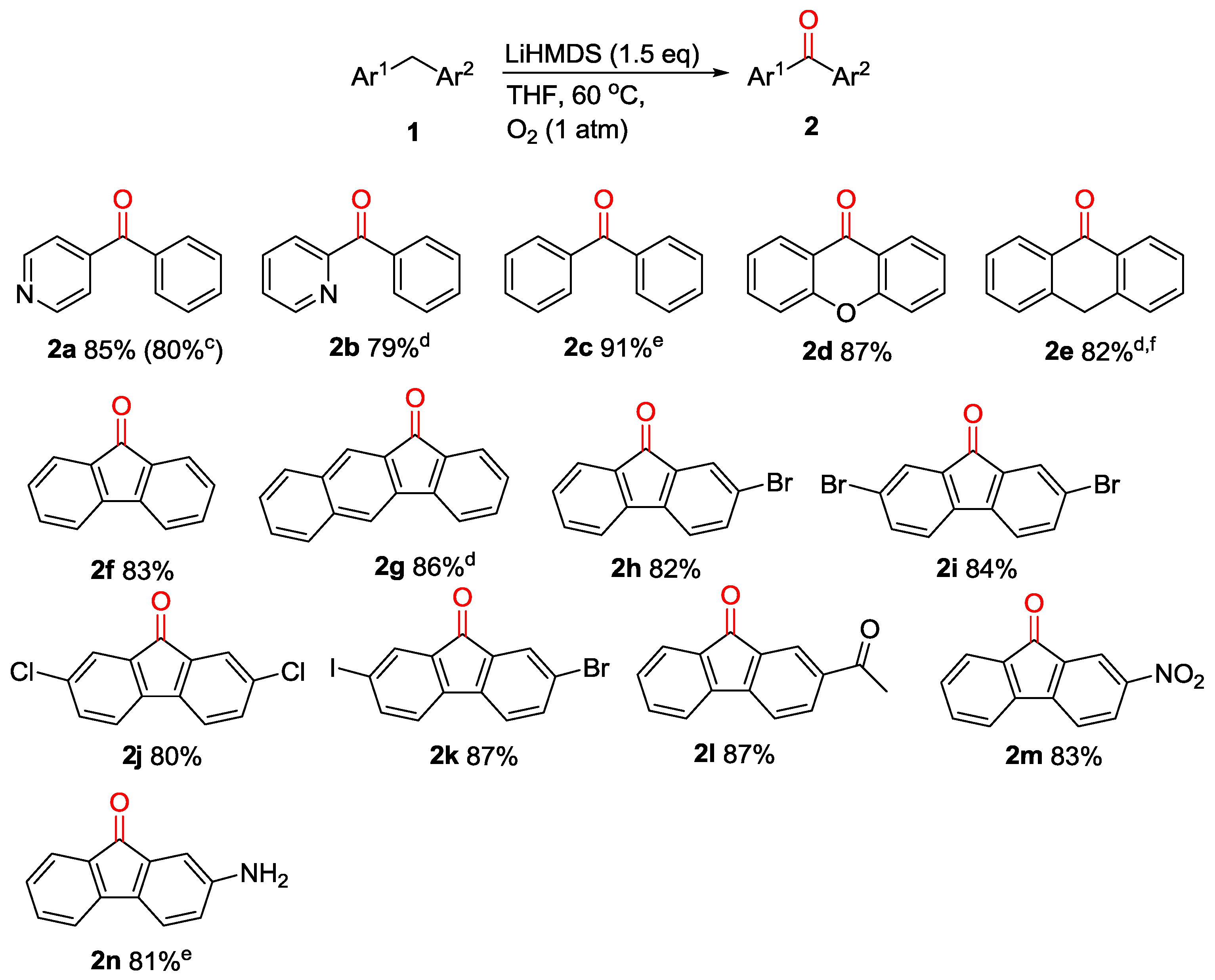
Abstract
An efficient direct C(sp3)–H oxidation of diarylmethanes has been demonstrated by this study. This method employs environment-friendly O2 as an oxidant and is promoted by commercially available MN(SiMe3)2 [M = K, Na or Li], which provides a facile method for the synthesis of various diaryl ketones in excellent yields. This protocol is metal-free, mild and compatible with a number of functional groups on substrates.
1. Introduction
The oxidation reaction is one of the most important transformations in organic synthesis by which oxygenated products of hydrocarbons were prepared and these compounds are valuable structural core in chemical and pharmaceutical industries [1]. Of these transformations, direct oxidation of methylene group of arylalkanes to ketones have attracted comprehensive attention, as diverse aryl ketone motifs are important structural units in numerous pharmaceuticals, naturally occurring molecules and organic functional materials [2,3,4,5,6]. Besides the traditional oxidation using KMnO4 as oxidant [7,8,9,10], advances in the synthetic method of aryl ketones were mainly based on three approaches: (1) classical Friedel-Crafts acylation of arenes [11], (2) oxidation of secondary alcohols [12] and CO insertion reactions [13,14]. Recently, significant progress has been made for the formation of aromatic ketones by transition-metal catalyzed oxidation of alkylarenes [15,16,17]. Notably, the latter represent a powerful tool in organic synthesis, while inevitably they will suffer from the use of toxic or expensive metals and ligands, harsh reaction conditions and generation of metal waste in most cases. Obviously, it is desirable to develop more practical protocols to achieve the synthesis of aryl ketones without the use of corrosive metal catalysts, hazardous stoichiometric oxidants and reductants [18,19,20,21]. Therefore, transition-metal-free methods for oxidation of alkylarenes are desirable in the pharmaceutical industry which can avoid the use of the heavy metal. For oxidation process, Molecular oxygen (O2) represents one of the best choices because of its low cost and has attracted substantial attention [22,23,24,25,26,27,28,29,30,31,32,33]. Herein, we wish to report the direct oxidation of diarylmethanes to diaryl ketones using O2-mediation by MN(SiMe3)2 [M = K, Na or Li], which represent a green and efficient synthetic method for this transformation.
2. Results
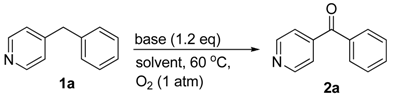
Initially, we commenced the reaction studies using 4-benzylpyridine (1a) as the model substrate. The control experiment was performed by stirring 4-benzylpyridine (1a) in THF under O2 at 60 °C for 16 h and no reaction occurred (entry 1, Table 1). To optimize the reaction conditions, various parameters such as temperature, bases, solvents were investigated. The strong bases play an important role in this transformation. As indicated in Table 1, the silylamides [MN(SiMe3)2, M = Li, Na, K] overwhelmed other bases (entries 2–8), giving the oxidative product in 76–85% yields. We anticipated that the inert sp3 C–H bond was transferred to carbanion before the direct insertion of O2, which needs the strong bases to achieve the deprotonation step. The solvent was next examined using LiN(SiMe3)2 as a base. Of the six solvents screened, THF showed the best performance (entries 4 and 9–13). Further screen of the reaction temperature indicated 60 °C is most appropriate. Only 35% yield of oxidation product 2a was obtained at 40 °C after 12 h (entry 14), while no elevated yield was observed when the reaction was conducted in higher temperature 80 °C (entry 15). Furthermore, there is no oxidation product if the O2 was replaced with N2.

Table 1.
Optimization of the reaction conditions.
With the optimal condition in hand, we then turn our attention to investigate the generality of this protocol. As presented in Figure 1, a variety of diarylmethanes were subjected to these reaction conditions. These reactions were conducted at 60 °C, except where noted. Diarylmethanes with different electronic and steric properties, such as 2-benzylpyridine (1b), diphenylmethane (1c), xanthene (1d), 9,10-dihydroanthracene (1e) and fluorene (1f), were proved to be good substrates, with corresponding products isolated in 79–91% yields. The family of fluorene analogues bearing various functional groups was examined next. The carbon-halo (electron-withdrawing) groups were compatible with this procedure and the oxidation products were obtained in excellent isolated yields (2h, 2i, 2j, 2k). Additionally, remarkable chemoselectivity is observed with fluorene derivatives containing acetal, nitro and amino moieties, which all underwent oxygenation delivering the corresponding functionalized products (2l, 2m, 2n) in 81–87% yields.
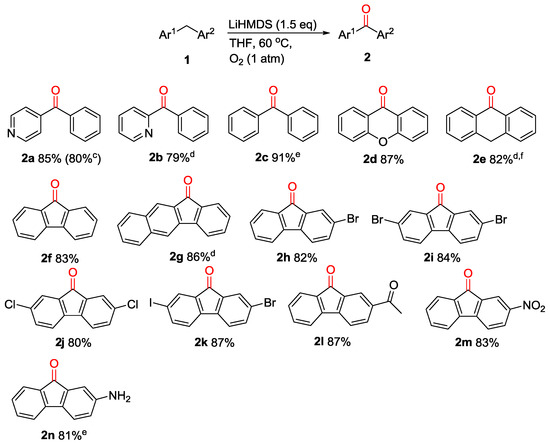
Figure 1.
Scope of diarylmethanes in metal-free oxidation a,b. a Reactions were conducted on a 0.1 mmol scale using 1 equiv of 1, 1.5 equiv of LiN(SiMe3)2 at 0.1 M. b Isolated yield. c Reaction conducted on 5 mmol scale. d LiHMDS was replaced with NaHMDS. e LiHMDS was replaved with KHMDS. f 80 °C.
To illustrate the scalablitlity of this methodology, we commenced the oxidation reaction of 4-benzylpyridine (1a) on a 5 mmol scale. The oxidation product 2a was isolated in 80% yield (0.73 g).
3. Materials and Methods
3.1. General
1H- and 13C-NMR spectra were obtained on Bruker AVANCE III 500 MHz and 600 MHz spectrometers (Bruker Co., Billerica, MA, USA) with TMS as the internal standard; MS spectra were measured on a Finnigan LCQDECA XP instrument and an Agilent Q-TOF 1290 LC/6224 MS system (Santa Clara, CA, USA); silica gel GF254 and H (10–40 mm, Qingdao Marine Chemical Factory, Qingdao, China) were used for TLC and CC. Unless otherwise noted, all reactions were carried out under an atmosphere of oxygen.
3.2. Representative Procedure for the Oxidation of Diarylmethane
To an 8 mL oven-dried vial, 4-benzylpyridine (0.1 mmol), dry THF (1 mL), LiHMDS (0.15 mmol) were added subsequently. The reaction system was sealed by a rubber septum with a needle connected with O2 balloon. After stirring at 60 °C for 12 h, the reaction mixture was passed through a short pad of silica gel and eluted with ethyl acetate (1 mL × 3). The combined organics were concentrated under reduced pressure. The residue was purified by flash chromatography to give the diarylketone 2a as white solid (15.6 mg, 85% yield). 1H NMR (500 MHz, CDCl3) δ 7.65 (d, J = 7.3 Hz, 2H), 7.54–7.43 (m, 4H), 7.33–7.25 (m, 2H). The 1H NMR data of 2a was identical to those reported in the literature [34].
Analogous compounds 2b–n were prepared according to the similar procedure for 2a. 2b: 1H NMR (500 MHz, CDCl3) δ 8.73 (d, J = 4.1 Hz, 1H), 8.10–8.03 (m, 3H), 7.90 (td, J = 7.8, 1.7 Hz, 1H), 7.60 (t, J = 7.4 Hz, 1H), 7.52–7.44 (m, 3H). The 1H NMR data of 2b was identical to those reported in the literature [34]. 2c: 1H NMR (500 MHz, CDCl3) δ 7.72 (dd, J = 5.2, 3.2 Hz, 2H), 7.54–7.47 (m, 1H), 7.43–7.36 (m, 2H). The 1H NMR data of 2c was identical to those reported in the literature [35]. 2d: 1H NMR (500 MHz, CDCl3) δ 8.34 (d, J = 7.9 Hz, 2H), 7.73 (t, J = 7.7 Hz, 2H), 7.49 (d, J = 8.4 Hz, 2H), 7.38 (t, J = 7.5 Hz, 2H). The 1H NMR data of 2d was identical to those reported in the literature [36]. 2e: 1H NMR (500 MHz, CDCl3): δ 8.26–8.37 (m, 2H), 7.56 (t, J = 7.4 Hz, 2H), 7.36–7.47 (m, 4H), 4.32 (s, 2H). The 1H NMR data of 2e was identical to those reported in the literature [37]. 2f: 1H NMR (500 MHz, CDCl3) δ 7.65 (d, J = 7.3 Hz, 2H), 7.54–7.43 (m, 4H), 7.33–7.25 (m, 2H). The 1H NMR data of 2f was identical to those reported in the literature [36]. 2g: 1H NMR (500 MHz, CDCl3): δ 8.14 (s, 1H), 7.88 (s, 1H), 7.85 (d, J = 7.5 Hz, 1H), 7.80 (d, J = 10.50 Hz, 1H), 7.73 (d, J = 9.5 Hz, 1H), 7.69 (d, J = 9.5 Hz, 1H), 7.75–7.55 (m, 2H), 7.45 (t, J = 9.2 Hz, 1H), 7.32 (t, J = 9.2 Hz, 1H). The 1H NMR data of 2g was identical to those reported in the literature [38]. 2h: 1H NMR (500 MHz, CDCl3): δ 7.78 (s, 1H), 7.68 (d, J = 7.3 Hz, 1H), 7.62 (d, J = 7.8 Hz, 1H), 7.53–7.52 (m, 2H), 7.41 (d, J = 7.8 Hz, 1H), 7.35–7.34 (m, 1H). The 1H NMR data of 2h was identical to those reported in the literature [39]. 2i: 1H NMR (500 MHz, CDCl3): δ 7.66 (d, J = 1.8 Hz, 2H), 7.52 (dd, J = 7.9 Hz, 1.8 Hz, 2H), 7.28 (d, J = 7.9 Hz, 2H). The 1H NMR data of 2i was identical to those reported in the literature [39]. 2j: 1H NMR (500 MHz, CDCl3) δ 7.62 (d, J = 1.8 Hz, 2H), 7.47 (dd, J = 8.0, 2.0 Hz, 2H), 7.44 (d, J = 7.9 Hz, 2H). The 1H NMR data of 2j was identical to those reported in the literature [40]. 2k: 1H NMR (500 MHz, CDCl3) δ 7.96 (d, J = 1.6 Hz, 1H), 7.84 (dd, J = 7.8, 1.6 Hz, 1H), 7.76 (d, J = 1.8 Hz, 1H), 7.63 (dd, J = 7.9, 1.9 Hz, 1H), 7.39 (d, J = 7.9 Hz, 1H), 7.28 (s, 1H). The 1H NMR data of 2k was identical to those reported in the literature [41]. 2l: 1H NMR (500 MHz, CDCl3) δ 8.17 (d, J = 0.98 Hz, 1H), 8.13 (dd, J = 7.8, 2.0 Hz, 1H), 7.70 (d, J = 7.3 Hz, 1H), 7.63–7.51 (m, 3H), 7.40–7.35 (m, 1H), 2.63 (s, 3H). The 1H NMR data of 2l was identical to those reported in the literature [42]. 2m: 1H NMR (500 MHz, CDCl3): δ 8.48 (d, J = 1.9 Hz, 1H), 8.43 (dd, J = 1.9 Hz, 8.2 Hz, 1H), 7.77 (d, J = 7.3 Hz, 1H), 7.72–7.67 (m, 2H), 7.62 (t, 1H), 7.46 (t, 1H). The 1H NMR data of 2m was identical to those reported in the literature [39]. 2n: 1H NMR (500 MHz, CDCl3) δ 7.49 (d, J = 7.3 Hz, 1H), 7.43–7.23 (m, 2H), 7.20 (d, J = 7.0 Hz, 1H), 7.16–7.00 (td, J = 7.2 Hz, 1.2 Hz, 1H), 6.89 (d, J = 2.3 Hz, 1H), 6.65 (dd, J = 7.9, 2.3 Hz, 1H), 3.82 (s, 2H). The 1H NMR data of 2n was identical to those reported in the literature [43].
4. Conclusions
In conclusion, we have developed a metal-free, environmentally benign method for C(sp3)–H oxidation of various diarylmethanes using silylamides [MN(SiMe3)2, M = Li, Na, K] as base and O2 as an oxidant. This protocol provides a complementary method to prepare diaryl ketones in good to excellent yields. The detailed mechanism study is still underway.
Author Contributions
J.L. conceived and designed the experiments and wrote the manuscript; F.Y., B.Z., P.C., D.Z., Q.L. and W.R. performed the experiments and analyzed the data; L.F. and L.L. interpreted the results and helped write the paper.
Funding
We are grateful to the National Natural Science Foundation of China (81302668) and Hangzhou Science and Technology Information Institute of China (20150633B45).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, B.C.; Zhou, P.; Davis, F.A.; Ciganek, E. Organic Reactions; Overman, L.E., Ed.; Wiley: New York, NY, USA, 2003. [Google Scholar]
- Wu, S.B.; Long, C.; Kennelly, E.J. Structural Diversity and Bioactivities of Natural Benzophenones. Nat. Prod. Rep. 2014, 31, 1158–1174. [Google Scholar] [CrossRef] [PubMed]
- Belluti, F.; de Simone, A.; Tarozzi, A.; Bartolini, M.; Djemil, A.; Bisi, A.; Gobbi, S.; Montanari, S.; Cavalli, A.; Andrisano, V.; et al. Fluorinated Benzophenone Derivatives: Balanced Multipotent Agents for Alzheimer’s Disease. Eur. J. Med. Chem. 2014, 78, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Vooturi, S.K.; Cheung, C.M.; Rybak, M.J.; Firestine, S.M. Design, Synthesis and Structure-Activity Relationships of Benzophenone-Based Tetraamides as Novel Antibacterial Agents. J. Med. Chem. 2009, 52, 5020–5031. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Yasuda, T.; Yang, Y.S.; Zhang, Q.; Adachi, C. Luminous Butterflies: Efficient Exciton Harvesting by Benzophenone Derivatives for Full-Color Delayed Fluorescence OLEDs. Angew. Chem. Int. Ed. 2014, 53, 6402–6406. [Google Scholar] [CrossRef] [PubMed]
- Ryabchun, A.; Sakhno, O.; Wegener, M. Conventional Elastomers Doped with Benzophenone Derivatives as Effective Media for All-optical Fabrication of Tunable Diffraction Elements. RSC Adv. 2016, 6, 51791–51800. [Google Scholar] [CrossRef]
- Al-hunaiti, A.; Raisanen, M.; Repo, T. From DNA to Catalysis: A Thymine-Acetate Ligated Non-Heme Iron(III) Catalyst for Oxidative Activation of Aliphatic C-H Bonds. Chem. Commun. 2016, 52, 2043–2046. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Li, S.; Pan, Y.; Xu, Y.; Wang, H. The Indium-Catalysed Hydration of Alkynes Using Substoichiometric Amounts of PTSA as Additive. Tetrahedron 2013, 69, 3775–3781. [Google Scholar] [CrossRef]
- Iosub, A.V.; Stahl, S.S. Palladium-Catalyzed Aerobic Oxidative Dehydrogenation of Cyclohexenes to Substituted Arene Derivatives. J. Am. Chem. Soc. 2015, 137, 3454–3457. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ferrate, O.; Britovsek, G.J.; Claver, C.; van Leeuwen, P.W. C-H Benzylic Oxidation Promoted by Dinuclear Iron DBDOC Iminopyridine Complexes. Inorg. Chim. Acta 2015, 431, 156–160. [Google Scholar] [CrossRef]
- Sartori, G.; Maggi, R. Advances in Friedel–Crafts Acylation Reactions: Catalytic and Green Processes; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Tojo, G.; Fernández, M.I. Oxidation of Alcohols to Aldehydes and Ketones: A Guide to Current Common Practice; Springer Science & Business Media: New York, NY, USA, 2006. [Google Scholar]
- Colquhoun, H.; Thompson, D.; Twigg, M.V. Carbonylation: Direct Synthesis of Carbonyl Compounds; Springer Science & Business Media: New York, NY, USA, 1991. [Google Scholar]
- Beller, M.; Wu, X.-F. Transition-Metal-Catalyzed Carbonylation Reactions; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Hughes, M.D.; Xu, Y.J.; Jenkins, P.; McMorn, P.; Landon, P.; Enache, D.I.; Carley, A.F.; Attard, G.A.; Hutchings, G.J.; King, F.; et al. Tunable Gold Catalysts for Selective Hydrocarbon Oxidation Under Mild Conditions. Nature 2005, 437, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Liu, M.J.; Wang, Y.Q.; Fan, H.Y.; Wu, J.; Huang, C.; Hou, H.W. Cu(I) Coordination Polymers as the Green Heterogeneous Catalysts for Direct C−H Bonds Activation of Arylalkanes to Ketones in Water with Spatial Confinement Effect. Inorg. Chem. 2017, 56, 13329–13336. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.L.; Liu, Y.; Ma, X.W.; Liu, P.; Gu, C.Z.; Dai, B. Cu(II)-Catalyzed Ligand-Free Oxidation of Diarylmethanes and Second Alcohols in Water. Chin. J. Chem. 2017, 35, 1391–1395. [Google Scholar] [CrossRef]
- Yanagisawa, S.; Itami, K. Tert-Butoxide-Mediated C-H Bond Arylation of Aromatic Compounds with Haloarenes. ChemCatChem 2011, 3, 827–829. [Google Scholar] [CrossRef]
- Shirakawa, E.; Hayashi, T. Transition-Metal-Free Coupling Reactions of Aryl Halides. Chem. Lett. 2012, 41, 130–134. [Google Scholar] [CrossRef]
- Mehta, V.P.; Punji, B. Recent Advances in Transition-Metal-Free Direct C-C and C-Heteroatom Bond Forming Reactions. RSC Adv. 2013, 3, 11957–11986. [Google Scholar] [CrossRef]
- Jin, F.; Han, W. Transition-Metal-Free, Ambient-Pressure Carbonylative Cross-Coupling Reactions of Aryl Halides with Potassium Aryltrifluoroborates. Chem. Commun. 2015, 51, 9133–9136. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.S. Palladium-Catalyzed Oxidation of Organic Chemicals with O2. Science 2005, 309, 1824–1826. [Google Scholar] [CrossRef] [PubMed]
- Que, L.; Tolman, W.B. Biologically Inspired Oxidation Catalysis. Nature 2008, 455, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Punniyamurthy, T.; Velusamy, S.; Iqbal, J. Recent Advances in Transition Metal Catalyzed Oxidation of Organic Substrates with Molecular Oxygen. Chem. Rev. 2005, 105, 2329–2363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Tang, C.; Jiao, N. Recent Advances in Copper-Catalyzed Dehydrogenative Functionalization via A Single Electron Transfer (SET) Process. Chem. Soc. Rev. 2012, 41, 3464–3484. [Google Scholar] [CrossRef] [PubMed]
- Pattillo, C.C.; Strambeanu, L.I.; Calleja, P.; Vermeulen, N.A.; Mizuno, T.; White, M.C. Aerobic Linear Allylic C-H Amination: Overcoming Benzoquinone Inhibition. J. Am. Chem. Soc. 2016, 138, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Saha, D.; Saha, D.; Guin, J. Aerobic Direct C(sp2)-H Hydroxylation of 2-Arylpyridines by Palladium Catalysis Induced with Aldehyde Auto-Oxidation. ACS Catal. 2016, 6, 6050–6054. [Google Scholar] [CrossRef]
- Anson, C.W.; Ghosh, S.; Hammes-Schiffer, S.; Stahl, S.S. Co(salophen)-Catalyzed Aerobic Oxidation of p-Hydroquinone: Mechanism and Implications for Aerobic Oxidation Catalysis. J. Am. Chem. Soc. 2016, 138, 4186–4193. [Google Scholar] [CrossRef] [PubMed]
- Brink, G.J.T.; Arends, I.W.C.E.; Sheldon, R.A. Green, Catalytic Oxidation of Alcohols in Water. Science 2000, 287, 1636–1639. [Google Scholar] [CrossRef] [PubMed]
- Brice, J.L.; Harang, J.E.; Timokhin, V.I.; Anastasi, N.R.; Stahl, S.S. Aerobic Oxidative Amination of Unactivated Alkenes Catalyzed by Palladium. J. Am. Chem. Soc. 2005, 127, 2868–2869. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Yu, J.Q. Pd(II)-Catalyzed Hydroxylation of Arenes with 1 atm of O2 or Air. J. Am. Chem. Soc. 2009, 131, 14654–14655. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Zhang, L.; Lee, J.Y. Copper-Catalyzed Synthesis of Azaspirocyclohexadienones from Alpha-Azido-N-Arylamides under an Oxygen Atmosphere. J. Am. Chem. Soc. 2010, 132, 7266–7267. [Google Scholar] [CrossRef] [PubMed]
- Lyons, T.W.; Reinhard, C.T.; Planavsky, N.J. The Rise of Oxygen in Earth’s Early Ocean and Atmosphere. Nature 2014, 506, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Sterckx, H.; Sambiagio, C.; Maes, B.U.W. Copper-Catalyzed Aerobic Oxygenation of Benzylpyridine N-Oxides and Subsequent Post-Functionalization. Adv. Synth. Catal. 2017, 359, 3226–3236. [Google Scholar] [CrossRef]
- Nambo, M.; Keske, E.C.; Rygus, J.P.G.; Yim, J.C.H.; Crudden, C.M. Development of Versatile Sulfone Electrophiles for Suzuki-Miyaura Cross-Coupling Reactions. ACS Catal. 2016, 7, 1108–1112. [Google Scholar] [CrossRef]
- Li, S.; Zhu, B.; Lee, R.; Qiao, B.; Jiang, Z. Visible light-induced selective aerobic oxidative transposition of vinyl halides using a tetrahalogenoferrate(III) complex catalyst. Org. Chem. Front. 2017, 5, 380–385. [Google Scholar] [CrossRef]
- Prebil, R.; Stavber, G.; Stavber, S. Aerobic Oxidation of Alcohols by Using a Completely Metal-Free Catalytic System. Eur. J. Org. Chem. 2014, 2014, 395–402. [Google Scholar] [CrossRef]
- Chinnagolla, R.K.; Jeganmohan, M. Regioselective Ortho-Arylation and Alkenylation of N-Alkyl Benzamides with Boronic Acids via Ruthenium-Catalyzed C–H Bond Activation: An Easy Route to Fluorenones Synthesis. Org. Lett. 2012, 14, 5246–5249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ji, X.; Jiang, S.S.; Liu, L.L.; Weeks, B.L.; Zhang, Z. Highly Efficient Synthesis of 9-Fluorenones from 9H-Fluorenes by Air Oxidation. Green Chem. 2011, 13, 1891–1896. [Google Scholar] [CrossRef]
- Yip, W.T.; Levy, D.H.; Kobetic, R.; Piotrowiak, P. Energy Transfer in Bichromophoric Molecules: The Effect of Symmetry and Donor/Acceptor Energy Gap. J. Phys. Chem. A 2009, 103, 10–20. [Google Scholar] [CrossRef]
- Valášek, M.; Edelmann, K.; Gerhard, L.; Fuhr, O.; Lukas, M.; Mayor, M. Synthesis of Molecular Tripods Based on A Rigid 9,9′-Spirobifluorene Scaffold. J. Org. Chem. 2014, 79, 7342–7357. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Oisaki, K.; Kanai, M. Chemoselective Aerobic Photo-Oxidation of 9H-Fluorenes for the Synthesis of 9-Fluorenones. Tetrahedron Lett. 2015, 46, 4736–4738. [Google Scholar] [CrossRef]
- Dufresne, S.; Callaghan, L.; Skene, W.G. Conjugated Fluorenes Prepared From Azomethines Connections-II: The Effect of Alternating Fluorenones and Fluorenes on the Spectroscopic and Electrochemical Properties. J. Phys. Chem. B 2009, 113, 15541–15549. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 2a–2n are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).