Cytotoxic and N-Acetyltransferase Inhibitory Meroterpenoids from Ganoderma cochlear
Abstract
:1. Introduction
2. Results and Discussion
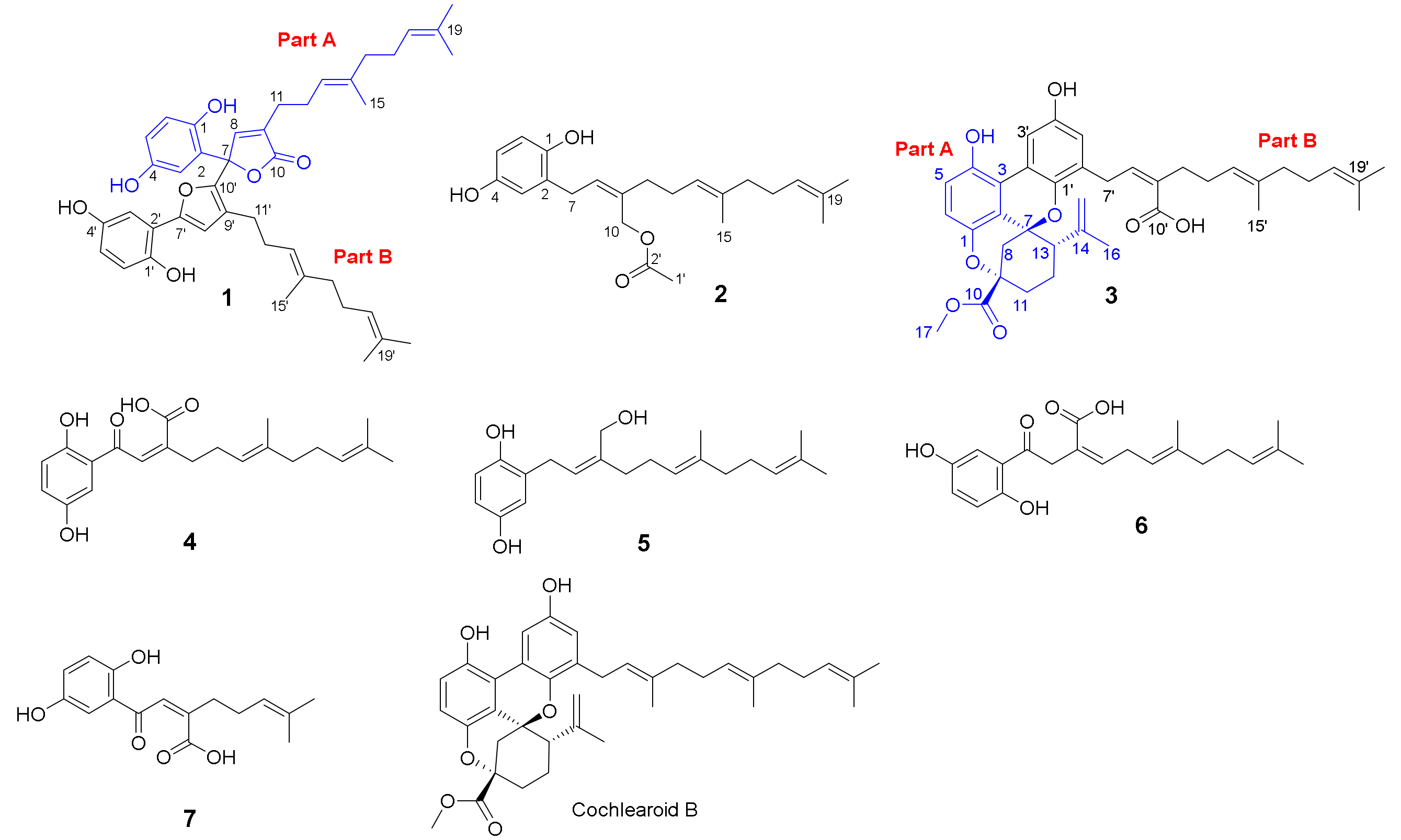
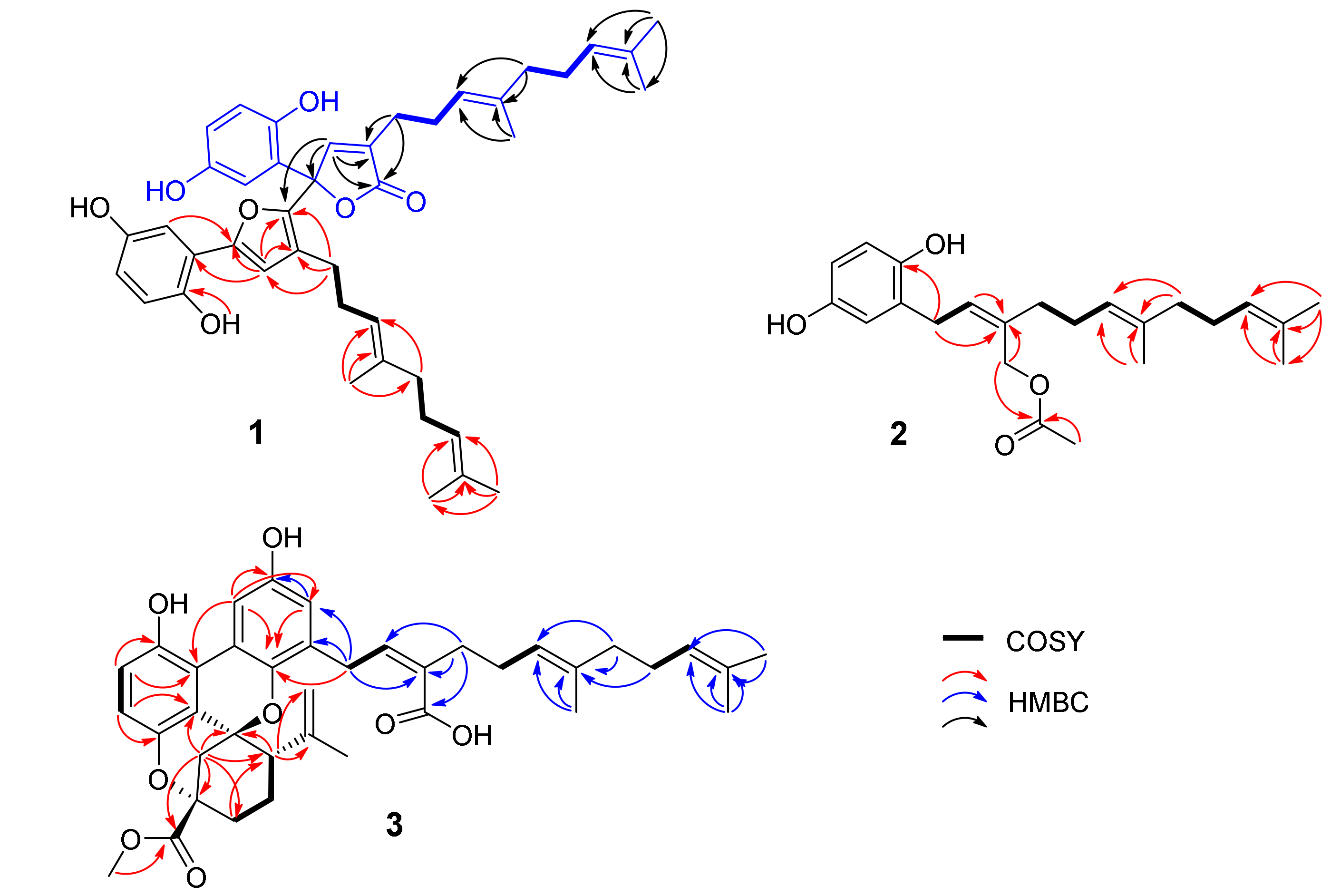
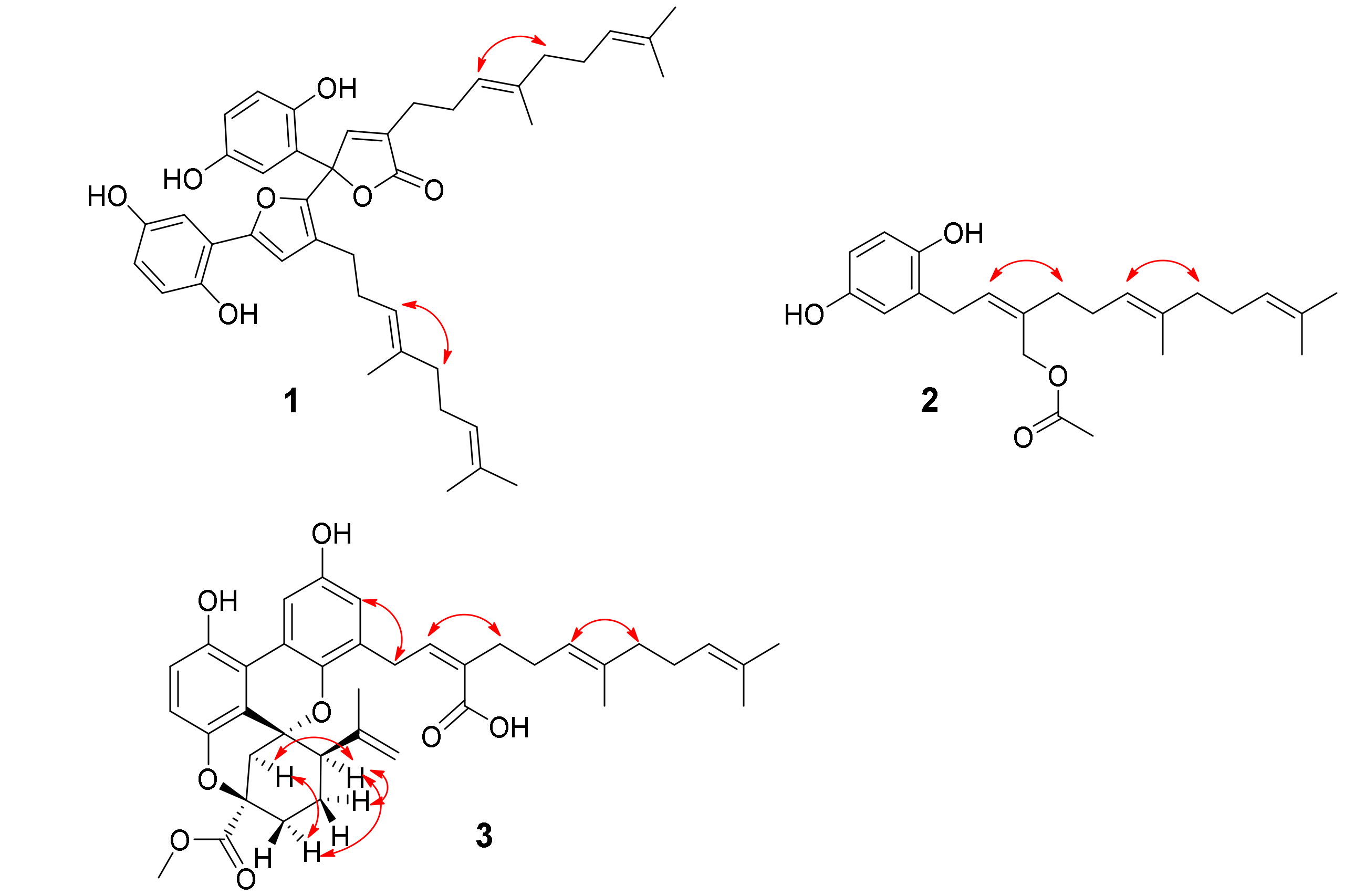
2.1. Structure Elucidation of the Compounds
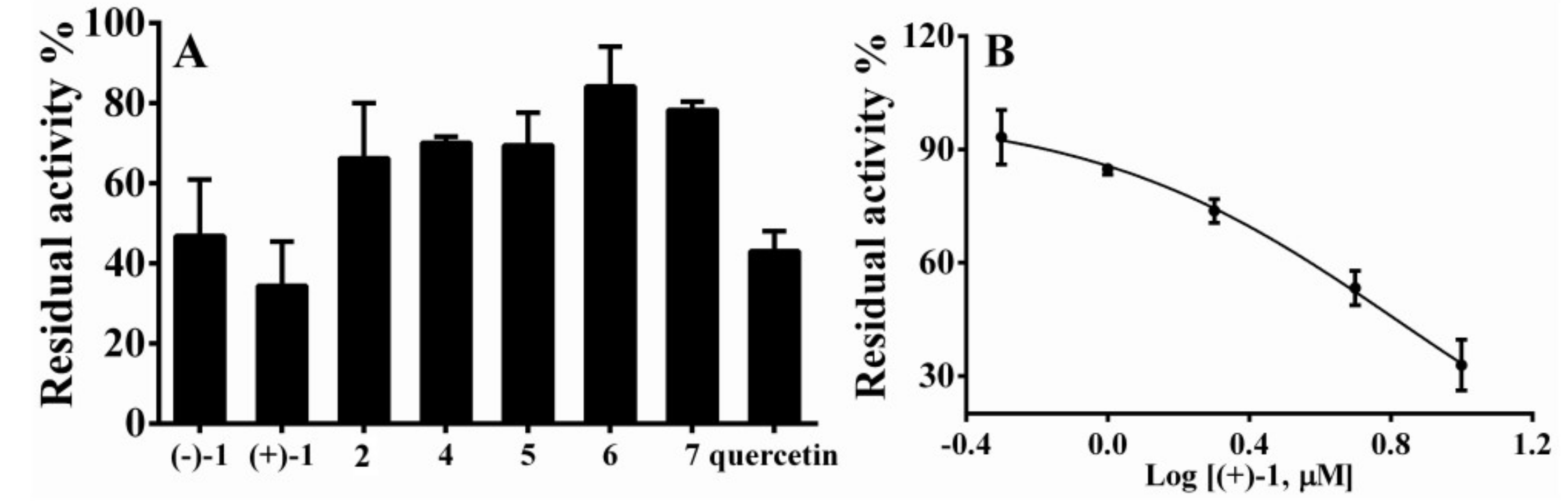
2.2. Biological Evaluation
3. Experimental Section
3.1. General Procedures
3.2. Fungal Material
3.3. Extraction and Isolation
3.4. Compound Characterization Data
3.5. Cell Viability
3.6. N-Acetyltransferase (NAT) Inhibition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, K.; Bao, L.; Ma, K.; Zhang, J.J.; Chen, B.S.; Han, J.J.; Ren, J.W.; Luo, H.J.; Liu, H.W. A novel class of α-glucosidase and HMG-CoA reductase inhibitors from Ganoderma leucocontextum and the anti-diabetic properties of ganomycin I in KK-Ay mice. Eur. J. Med. Chem. 2017, 127, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.J.; Fang, P.S.; Chen, D.H.; Chen, K.D.; Yen, G.C. Anti-invasive effect of a rare mushroom, Ganoderma colossum, on human hepatoma cells. J. Agric. Food Chem. 2010, 58, 7657–7663. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.C.; Yang, F.L.; Huang, Z.Y.; Chen, C.S.; Yang, Y.L.; Hua, K.F.; Li, J.J.; Chen, S.T.; Wu, S.H. Oligosaccharide and peptidoglycan of Ganoderma lucidum activate the immune response in human mononuclear cells. J. Agric. Food Chem. 2012, 60, 2830–2837. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; He, H.; Xie, B.J. Novel antioxidant peptides from fermented mushroom Ganoderma lucidum. J. Agric. Food Chem. 2004, 52, 6646–6652. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Z.; Cheng, B.H.; Ma, Q.Y.; Wang, Q.; Kong, F.D.; Dai, H.F.; Qiu, S.H.; Zheng, Y.P.; Liu, Z.Q.; Zhao, Y.X. Anti-allergic prenylated hydroquinones and alkaloids from the fruiting body of Ganoderma calidophilum. RSC Adv. 2016, 6, 21139–21147. [Google Scholar] [CrossRef]
- Yan, Y.M.; Ai, J.; Zhou, L.L.; Arthur, C.K.C.; Li, R.; Nie, J.; Fang, P.; Wang, X.L.; Luo, J.; Hu, Q.; et al. Lingzhiols, unprecedented rotary door-shaped meroterpenoids as potent and selective inhibitors of -Smad3 from Ganoderma lucidum. Org. Lett. 2013, 15, 5488–5491. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Di, L.; Dai, W.F.; Lu, Q.; Yan, Y.M.; Yang, Z.L.; Li, R.T.; Cheng, Y.X. Applanatumin A, a new dimeric meroterpenoid from Ganoderma applanatum that displays potent antifibrotic activity. Org. Lett. 2015, 17, 1110–1113. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Di, L.; Yang, X.H.; Cheng, Y.X. Applanatumols A and B, meroterpenoids with unprecedented skeletons from Ganoderma applanatum. RSC Adv. 2016, 6, 45963–45967. [Google Scholar] [CrossRef]
- Zhou, F.J.; Nian, Y.; Yan, Y.M.; Gong, Y.Y.; Luo, Q.; Zhang, Y.; Hou, B.; Zuo, Z.L.; Wang, S.M.; Jiang, H.H.; et al. Two new classes of T-type calcium channel inhibitors with new chemical scaffolds from Ganoderma cochlear. Org. Lett. 2015, 17, 3082–3085. [Google Scholar] [CrossRef] [PubMed]
- Lou, Q.; Tian, L.; Di, L.; Yan, Y.M.; Wei, X.Y.; Wang, X.F.; Cheng, Y.X. (±)-Sinensilactam A, a pair of rare hybrid metabolites with Smad3 phosphorylation inhibition from Ganoderma sinensis. Org. Lett. 2015, 17, 1565–1568. [Google Scholar] [CrossRef]
- Yan, Y.M.; Wang, X.L.; Zhou, L.L.; Zhou, F.J.; Li, R.; Tian, Y.; Zuo, Z.L.; Fang, P.; Chung, A.C.K.; Hou, F.F.; et al. Lingzhilactones from Ganoderma lingzhi ameliorate adriamycin-induced nephropathy in mice. J. Ethnopharmacol. 2015, 176, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Wang, X.L.; Di, L.; Yan, Y.M.; Lu, Q.; Yang, X.H.; Hu, D.B.; Cheng, Y.X. Isolation and identification of renoprotective substances from the mushroom Ganoderma lucidum. Tetrahedron 2015, 71, 840–845. [Google Scholar] [CrossRef]
- Luo, Q.; Yang, X.H.; Yang, Z.L.; Tu, Z.C.; Cheng, Y.X. Miscellaneous meroterpenoids from Ganoderma applanatum. Tetrahedron 2016, 72, 4564–4574. [Google Scholar] [CrossRef]
- Luo, Q.; Tu, Z.C.; Cheng, Y.X. Two rare meroterpenoidal rotamers from Ganoderma applanatum. RSC Adv. 2017, 7, 3413–3418. [Google Scholar] [CrossRef]
- Luo, Q.; Wei, X.Y.; Yang, J.; Luo, J.F.; Liang, R.; Tu, Z.C.; Cheng, Y.X. Spiro meroterpenoids from Ganoderma applanatum. J. Nat. Prod. 2017, 80, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Zhou, F.J.; Dou, M.; Yan, Y.M.; Wang, S.M.; Di, L.; Cheng, Y.X. Cochlearoids F–K: Phenolic meroterpenoids from the fungus Ganoderma cochlear and their renoprotective activity. Bioorg. Med. Chem. Lett. 2016, 26, 5507–5512. [Google Scholar] [CrossRef] [PubMed]
- Dou, M.; Di, L.; Zhou, L.L.; Yan, Y.M.; Wang, X.L.; Zhou, F.J.; Yang, Z.L.; Li, R.T.; Hou, F.F.; Cheng, Y.X. Cochlearols A and B, polycyclic meroterpenoids from the fungus Ganoderma cochlear that have renoprotective activities. Org. Lett. 2014, 16, 6064–6067. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Dou, M.; Luo, Q.; Cheng, L.Z.; Yan, Y.M.; Li, R.T.; Cheng, Y.X. Racemic alkaloids from the fungus Ganoderma cochlear. Fitoterapia 2017, 116, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Dine, R.S.E.; Halawany, A.M.E.; Ma, C.M.; Hattori, M. Inhibition of the dimerization and active site of HIV-1 protease by secondary metabolites from the vietnamese mushroom Ganoderma colossum. J. Nat. Prod. 2009, 72, 2019–2023. [Google Scholar] [CrossRef] [PubMed]
- Yazaki, K.; Fukui, H.; Tabata, M. Isolation of the intermediates and related metabolites of shikonin biosynthesis from Lithospermum erythrorhizon cell cultures. Chem. Pharm. Bull. 1986, 34, 2290–2293. [Google Scholar] [CrossRef]
- Cao, W.W.; Luo, Q.; Cheng, Y.X.; Wang, S.M. Meroterpenoid enantiomers from Ganoderma sinensis. Fitoterapia 2016, 110, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.R.; Liu, J.Q.; Wang, C.F.; Han, Z.H.; Shu, Y.; Li, X.Y.; Zhou, L.; Qiu, M.H. Unusual prenylated phenols with antioxidant activities from Ganoderma cochlear. Food Chem. 2015, 171, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Dou, M.; Li, R.T.; Cheng, Y.X. Minor compounds from fungus Ganoderma cochlear. Chin. Herb. Med. 2016, 8, 85–88. [Google Scholar] [CrossRef]
- Bhakta, S.; Besra, G.S.; Upton, A.M.; Parish, T.; Vernon, C.S.D.; Gibson, K.J.C.; Knutton, S.; Gordon, S.; Silva, R.P.; Anderton, M.C.; et al. Arylamine N-acetyltransferase is required for synthesis of mycolic acids and complex lipids in Mycobacterium bovis BCG and represents a novel drug target. J. Exp. Med. 2004, 199, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.W.; Lu, W.Q.; Chen, G.L.; Cheng, F.X.; Su, H.; Chen, Y.H.; Liu, M.Y.; Pang, X.F. Inhibition of histone deacetylases sensitizes EGF receptor-TK inhibitor-resistant non-small cell lung cancer cells to erlotinib in vitro and in vivo. Br. J. Pharmacol. 2017, 174, 3608–3622. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cui, L.; Zhou, N.N.; Zhu, W.P.; Wang, R.; Qian, X.H.; Xu, Y.F. A highly selective and sensitive near-infrared fluorescence probe for arylamine N-acetyltransferase 2 in vitro and in vivo. Chem. Sci. 2013, 4, 2936–2940. [Google Scholar] [CrossRef]
- Kukongviriyapan, V.; Phromsopha, N.; Tassaneeyakul, W.; Kukongviriyapan, U.; Sripa, B.; Hahnvajanawong, V.; Bhudhisawasdi, V. Inhibitory effects of polyphenolic compounds on human arylamine N-acetyltransferase 1 and 2. Xenobiotica 2006, 36, 15–28. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–7 are available from the authors. |
| Position | δH | δC | Position | δH | δC |
|---|---|---|---|---|---|
| 1 | 146.4 | 1′ | 146.7 | ||
| 2 | 124.3 | 2′ | 117.2 | ||
| 3 | 6.84 (d, 2.2) | 111.9 | 3′ | 6.79 (d, 2.5) | 110.8 |
| 4 | 149.9 | 4′ | 149.7 | ||
| 5 | 6.57 (dd, 8.5, 2.2) | 115.8 | 5′ | 6.49 (d, 8.7, 2.5) | 115.2 |
| 6 | 6.61 (d, 8.5) | 116.6 | 6′ | 6.69 (d, 8.7) | 116.7 |
| 7 | 84.6 | 7′ | 148.4 | ||
| 8 | 7.96 (s) | 149.2 | 8′ | 6.76 (s) | 112.8 |
| 9 | 130.7 | 9′ | 124.9 | ||
| 10 | 172.4 | 10′ | 143.1 | ||
| 11 | 2.30 (m) | 24.8 | 11′ | 2.12 (m) | 24.8 |
| 12 | 2.22 (m) | 25.5 | 12′ | 1.97 (m) | 26.1 |
| 13 | 5.09 (t, 6.5) | 122.7 | 13′ | 4.98 (overlap) | 123.5 |
| 14 | 136.0 | 14′ | 135.0 | ||
| 15 | 1.47 (s) | 15.8 | 15′ | 1. 49 (s) | 15.9 |
| 16 | 1.85 (m) | 39.1 | 16′ | 1.88 (m) | 39.1 |
| 17 | 1.98 (m) | 26.2 | 17′ | 1.93 (m) | 28.5 |
| 18 | 5.04 (t, 6.8) | 124.1 | 18′ | 4.99 (overlap) | 124.1 |
| 19 | 130.7 | 19′ | 130.7 | ||
| 20 | 1.52 (s) | 17.7 | 20′ | 1.50 (s) | 17.6 |
| 21 | 1.60 (s) | 25.5 | 21′ | 1.59 (s) | 25.5 |
| 1-OH | 9.09 (s) | 1′-OH | 9.35 (s) | ||
| 4-OH | 8.86 (s) | 4′-OH | 8.81 (s) |
| Position | δH | δC | Position | δH | δC |
|---|---|---|---|---|---|
| 1 | 149.0 | 13 | 5.10 (t-like, 5.0) | 125.0 | |
| 2 | 129.1 | 14 | 136.4 | ||
| 3 | 6.52 (d, 2.9) | 117.4 | 15 | 1. 57 (s) | 16.1 |
| 4 | 151.1 | 16 | 1.94 (m) | 40.8 | |
| 5 | 6.45 (dd, 8.5, 2.9) | 114.2 | 17 | 2.02 (m) | 27.7 |
| 6 | 6.58 (d, 8.5) | 116.5 | 18 | 5.03 (t-like, 6.9) | 125.5 |
| 7 | 3.33 (m) | 29.3 | 19 | 132.0 | |
| 8 | 5.57 (t, 7.7) | 130.5 | 20 | 1.57 (s) | 17.8 |
| 9 | 135.2 | 21 | 1.65 (s) | 25.9 | |
| 10 | 4.74 (s) | 63.1 | 1′ | 2.04 (s) | 20.9 |
| 11 | 2.12 (overlap) | 36.5 | 2′ | 173.0 | |
| 12 | 2.12 (overlap) | 27.7 |
| Position | δH | δC | Position | δH | δC |
|---|---|---|---|---|---|
| 1 | 147.1 | 1′ | 146.4 | ||
| 2 | 119.4 | 2′ | 128.8 | ||
| 3 | 117.5 | 3′ | 7.90 (d, 2.9) | 114.0 | |
| 4 | 149.1 | 4′ | 151.3 | ||
| 5 | 6.76 (d, 8.8) | 118.3 | 5′ | 6.47 (d, 2.9) | 116.6 |
| 6 | 6.72 (d, 8.8) | 116.2 | 6′ | 123.2 | |
| 7 | 76.1 | 7′ | 3.83 (dd, 15.7, 7.9) | 31.7 | |
| 8 | 2.47 (d, 12.5) | 41.7 | 3.66 (dd, 15.7, 7.9) | ||
| 2.33 (dd, 12.5, 2.2) | 8′ | 5.95 (t-like, 7.9) | 140.5 | ||
| 9 | 81.0 | 9′ | 133.5 | ||
| 10 | 173.9 | 10′ | 172.0 | ||
| 11 | Ha: 2.09 (m) | 36.3 | 11′ | 2.29 (t, 7.3) | 36.1 |
| Hb: 1.97 (m) | 12′ | 2.15 (m) | 28.5 | ||
| 12 | Ha: 1.66 (m) | 26.7 | 13′ | 5.10 (t, 6.9) | 124.6 |
| Hb: 1.47 (m) | 14′ | 136.9 | |||
| 13 | 2.63 (dd, 13.2, 3.3) | 58.2 | 15′ | 1.55 (s) | 16.2 |
| 14 | 145.7 | 16′ | 1.91 (m) | 40.7 | |
| 15 | 4.46 (s) | 114.2 | 17′ | 2.10 (m) | 27.8 |
| 4.27 (s) | 18′ | 5.04 (t, 6.9) | 125.4 | ||
| 16 | 1.23 (s) | 21.1 a | 19′ | 132.0 | |
| 17 | 3.80 (s) | 53.2 | 20′ | 1.56 (s) | 17.8 |
| 21′ | 1.63 (s) | 25.9 | |||
| Compound | IC50 (μM) | ||
|---|---|---|---|
| H1975 | PC9 | A549 | |
| 2 | 32.43 | 40.57 | 30.65 |
| 5 | 19.47 | 35.70 | 21.60 |
| Positive control a | 7.66 | 0.085 | 4.59 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, L.-Z.; Qin, F.-Y.; Ma, X.-C.; Wang, S.-M.; Yan, Y.-M.; Cheng, Y.-X. Cytotoxic and N-Acetyltransferase Inhibitory Meroterpenoids from Ganoderma cochlear. Molecules 2018, 23, 1797. https://doi.org/10.3390/molecules23071797
Cheng L-Z, Qin F-Y, Ma X-C, Wang S-M, Yan Y-M, Cheng Y-X. Cytotoxic and N-Acetyltransferase Inhibitory Meroterpenoids from Ganoderma cochlear. Molecules. 2018; 23(7):1797. https://doi.org/10.3390/molecules23071797
Chicago/Turabian StyleCheng, Li-Zhi, Fu-Ying Qin, Xiao-Chi Ma, Shu-Mei Wang, Yong-Ming Yan, and Yong-Xian Cheng. 2018. "Cytotoxic and N-Acetyltransferase Inhibitory Meroterpenoids from Ganoderma cochlear" Molecules 23, no. 7: 1797. https://doi.org/10.3390/molecules23071797
APA StyleCheng, L. -Z., Qin, F. -Y., Ma, X. -C., Wang, S. -M., Yan, Y. -M., & Cheng, Y. -X. (2018). Cytotoxic and N-Acetyltransferase Inhibitory Meroterpenoids from Ganoderma cochlear. Molecules, 23(7), 1797. https://doi.org/10.3390/molecules23071797






