Meyer, S.E.; Masi, M.; Clement, S.; Davis, T.L.; Beckstead, J. Mycelial growth rate and toxin production in the seed pathogen Pyrenophora semeniperda: Resource trade-offs and temporally varying selection. Plant Pathol. 2015, 64, 1450–1460. [Google Scholar] [CrossRef]
- Masi, M.; Evidente, A.; Meyer, S.; Nicholson, J.; Muñoz, A. Effect of strain and cultural conditions on the production of cytochalasin B by the potential mycoherbicide Pyrenophora semeniperda (Pleosporaceae, Pleosporales). Biocontrol Sci. Technol. 2014, 24, 53–64. [Google Scholar] [CrossRef]
- Masi, M.; Meyer, S.; Cimmino, A.; Andolfi, A.; Evidente, A. Pyrenophoric acid, a phytotoxic sesquiterpenoid penta-2,4-dienoic acid produced by a potential mycoherbicide. Pyrenophora semeniperda. J. Nat. Prod. 2014, 77, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Meyer, S.; Cimmino, A.; Clement, S.; Black, B.; Evidente, A. Pyrenophoric acids B. and C., two new phytotoxic sesquiterpenoids produced by Pyrenophora semeniperda. J. Nat. Prod. 2014, 62, 10304–10311. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Meyer, S.; Clement, S.; Andolfi, A.; Cimmino, A.; Evidente, A. Spirostaphylotrichin W, a spirocyclic γ-lactam isolated from liquid culture of Pyrenophora semeniperda, a potential mycoherbicide for cheatgrass (Bromus tectorum) biocontrol. Tetrahedron 2014, 70, 1497–1501. [Google Scholar] [CrossRef]
- Cimmino, A.; Masi, M.; Evidente, M.; Superchi, S.; Evidente, A. Fungal phytotoxins with potential herbicidal activity: Chemical and biological characterization. Nat. Prod. Rep. 2015, 32, 1629–1653. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Meyer, S.; Pescitelli, G.; Cimmino, A.; Clement, S.; Peacock, B.; Evidente, A. Phytotoxic activity against Bromus tectorum for secondary metabolites of a seed-pathogenic Fusarium strain belonging to the F. tricinctum species complex. Nat. Prod. Res. 2017, 31, 2768–2777. [Google Scholar] [CrossRef] [PubMed]
- Pearce, J.F.; Meyer, S.E.; Geary, B.D.; Ricks, N.; Coleman, C.E. The fungal pathogen Rutstroemia capillus-albis sp.n. is the causal agent of bleach blonde syndrome on Bromus tectorum. Mycologia in review.
- Berger, S.; Braun, S. 200 and More Basic NMR Experiments: A Practical Course, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Dame, Z.T.; Silima, B.; Gryzenhout, M.; van Ree, T. Bioactive compounds from the endophytic fungus Fusarium proliferatum. Nat. Prod. Res. 2016, 30, 1301–1304. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, S.; Beale, M.H.; Ward, J.L.; Strange, R.N. Chickpea wilt: Identification and toxicity of 8-O-methylfusarubin from Fusarium acutatum. Phytochemistry 2005, 66, 1536–1539. [Google Scholar] [CrossRef] [PubMed]
- Steyn, P.S.; Wessels, P.L.; Marasas, W.F. Pigments from Fusarium moniliforme sheldon: Structure and 13C nuclear magnetic resonance assignments of an azaanthraquinone and three naphthoquinones. Tetrahedron 1979, 35, 1551–1555. [Google Scholar] [CrossRef]
- Tatum, J.H.; Baker, R.A.; Berry, R.E. Naphthofurans produced by Fusarium oxysporum isolated from citrus. Phytochemistry 1987, 26, 2499–2500. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chang, F.R.; Teng, C.M.; Wu, Y.C. Cheritamine, a new N-fatty acyl tryptamine and other constituents from the stems of Annona cherimola. J. Chin. Chem. Soc. 1999, 46, 77–86. [Google Scholar] [CrossRef]
- Cimmino, A.; Sarrocco, S.; Masi, M.; Diquattro, S.; Evidente, M.; Vannacci, G.; Evidente, A. Fusaproliferin, terpestacin and their derivatives display variable allelopathic activity against some ascomycetous fungi. Chem. Biodivers. 2016, 13, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision, A.03; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Bruhn, T.; Schaumloeffel, A.; Hemberger, Y.; Bringmann, G. SpecDis: Quantifying the comparison of calculated and experimental electronic circular dichroism spectra. Chirality 2013, 25, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Pescitelli, G. SpecDis, version 1.71; Berlin, Germany, 2017. Available online: https://specdis-software.jimdo.com/ (accessed on 20 June 2018).
- Pretsch, E.; Buhlmann, P.; Badertscher, M. Structure Determination of Organic Compounds−Tables of Spectral Data, 4th ed.; Springer-Verlag: Berlin, Germany, 2009. [Google Scholar]
- Breitmaier, E.; Voelter, W. Carbon-13 NMR Spectroscopy; VCH Verlagsgesellschaft: Weinheim, Germany, 1987; pp. 183–280. [Google Scholar]
- Visconti, A.; Surico, G.; Iacobellis, N.S.; Bottalico, A. Production of pigments by isolates of Fusarium moniliforme Sheld. from cereals in Italy and their antibacterial acitivity. Phytopathol. Mediterr. 1983, 22, 152–156. [Google Scholar]
- Bauer, J.D.; Cutler, H.G.; Hugdahl, J.D.; Garcia, E.L.; Matesic, D.F.; Cutler, S.J. Biological evaluation of a secondary metabolite, 9-O-methylfusarubin, from Fusarium oxysporum. Med. Chem. Res. 2005, 14, 369–381. [Google Scholar] [CrossRef]
- Wijeratne, E.K.; Bashyal, B.P.; Gunatilaka, M.K.; Arnold, A.E.; Gunatilaka, A.L. Maximizing chemical diversity of fungal metabolites: Biogenetically related heptaketides of the endolichenic fungus Corynespora sp. (1). J. Nat. Prod. 2010, 73, 1156–1159. [Google Scholar] [CrossRef] [PubMed]
- Geng, W.L.; Wang, X.Y.; Kurtán, T.; Mándi, A.; Tang, H.; Schulz, B.; Sung, P.; Zhang, W. Herbarone, a rearranged heptaketide derivative from the sea hare associated fungus Torula herbarum. J. Nat. Prod. 2012, 75, 1828–1832. [Google Scholar] [CrossRef] [PubMed]
- Pescitelli, G.; Bruhn, T. Good computational practice in the assignment of absolute configurations by TDDFT calculations of ECD spectra. Chirality 2016, 28, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Superchi, S.; Scafato, P.; Górecki, M.; Pescitelli, G. Absolute configuration determination by quantum mechanical calculation of chiroptical spectra: Basics and applications to fungal metabolites. Curr. Med. Chem. 2018, 25, 287–320. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; Mahmood Ahmed, K.; Gaggeri, R.; Della Volpe, S.; Maggi, L.; Mazzeo, G.; Longhi, G.; Abbate, S.; Corana, F.; Martino, E.; et al. (R)-(–)-Aloesaponol III 8-methyl ether from Eremurus persicus: A novel compound against leishmaniosis. Molecules 2017, 22, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.A.; Tatum, J.H.; Nemec, S. Antimicrobial activity of naphthoquinones from Fusaria. Mycopathologia 1990, 111, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, J.; Motohashi, K.; Sakamoto, K.; Hashimoto, S.; Yamanouchi, M.; Tanaka, H.; Takahashi, T.; Takagi, M.; Shin-ya, K. Screening and evaluation of new inhibitors of hepatic glucose production. J. Antibiot. 2009, 62, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.M.; Liu, S.X.; Huang, C.H.; Pang, J.Y.; Lin, Y.C. Secondary metabolites of a mangrove endophytic fungus Aspergillus terreus (No. GX7-3B) from the South China Sea. Mar. Drugs 2013, 11, 2616–2624. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Masi, M.; Evidente, M.; Superchi, S.; Evidente, A. Application of Mosher’s method for absolute configuration assignment to bioactive plants and fungi metabolites. J. Pharm. Biomed. Anal. 2017, 144, 59–89. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3th ed.; John Wiley & Sons: Chichester, UK, 2009. [Google Scholar]
- D’Abrosca, B.; Dellagreca, M.; Fiorentino, A.; Isidori, M.; Monaco, P.; Pacifico, S. Chemical constituents of the aquatic plant Schoenoplectus lacustris: Evaluation of phytotoxic effects on the green alga Selenastrum capricornutum. J. Chem. Ecol. 2006, 32, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Oka, M.; Iimura, S.; Narita, Y.; Furumai, T.; Konishi, M.; Oki, T.; Gao, Q.; Kakisawa, H. Stereochemistry and biosynthesis of terpestacin, a new syncytium formation inhibitor. J. Org. Chem. 1993, 58, 1875–1881. [Google Scholar] [CrossRef]
- Liu, D.; Li, X.M.; Li, C.S.; Wang, B.G. Sesterterpenes and 2H-pyran-2-ones (= α-pyrones) from the mangrove-derived endophytic fungus Fusarium proliferatum MA-84. Helv. Chim. Acta 2013, 96, 437–444. [Google Scholar] [CrossRef]
- Graziani, S.; Vasnier, C.; Daboussi, M.J. Novel polyketide synthase from Nectria haematococca. Appl. Environ. Microbiol. 2004, 70, 2984–2988. [Google Scholar] [CrossRef] [PubMed]
- Studt, L.; Wiemann, P.; Kleigrewe, K.; Humpf, H.U.; Tudzynski, B. Biosynthesis of fusarubins accounts for pigmentation of Fusarium fujikuroi perithecia. Appl. Environ. Microbiol. 2012, 78, 4468–4480. [Google Scholar] [CrossRef] [PubMed]
- Duval, J.; Pecher, V.; Poujol, M.; Lesellier, E. Research advances for the extraction, analysis and uses of anthraquinones: A. review. Ind. Crops Prod. 2016, 94, 812–833. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–5 are available from the authors. |






| 6 | 7 | |
|---|---|---|
| Position | δH (J in Hz) | δH (J in Hz) |
| 3 | 8.062 (1H, s) | 7.998 (1H, s) |
| MeO-5 b | 4.042 (3H, s) | 4.041 (3H, s) |
| 6 | 6.833 (1H, s) | 6.827 (1H, s) |
| MeO-7 b | 4.014 (3H, s) | 4.012 (3H, s) |
| 1’ | 5.976 (1H, q, J = 8.0) | 5.824 (1H, q, J = 8.0) |
| 2’ | 1.717 (3H, d, J = 8.0) | 1.786 (3H, d, J = 8.0) |
| MeO | 3.580 (3H, s) | 3.569 (3H, s) |
| Ph | 7.505–7.380 (5H, m) | 7.491–7.367 (5H, m) |
| OH-8 | 13.481 (br s) | 13.480 (br s) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masi, M.; Meyer, S.; Górecki, M.; Pescitelli, G.; Clement, S.; Cimmino, A.; Evidente, A. Phytotoxic Activity of Metabolites Isolated from Rutstroemia sp.n., the Causal Agent of Bleach Blonde Syndrome on Cheatgrass (Bromus tectorum). Molecules 2018, 23, 1734. https://doi.org/10.3390/molecules23071734
Masi M, Meyer S, Górecki M, Pescitelli G, Clement S, Cimmino A, Evidente A. Phytotoxic Activity of Metabolites Isolated from Rutstroemia sp.n., the Causal Agent of Bleach Blonde Syndrome on Cheatgrass (Bromus tectorum). Molecules. 2018; 23(7):1734. https://doi.org/10.3390/molecules23071734
Chicago/Turabian StyleMasi, Marco, Susan Meyer, Marcin Górecki, Gennaro Pescitelli, Suzette Clement, Alessio Cimmino, and Antonio Evidente. 2018. "Phytotoxic Activity of Metabolites Isolated from Rutstroemia sp.n., the Causal Agent of Bleach Blonde Syndrome on Cheatgrass (Bromus tectorum)" Molecules 23, no. 7: 1734. https://doi.org/10.3390/molecules23071734
APA StyleMasi, M., Meyer, S., Górecki, M., Pescitelli, G., Clement, S., Cimmino, A., & Evidente, A. (2018). Phytotoxic Activity of Metabolites Isolated from Rutstroemia sp.n., the Causal Agent of Bleach Blonde Syndrome on Cheatgrass (Bromus tectorum). Molecules, 23(7), 1734. https://doi.org/10.3390/molecules23071734