Interaction between Different Pharmaceutical Excipients in Liquid Dosage Forms—Assessment of Cytotoxicity and Antimicrobial Activity
Abstract
1. Introduction
2. Results
2.1. Cytotoxicity Tests
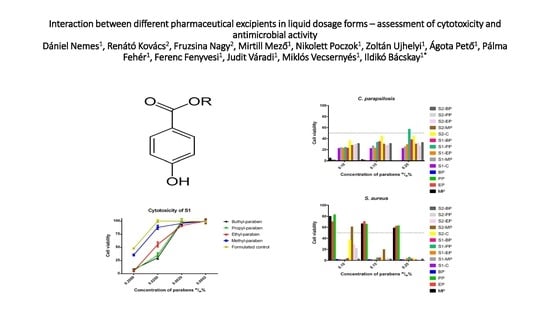
2.1.1. Cytotoxicity of Parabens
2.1.2. Cytotoxicity of Solvents
2.1.3. Cytotoxicity of Formulated Systems
2.2. Antimicrobial Tests
2.2.1. Antifungal Tests
2.2.2. Antibacterial Tests
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Cytotoxicity Tests
4.4. Antimicrobial Tests
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Behrens, M.; Blank, K.; Meyerhof, W. Blends of Non-caloric Sweeteners Saccharin and Cyclamate Show Reduced Off-Taste due to TAS2R Bitter Receptor Inhibition. Cell Chem. Biol. 2017, 24, 1199–1204. [Google Scholar] [CrossRef] [PubMed]
- Maillard, J.-Y. Bacterial target sites for biocide action. J. Appl. Microbiol. 2002, 92, 16–27. [Google Scholar] [CrossRef]
- Nakawaga, Y.; Moore, G. Role of mitochondrial membrane permeability transition in p-hydroxybenzoate ester-induced cytotoxicity in rat hepatocytes. Biochem. Pharmacol. 1999, 58, 811–816. [Google Scholar] [CrossRef]
- Baardseth, P.; Russwurm, H. Content of some organic acids in cloudberry (Rubus chamaemorus L.). Food Chem. 1978, 3, 43–46. [Google Scholar] [CrossRef]
- Jianmei, C.; Bo, L.; Zheng, C.; Huai, S.; Guohong, L.; Cibin, G. Identification of ethylparaben as the antimicrobial substance produced by Brevibacillus brevis FJAT-0809-GLX. Microbiol. Res. 2015, 172, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.M.; Wang, W.; Zhang, J.J.; Wang, Z.R.; Wang, Y.; Hao, W.J.; Huang, W.Y. Antibacterial Constituents of Hainan Morinda citrifolia (Noni) Leaves. J. Food. Sci. 2016, 81, 1192–1196. [Google Scholar] [CrossRef] [PubMed]
- Soni, M.G.; Carabin, I.G.; Burdock, G.A. Safety assessment of esters of p-hydroxybenzoic acid (parabens). Food Chem. Toxicol. 2005, 43, 985–1015. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, F.; Maibach, H. An overview of parabens and allergic contact dermatitis. Skin Ther. Lett. 2013, 18, 5–7. [Google Scholar]
- Yim, E.; Baquerizo, N.K.L.; Tosti, A. Contact dermatitis caused by preservatives. Dermatitis 2014, 25, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Warshaw, E.M.; Maibach, H.I.; Taylor, J.S.; Sasseville, D.; De Koven, J.G.; Zirwas, M.J.; Fransway, A.F.; Mathias, C.G.; Zug, K.A.; De Leo, V.A.; et al. North American contact dermatitis group patch test results: 2011–2012. Dermatitis 2015, 26, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Dinkloh, A.; Worm, M.; Geier, J.; Schnuch, A.; Wollenberg, A.J. Contact sensitization in patients with suspected cosmetic intolerance: Results of the IVDK 2006–2011. Eur. Acad. Dermatol. Venereol. 2015, 29, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Spanier, A.J.; Fausnight, T.; Camacho, T.F.; Braun, J.M. The associations of triclosan and paraben exposure with allergen sensitization and wheeze in children. Allergy Asthma Proc. 2014, 35, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Darbre, P.D.; Harvey, P.W. Paraben esters: Review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human health risks. J. Appl. Toxicol. 2008, 28, 561–578. [Google Scholar] [CrossRef] [PubMed]
- Roszak, J.; Smok-Pieniążek, A.; Domeradzka-Gajda, K.; Grobelny, J.; Tomaszewska, E.; Ranoszek-Soliwoda, K.; Celichowski, G.; Stępnik, M. Inhibitory effect of silver nanoparticles on proliferation of estrogen-dependent MCF-7/BUS human breast cancer cells induced by butyl paraben or di-n-butyl phthalate. Toxicol. Appl. Pharmacol. 2017, 25, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Nishihama, Y.; Yoshinaga, J.; Iida, A.; Konishi, S.; Imai, H.; Yoneyama, M.; Nakajima, D.; Shiraishi, H. Association between paraben exposure and menstrual cycle in female university students in Japan. Reprod. Toxicol. 2016, 63, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Sandanger, T.M.; Huber, S.; Moe, M.K.; Braathen, T.; Leknes, H.; Lund, E. Plasma concentrations of parabens in postmenopausal women and self-reported use of personal care products: The NOWAC postgenome study. J. Expo. Sci. Environ. Epidemiol. 2011, 21, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Janjua, N.R.; Mortensen, G.K.; Andersson, A.M.; Kongshoj, B.; Skakkebaek, N.E.; Wulf, H.C. Systemic uptake of diethyl phthalate, dibutyl phthalate, and butyl paraben following whole-body topical application and reproductive and thyroid hormone levels in humans. Environ. Sci. Technol. 2007, 41, 5564–5570. [Google Scholar] [CrossRef] [PubMed]
- Epstein, S.P.; Ahdoot, M.; Marcus, E.; Asbell, P.A. Comparative toxicity of preservatives on immortalized corneal and conjunctival epithelial cells. J. Ocul. Pharmacol. Ther. 2009, 25, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.M.; Menezes, P.F.; Letenski, G.C.; Praes, C.E.; Feferman, I.H.; Lorencini, M. In vitro induction of apoptosis, necrosis and genotoxicity by cosmetic preservatives: Application of flow cytometry as a complementary analysis by NRU. Int. J. Cosmet. Sci. 2012, 34, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.H.; Verma, R.J. Butyl p-hydroxybenzoic acid induces oxidative stress in mice liver—An in vivo study. Acta Pol. Pharm. 2011, 68, 875–879. [Google Scholar] [PubMed]
- Nellis, G.; Metsvaht, T.; Varendi, H.; Toompere, K.; Lass, J.; Mesek, I.; Nunn, A.J.; Turner, M.A.; Lutsar, I. Potentially harmful excipients in neonatal medicines: A pan-European observational study. Arch. Dis Child. 2015, 100, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Towers, C.V.; Terry, P.D.; Lewis, D.; Howard, B.; Chambers, W.; Armistead, C.; Weitz, B.; Porter, S.; Borman, C.J.; Kennedy, R.C.; et al. Transplacental passage of antimicrobial paraben preservatives. J. Expo. Sci. Environ. Epidemiol. 2015, 25, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Hines, E.P.; Mendola, P.; von Ehrenstein, O.S.; Ye, X.; Calafat, A.M.; Fenton, S.E. Concentrations of environmental phenols and parabens in milk, urine and serum of lactating North Carolina women. Reprod. Toxicol. 2015, 54, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Nemes, D.; Ujhelyi, Z.; Arany, P.; Pető, A.; Fehér, P.; Váradi, J.; Fenyvesi, F.; Vecsernyés, M.; Bácskay, I. Biocompatibility investigation of different pharmaceutical excipients used in liquid dosage forms. Pharmazie 2018, 73, 16–18. [Google Scholar] [PubMed]
- Ujhelyi, Z.; Fenyvesi, F.; Váradi, J.; Fehér, P.; Kiss, T.; Veszelka, S.; Deli, M.; Vecsernyés, M.; Bácskay, I. Evaluation of cytotoxicity of surfactants used in self-micro emulsifying drug delivery systems and their effects on paracellular transport in Caco-2 cell monolayer. Eur. J. Pharm. Sci. 2012, 47, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Bu, P.; Ji, Y.; Narayanan, S.; Dalrymple, D.; Cheng, X.; Serajuddin, A.T.M. Assessment of cell viability and permeation enhancement in presence of lipid-based self-emulsifying drug delivery systems using Caco-2 cell model: Polysorbate 80 as the surfactant. Eur. J. Pharm. Sci. 2017, 99, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, A.J. Epidemiology of Invasive Candidiasis: A Persistent Public Health Problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Atemnkeng, M.A.; De Cock, K.; Plaizier-Vercammen, J. Post-marketing assessment of content and efficacy of preservatives in artemisinin-derived antimalarial dry suspensions for paediatric use. Malar. J. 2007, 6. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency Committee for Medicinal Products for Human Use Formulations of Choice for the Paediatric Population Reflection Paper. EMEA/CHMP/PEG/194810/2005. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003782.pdf (accessed on 10 July 2018).
- US Code of Federal Regulations Title 21, Chapter I, Subchapter D, Part 328 §328.10. Available online: https://www.ecfr.gov/cgi-bin/text-idx?SID=3d64a4a03b70bbe84ac1263cbd2e6b77&mc=true&node=sp21.5.328.b&rgn=div6 (accessed on 10 July 2018).
- Seedher, N.; Bhatia, S. Solubility enhancement of Cox-2 inhibitors using various solvent systems. AAPS PharmSciTech 2003, 4, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Barka, B.Z.; Grintzalis, K.; Polet, M.; Heude, C.; Sommer, U.; Miled, B.H.; Rhouma, B.K.; Mohsen, S.; Tebourbi, O.; Schneider, Y.J. A combination of NMR and liquid chromatography to characterize the protective effects of Rhus tripartita extracts on ethanol-induced toxicity and inflammation on intestinal cells. J. Pharm. Biomed. Anal. 2018, 150, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Santoveña, A.; Sánchez-Negrín, E.; Charola, L.; Llabrés, M.; Fariña, J.B. Study of quality and stability of ursodeoxycholic acid formulations for oral pediatric administration. Int. J. Pharm. 2014, 477, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Ribes, S.; Fuentes, A.; Talens, P.; Barat, J.M.; Ferrari, G.; Donsì, F. Influence of emulsifier type on the antifungal activity of cinnamon leaf, lemon and bergamot oil nanoemulsions against Aspergillus niger. Food Control 2017, 73, 784–795. [Google Scholar] [CrossRef]
- Han, J.; Washington, C. Partition of antimicrobial additives in an intravenous emulsion and their effect on emulsion physical stability. Int. J. Pharm. 2005, 288, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Lakeram, M.; Paine, A.J.; Lockley, D.J.; Sanders, D.J.; Pendlington, R.; Forbes, B. Transesterification of p-hydroxybenzoate esters (parabens) by human intestinal (Caco-2) cells. Xenobiotica 2006, 36, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Tománková, H.; Pinkasová, M. Determination of Parabens and their Degradation Product p-Hydroxy-Benzoic Acid in Pharmaceutical Dosage Forms by Hptlc Densitometry. Anal. Lett. 1990, 23, 1319–1332. [Google Scholar] [CrossRef]
- Dagher, Z.; Borgie, M.; Magdalou, J.; Chahine, R.; Greige-Gerges, H. p-Hydroxybenzoate esters metabolism in MCF7 breast cancer cells. Food Chem. Toxicol. 2012, 50, 4109–4114. [Google Scholar] [CrossRef] [PubMed]
- Armitage, W.J.; Mazur, P. Toxic and osmotic effects of glycerol on human granulocytes. Am. J. Physiol. 1984, 247, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Eskandani, M.; Hamishehkar, H.; Ezzati, N.D.J. Cyto/Genotoxicity Study of Polyoxyethylene (20) Sorbitan Monolaurate (Tween 20). DNA Cell Biol. 2013, 32, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Dimitrijevic, D.; Shaw, A.J.; Florence, A.T. Effects of some non-ionic surfactants on transepithelial permeability in Caco-2 cells. J. Pharm. Pharmacol. 2000, 52, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Bredin, J.; Davin-Régli, A.; Pagés, J.M. Propyl paraben induces potassium efflux in Escherichia coli. J. Antimicrob. Chemother. 2005, 55, 1013–1015. [Google Scholar] [CrossRef] [PubMed]
- Flasiński, M.; Gawryś, M.; Broniatowski, M.; Wydrob, P. Studies on the interactions between parabens and lipid membrane components in monolayers at the air/aqueous solution interface. Biochim. Biophys. Acta 2016, 1858, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Nygård, Y.; Mojzita, D.; Toivari, M.; Penttilä, M.; Wiebe, M.G.; Ruohonen, L. The diverse role of Pdr12 in resistance to weak organic acids. Yeast 2014, 6, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Thong, C.H.; Draughon, F.A. Inhibition by antimicrobial food additives of ochratoxin A production by Aspergillus sulphureus and Penicillium viridicatum. Appl. Environ. Microbiol. 1985, 49, 1407–1411. [Google Scholar]
- Er, B.; Demirhan, B.; Onurdag, F.K.; Ozgacar, S.Ö.; Oktem, A.B. Antimicrobial and antibiofilm effects of selected food preservatives against Salmonella spp. isolated from chicken samples. Poult. Sci. 2014, 93, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Koseki, T.; Mihara, K.; Murayama, T.; Shiono, Y. A novel Aspergillus oryzae esterase that hydrolyzes 4-hydroxybenzoic acid esters. FEBS Lett. 2010, 584, 4032–4036. [Google Scholar] [CrossRef] [PubMed]
- Lakkis, C.; Fleiszig, S.M.J. Resistance of Pseudomonas aeruginosa Isolates to Hydrogel Contact Lens Disinfection Correlates with Cytotoxic Activity. J. Clin. Microbiol. 2001, 39, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Smaoui, S.; Slima, H.B. Effects of parabens and isothiazolinone on the microbiological quality of baby shampoo: The challenge test. Biocontrol Sci. 2012, 17, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Charnock, C.; Finsrud, T. Combining esters of para-hydroxy benzoic acid (parabens) to achieve increased antimicrobial activity. J. Clin. Pharm. Ther. 2007, 32, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Jiménez, D.F.; Pérez-García, L.A.; Martínez-Álvarez, J.A.; Mora-Montes, H.M. Role of the Fungal Cell Wall in Pathogenesis and Antifungal Resistance. Curr. Fungal Infect. Rep. 2012, 6, 275–282. [Google Scholar] [CrossRef]
- De Groot, P.W.J.; Kraneveld, E.A.; Yin, Q.Y.; Dekker, H.L.; Groß, U.; Crielaard, W.; de Koster, C.G.; Bader, O.; Klis, F.M.; Weig, M. The Cell Wall of the Human Pathogen Candida glabrata: Differential Incorporation of Novel Adhesin-Like Wall Proteins. Eukaryote Cell. 2008, 7, 1951–1964. [Google Scholar] [CrossRef] [PubMed]
- Pérez-García, L.A.; Csonka, K.; Flores-Carreón, A.; Estrada-Mata, E.; Mellado-Mojica, E.; Németh, T.; López-Ramírez, L.A.; Toth, R.; López, M.G.; Vizler, C.; et al. Role of Protein Glycosylation in Candida parapsilosis Cell Wall Integrity and Host Interaction. Front. Microbiol. 2016, 6, 306. [Google Scholar] [CrossRef] [PubMed]
- Matos, T.J.; Jensen, B.B.; Bernardo, F.M.; Barreto, A.H.; Hojberg, O. Mycoflora of two types of Portuguese dry-smoked sausages and inhibitory effect of sodium benzoate, potassium sorbate, and methyl p-hydroxybenzoate on mold growth rate. J. Food. Prot. 2007, 70, 1468–1474. [Google Scholar] [CrossRef] [PubMed]
- Shinsaku, I.; Satoru, Y.; Yasutaka, N.; Yasuyuki, S.; Shunsuke, Y. Effects of alkyl parabens on plant pathogenic fungi. Bioorg. Med. Chem. Lett. 2015, 25, 1774–1777. [Google Scholar]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 2000, 6, 509–515. [Google Scholar]
- Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). Method for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Yeasts. 2017. Available online: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Files/EUCAST_E_Def_7_3_1_Yeast_testing__definitive.pdf (accessed on 15 June 2018).
Sample Availability: Not available. |













| Component | S1 | S2 |
|---|---|---|
| Paraben | 0.2 w/w%, 0.02 w/w%, 0.002 w/w%, 0.0002 w/w% | |
| Glycerol | 30 v/v%, 3 v/v%, 0.3 v/v%, 0.03 v/v% | - |
| Polysorbate 20 | 0.002 v/v%, 0.0002 v/v%, 0.00002 v/v%, 0.000002 v/v% | - |
| Capryol PGMC™ | - | 0.5 v/v%, 0.05 v/v%, 0.005 v/v%, 0.0005 v/v% |
| Ethanol | - | 1.4 v/v%, 0.14 v/v%, 0.014 v/v%, 0.0014 v/v% |
| PBS | solvent, used for tenfold, hundredfold, thousand-fold dilution | |
| Component | S1 | S2 | Control |
|---|---|---|---|
| Paraben | 0.1 w/w%, 0.15 w/w%, 0.25 w/w% | ||
| Glycerol | 30 v/v% | - | - |
| Polysorbate 20 | 0.002 v/v% | - | - |
| Capryol PGMC™ | - | 0.5 v/v% | - |
| Ethanol | - | 0.7 v/v%, 1.05 v/v%, 1.75 v/v% | 0.7 v/v%, 1.05 v/v%, 1.75 v/v% |
| RPMI-1640 | solvent for antifungal tests | ||
| Mueller-Hinton broth | solvent for antibacterial tests | ||
| ANOVA Followed by Tukey’s Multiple Comparisons Test | Level of Significance |
|---|---|
| Parabens alone, 0.2% Methyl vs. Ethyl | ns |
| Parabens alone, 0.2% Methyl vs. Propyl | ** |
| Parabens alone, 0.2% Methyl vs. Butyl | **** |
| Parabens alone, 0.2% Ethyl vs. Propyl | ** |
| Parabens alone, 0.2% Ethyl vs. Butyl | **** |
| Parabens alone, 0.2% Propyl vs. Butyl | ns |
| Parabens alone, 0.02% Methyl vs. Ethyl | ns |
| Parabens alone, 0.02% Methyl vs. Propyl | ** |
| Parabens alone, 0.02% Methyl vs. Butyl | * |
| Parabens alone, 0.02% Ethyl vs. Propyl | * |
| Parabens alone, 0.02% Ethyl vs. Butyl | ns |
| Parabens alone, 0.02% Propyl vs. butyl | ns |
| Parabens alone, lower concentrations | all are insignificant |
| S1, 0.2% Formulated control vs. Methyl | * |
| S1, 0.2% FC vs. Ethyl | **** |
| S1, 0.2% FC vs. Propyl | **** |
| S1, 0.2% FC vs. Butyl | **** |
| S1, 0.2% Methyl vs. Ethyl | **** |
| S1, 0.2% Methyl vs. Propyl | **** |
| S1, 0.2% Methyl vs. Butyl | **** |
| S1, 0.2% Ethyl vs. Propyl | ns |
| S1, 0.2% Ethyl vs. Butyl | ns |
| S1, 0.2% Propyl vs. Butyl | ns |
| S1, 0.02% FC vs. Methyl | * |
| S1, 0.02% FC vs. Ethyl | **** |
| S1, 0.02% FC vs. Propyl | **** |
| S1, 0.02% FC vs. Butyl | **** |
| S1, 0.02% Methyl vs. Ethyl | **** |
| S1, 0.02% Methyl vs. Propyl | **** |
| S1, 0.02% Methyl vs. Butyl | **** |
| S1, 0.02% Ethyl vs. Propyl | *** |
| S1, 0.02% Ethyl vs. Butyl | *** |
| S1, 0.02% Propyl vs. Butyl | ns |
| S1 lower concentrations | all are insignificant |
| S2, 0.2% Formulated control vs. Methyl | **** |
| S2, 0.2% FC vs. Ethyl | **** |
| S2, 0.2% FC vs. Propyl | **** |
| S2, 0.2% FC vs. Butyl | **** |
| S2, 0.2% Methyl vs. Ethyl | ns |
| S2, 0.2% Methyl vs. Propyl | ns |
| S2, 0.2% Methyl vs. Butyl | ** |
| S2, 0.2% Ethyl vs. Propyl | ns |
| S2, 0.2% Ethyl vs. Butyl | ** |
| S2, 0.2% Propyl vs. Butyl | ** |
| S2, 0.02% FC vs. Methyl | * |
| S2, 0.02% FC vs. Ethyl | ns |
| S2, 0.02% FC vs. Propyl | ns |
| S2, 0.02% FC vs. Butyl | ** |
| S2, 0.02% Methyl vs. Ethyl | * |
| S2, 0.02% Methyl vs. Propyl | * |
| S2, 0.02% Methyl vs. Butyl | ns |
| S2, 0.02% Ethyl vs. Propyl | ns |
| S2, 0.02% Ethyl vs. Butyl | * |
| S2, 0.02% Propyl vs. Butyl | * |
| S2 lower concentrations | all are insignificant |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemes, D.; Kovács, R.; Nagy, F.; Mező, M.; Poczok, N.; Ujhelyi, Z.; Pető, Á.; Fehér, P.; Fenyvesi, F.; Váradi, J.; et al. Interaction between Different Pharmaceutical Excipients in Liquid Dosage Forms—Assessment of Cytotoxicity and Antimicrobial Activity. Molecules 2018, 23, 1827. https://doi.org/10.3390/molecules23071827
Nemes D, Kovács R, Nagy F, Mező M, Poczok N, Ujhelyi Z, Pető Á, Fehér P, Fenyvesi F, Váradi J, et al. Interaction between Different Pharmaceutical Excipients in Liquid Dosage Forms—Assessment of Cytotoxicity and Antimicrobial Activity. Molecules. 2018; 23(7):1827. https://doi.org/10.3390/molecules23071827
Chicago/Turabian StyleNemes, Dániel, Renátó Kovács, Fruzsina Nagy, Mirtill Mező, Nikolett Poczok, Zoltán Ujhelyi, Ágota Pető, Pálma Fehér, Ferenc Fenyvesi, Judit Váradi, and et al. 2018. "Interaction between Different Pharmaceutical Excipients in Liquid Dosage Forms—Assessment of Cytotoxicity and Antimicrobial Activity" Molecules 23, no. 7: 1827. https://doi.org/10.3390/molecules23071827
APA StyleNemes, D., Kovács, R., Nagy, F., Mező, M., Poczok, N., Ujhelyi, Z., Pető, Á., Fehér, P., Fenyvesi, F., Váradi, J., Vecsernyés, M., & Bácskay, I. (2018). Interaction between Different Pharmaceutical Excipients in Liquid Dosage Forms—Assessment of Cytotoxicity and Antimicrobial Activity. Molecules, 23(7), 1827. https://doi.org/10.3390/molecules23071827