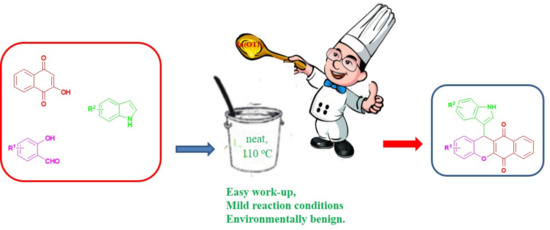
3.2. General Procedure for the Synthesis of Compounds 4
To a mixture of indole (1 mmol), substituted salicylic aldehyde (1 mmol) and 2-hydroxy-1,4-naphthoquinone (1 mmol), In(OTf)
3 (0.1 mmol) was added. The mixture was stirred at 110 °C for 5–7 h. After completion of the reaction (TLC), the reaction mixture is cooled to room temperature, treated with water (10 mL), extracted with CH
2Cl
2 (2 × 10 mL), and filtered, and the solvent is evaporated in vacuo. Solvent was evaporated and the crude product purified by silica gel column chromatography using petroleum ether: dichloromethane (v:v = 1:3) as eluent to afford the pure product 4 (the copy of IR,
1H NMR,
13C NMR, and HMRS of compounds 4 see
Supplementary Materials).
12-(2-Phenyl-1H-indol-3-yl)-12H-benzo[b]xanthene-6,11-dione (4a): Reddish-brown powder, m.p. 260–262 °C, 1H NMR (400 MHz,DMSO-d6) δ: 11.39 (s, 1H), 8.07–8.00 (m, 3H), 7.90–7.80 (m, 3H), 7.59 (t, 2H, J = 7.6 Hz), 7.49 (t, 1H, J = 7.2 Hz), 7.31–7.28 (m, 4H), 7.23–7.21 (m, 2H), 7.19 (t, 2H, J = 1.2 Hz), 5.82 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ: 183.9, 178.2, 150.9, 148.2, 136.3, 135.3, 135.2, 134.4, 133.4, 131.8, 130.7, 129.8, 129.6 (2C), 129.2 (2C), 128.7, 128.6, 126.9, 126.5, 126.3, 126.1, 124.1, 121.8, 121.3, 119.8, 118.1, 117.2, 115.2, 112.0, 29.5; IR (KBr): v 3360, 1632, 1650 cm−1; HRMS-ESI (m/z): calcd for C31H19NNaO3 [M + Na]+: 476.1263, found: 476.1262.
2-Bromo-12-(2-Phenyl-1H-indol-3-yl)-12H-benzo[b]xanthene-6,11-dione (4b): Red powder, m.p. 279–280 °C, 1H NMR (400 MHz,DMSO-d6) δ: 11.46 (s, 1H), 8.04–8.02 (m, 1H), 7.93–7.78 (m, 5H), 7.58 (t, J = 7.6 Hz, 2H), 7.48 (t, J = 7.2 Hz, 1H), 7.37–7.32 (m, 3H), 7.24 (d, J = 8.8 Hz ,1H), 7.04 (t, J = 7.6 Hz ,1H), 6.92–6.89 (m, 2H), 5.79 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ: 183.6, 177.8, 150.5, 147.3, 136.2, 135.6, 135.2, 134.5, 133.2, 132.0, 131.6 (2C), 130.6, 129.5 (2C), 129.3 (2C), 128.7, 126.8, 126.6, 126.5, 126.3, 122.0, 120.9, 120.0, 119.6, 118.0, 117.4, 114.8, 112.2, 19.4; IR (KBr): v 3379, 1671, 1634 cm−1; HRMS-ESI (m/z): calcd for C31H18BrNNaO3 [M + Na]+: 554.0368, found: 554.0362.
2-Chloro-12-(2-Phenyl-1H-indol-3-yl)-12H-benzo[b]xanthene-6,11-dione (4c): Red powder, m.p. 275-276 °C, 1H NMR (400 MHz,DMSO-d6) δ: 11.46 (s, 1H), 8.05–8.03 (m, 1H), 7.93–7.80 (m, 5H), 7.58 (t, J = 7.2 Hz, 2H), 7.49 (t, J = 7.2 Hz, 1H), 7.34–7.32 (m, 3H), 7.27–7.24 (m, 1H), 7.04 (d, J = 7.6 Hz, 1H), 6.92 (t, J = 7.6 Hz ,1H), 6.77 (d, J = 2.0 Hz ,1H), 6.92–6.89 (m, 2H), 5.81 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ: 183.6, 177.9, 150.6, 146.9, 136.2, 135.6, 135.2, 134.5, 133.2, 131.7, 130.7, 129.5 (2C), 129.4 (2C), 129.3, 129.0, 128.8, 128.7, 126.8, 126.5, 126.3, 126.2, 122.0, 120.8, 120.0, 119.3, 118.0, 114.8, 112.2, 29.6; IR (KBr): v 3377, 1675, 1634 cm−1; HRMS-ESI (m/z): calcd for C31H18ClNNaO3 [M + Na]+: 510.0873, found: 510.0858.
4-Bromo-2-chloro-12-(2-Phenyl-1H-indol-3-yl)-12H-benzo[b]xanthene-6,11-dione (4d): Red powder, m.p. 243–244 °C, 1H NMR (400 MHz,DMSO-d6) δ: 11.49 (s, 1H), 8.07–8.04 (m, 1H), 7.90–7.80 (m, 5H), 7.67 (d, J = 2.4 Hz, 1H), 7.57–7.46 (m, 3H), 7.39–7.32 (m, 2H), 7.08–7.04 (m, 1H), 6.97–6.93 (m, 1H), 6.78 (d, J = 2.4 Hz ,1H), 5.85 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ: 183.5, 177.4, 150.3, 144.2, 136.1, 136.0, 135.2, 134.6, 133.0, 131.6, 130.7, 129.6, 129.5 (2C), 129.2 (2C), 128.8, 128.5, 127.7, 127.4, 126.8, 126.5, 126.4, 122.1, 121.3, 120.1, 118.0, 114.6, 112.2, 112.0, 30.0; IR (KBr): v 3288, 1685, 1637 cm−1; HRMS-ESI (m/z): calcd for C31H17BrClNNaO3 [M + Na]+: 587.9978, found: 587.9968.
2-Methyl-12-(2-Phenyl-1H-indol-3-yl)-12H-benzo[b]xanthene-6,11-dione (4e): Orange-red powder, m.p. 244–245 °C, 1H NMR (400 MHz,DMSO-d6) δ: 11.38 (s, 1H), 8.03–7.99 (m, 3H), 7.86–776 (m, 3H), 7.60 (t, J = 7.6 Hz, 2H), 7.50 (t, J = 7.2 Hz, 1H), 7.32–7.26 (m, 2H), 7.13 (d, J = 8.0 Hz ,1H), 7.03–6.96 (m, 2H), 6.86 (t, J = 7.2 Hz, 2H), 6.63 (s, 3H), 5.72 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ: 183.7, 178.2, 150.9, 146.2, 136.3, 135.3, 135.1, 134.3, 133.5, 131.8, 130.7, 129.8, 129.5 (2C), 129.4 (2C), 129.2, 128.5, 126.9, 126.4, 126.3, 123.7, 121.8, 121.1, 119.7, 118.1, 117.0, 115.2, 112.0, 29.5, 20.8; IR (KBr): v 3372, 1677, 1631 cm−1; HRMS-ESI (m/z): calcd for C32H21NNaO3 [M + Na]+: 490.1419, found: 490.1410.
2-Nitro-12-(2-Phenyl-1H-indol-3-yl)-12H-benzo[b]xanthene-6,11-dione (4f): Yellow powder, m.p. 245–246 °C, 1H NMR (400 MHz, DMSO-d6) δ: 11.49 (s, 1H), 8.07–8.04 (m, 1H), 7.90–7.80 (m, 5H), 7.67 (d, J = 2.4 Hz, 1H), 7.57–7.46 (m, 3H), 7.39–7.32 (m, 2H), 7.08–7.04 (m, 1H), 6.97–6.93 (m, 1H), 6.78 (d J = 2.4 Hz, 1H), 5.85 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ: 183.6, 177.6, 152.3, 150.2, 144.7, 136.2, 136.0, 135.3, 134.7, 133.0, 131.6, 130.7, 129.5 (2C), 129.3 (2C), 128.9, 126.8, 126.6, 126.4, 125.7, 125.5, 124.4, 122.1, 121.3, 120.1, 118.7, 118.1, 114.9, 112.2, 29.6; IR (KBr): v 3358, 1680, 1665, 1644 cm−1; HRMS-ESI (m/z): calcd for C31H18N2NaO5 [M + Na]+: 521.113, found: 521.1106.
2-Methoxy-12-(2-Phenyl-1H-indol-3-yl)-12H-benzo[b]xanthene-6,11-dione (4g): Yellow powder, m.p. 211–212 °C, 1H NMR (400 MHz,DMSO-d6) δ: 11.35 (s, 1H), 8.06–7.98 (m, 3H), 7.88–7.78 (m, 3H), 7.59 (d, J = 7.6 Hz, 2H), 7.48 (d, J = 7.6 Hz, 1H), 7.31–7.26 (m, 2H), 7.02–6.98 (m, 1H), 6.88–6.83 (m, 2H), 6.73 (d J = 7.4 Hz, 1H), 6.60–6.58 (m, 1H), 5.72 (s, 1H), 3.71 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ: 183.8, 178.2, 159.4, 150.9, 148.7, 136.3, 135.2, 135.1, 134.4, 133.5, 131.8, 130.7, 130.3, 129.5 (2C), 129.2 (2C), 128.5, 126.9, 126.4, 126.3, 121.8, 121.6, 119.7, 118.2, 115.9, 115.4, 113.2, 112.0, 101.7, 55.9, 29.0; IR (KBr): v 3326, 1682, 1638 cm−1; HRMS-ESI (m/z): calcd for C32H21NNaO4 [M + Na]+: 506.1368, found: 506.1358.
2,4-Dibromo-12-(2-phenyl-1H-indol-3-yl)-12H-benzo[b]xanthene-6,11-dione (4h): Red powder, m.p. 252–253 °C, 1H NMR (400 MHz,DMSO-d6) δ: 11.50 (s, 1H), 8.06 (dd, J = 2.0, 7.6 Hz, 1H), 7.88–7.82 (m, 5H), 7.77 (d, J = 2.4 Hz, 1H), 7.57–7.47 (m, 3H), 7.39–7.33 (m, 2H), 7.08–7.04 (m, 1H), 6.96 (t, J = 7.6 Hz, 1H), 6.90 (d, J = 7.0 Hz, 1H), 5.85 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ: 183.5, 177.4, 150.3, 144.6, 136.1, 136.0, 135.2, 134.6, 134.2, 133.0, 131.6, 131.5, 130.7, 129.5 (2C), 129.3 (2C), 128.8, 128.1, 126.8, 126.5, 126.4, 122.1, 121.4, 120.1, 118.0, 117.3, 114.7, 112.2, 112.1, 29.9; IR (KBr): v 3289, 1683, 1635 cm−1; HRMS-ESI (m/z): calcd for C31H17Br2NNaO3 [M + Na]+: 631.9473, found: 631.9462.
3-Methoxy-12-(2-phenyl-1H-indol-3-yl)-12H-benzo[b]xanthene-6,11-dione (4i): Orange-red powder, m.p. 244–245 °C, 1H NMR (400 MHz, DMSO-d6) δ: 11.40 (s, 1H), 8.05–8.00 (m, 3H), 7.88–7.78 (m, 3H), 7.61 (d, J = 7.6 Hz, 2H), 7.50 (t, J = 7.6 Hz, 1H), 7.32–7.20 (m, 3H), 7.03–6.99 (m, 1H), 6.88–6.84 (m, 1H), 6.77 (dd, J = 2.8, 8.8 Hz, 1H), 5.75 (s, 1H), 3.51 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ: 183.8, 178.2, 156.8, 151.2, 142.1, 136.3, 135.3, 135.1, 134.3, 133.4, 131.8, 130.7, 129.6 (2C), 129.3 (2C), 128.6, 126.8, 126.4, 126.3, 125.1, 121.8, 120.2, 119.7, 118.3, 118.1, 115.0, 114.1, 114.0, 112.1, 55.5, 29.9; IR (KBr): v 3304, 1683, 1638 cm−1; HRMS-ESI (m/z): calcd for C32H21NNaO4 [M + Na]+: 506.1368, found: 506.1357.
4-Bromo-2-chloro-12-(1H-indol-3-yl)-12H-benzo[b]xanthene-6,11-dione (4j): Yellow-brown powder, m.p. 248–250 °C, 1H NMR (400 MHz, DMSO-d6) δ: 11.08 (s, 1H), 8.10 (d, J = 7.2 Hz, 1H), 7.92–7.84 (m, 3H), 7.73 (s, 1H), 7.68 (d, J = 8.0 Hz, 1H), 7.52 (s, 1H), 7.38 (s, 1H), 7.33–7.31 (d, J = 7.6 Hz, 1H), 7.08–7.00 (m, 2H), 5.71 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ: 183.2, 177.7, 150.5, 144.9, 136.7, 135.0, 134.6, 131.7, 131.3, 131.2, 129.6, 129.0, 128.6, 126.6, 126.2, 125.5, 124.9, 122.1, 121.8, 119.7, 118.5, 118.2, 112.3, 111.8, 29.9; IR (KBr): v 3403, 1633, 1680 cm−1; HRMS-ESI (m/z): calcd for C25H13BrClNNaO3 [M + Na]+: 511.9665, found: 511.9664.
2-chloro-12-(1H-indol-3-yl)-12H-benzo[b]xanthene-6,11-dione (4k): Brown powder, m.p. 343–345 °C, 1H NMR (400 MHz, DMSO-d6) δ: 11.04 (s, 1H), 7.91–7.89 (m, 1H), 7.85–7.82 (m, 3H), 7.61 (d, J = 8.0 Hz, 1H), 7.46–7.30 (m, 5H), 7.06–6.96 (m, 2H), 5.67 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ: 183.4, 178.2, 150.5, 147.6, 136.7, 135.0, 134.4, 131.8, 131.1, 129.5, 129.4, 128.5, 127.1, 126.5, 126.2, 125.6, 124.6, 121.7, 119.5, 119.3, 118.5 (2C), 112.3, 29.5; IR (KBr): v 3410, 1634, 1685 cm−1; HRMS-ESI (m/z): calcd for C25H14ClNNaO3 [M + Na]+: 434.0560, found: 434.0559.
2-Bromo-12-(1H-indol-3-yl)-12H-benzo[b]xanthene-6,11-dione (4l): Reddish-brown powder, m.p. 262–264 °C, 1H NMR (400 MHz, DMSO-d6) δ: 8.10–8.07 (m, 1H), 7.91–7.82 (m, 3H), 7.61–7.58 (m, 2H), 7.46–7.43 (m, 1H), 7.36–7.30 (m, 3H), 7.04 (t, J = 7.2 Hz, 1H), 6.98 (t, J = 7.6 Hz, 1H); 13C NMR (100 MHz, DMSO-d6) δ: 183.3, 178.2, 150.5, 148.1, 136.7, 135.0, 134.4, 132.5, 131.8, 131.4, 131.1, 127.5, 126.5, 126.2, 125.6, 124.6, 121.8, 121.7, 119.7, 119.5 (2C), 118.5, 117.4, 112.3, 29.4; IR (KBr): v 3410, 1633, 1683 cm−1; HRMS-ESI (m/z): calcd for C25H14BrNNaO3 [M + Na]+: 478.0055, found: 478.0056.
2-Fluoro-12-(1H-indol-3-yl)-12H-benzo[b]xanthene-6,11-dione (4m): Reddish-brown powder, m.p. 330–332 °C, 1H NMR (400 MHz, DMSO-d6) δ: 11.02 (s, 1H), 8.10–8.08 (m, 1H), 7.92–7.82 (m, 3H), 7.61 (d, J = 8.8 Hz, 1H), 7.43–7.40 (m, 1H), 7.35–7.22 (m, 3H), 7.15–7.10 (m, 1H), 7.04 (t, J = 7.2 Hz, 1H), 6.97 (t, J = 6.8 Hz, 1H), 5.66 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ: 183.4, 178.4, 160.6, 150.7, 145.2, 136.8, 135.0, 134.4, 131.8, 131.1, 126.5, 126.2, 125.6, 124.5, 121.6, 121.2, 119.5, 119.2, 119.1, 118.5, 118.4, 115.8, 115.7, 112.2, 29.8; IR (KBr): v 3416, 1650, 1638 cm−1; HRMS-ESI (m/z): calcd for C25H14FNNaO3 [M + Na]+: 418.0855, found: 418.0858.
2-Chloro-12-(2-methyl-1H-indol-3-yl)-12H-benzo[b]xanthene-6,11-dione (4n): Reddish-brown powder, m.p. 264–265 °C, 1H NMR (400 MHz, DMSO-d6) δ: 11.00 (s, 1H), 8.05–7.79 (m, 4H), 7.41 (d, J = 8.8 Hz, 1H), 7.33–7.30 (m, 1H), 7.19 (d, J = 7.6 Hz, 2H), 7.07 (d, J = 7.6 Hz, 1H), 6.88 (d, J = 7.2 Hz, 1H), 6.79 (d, J = 7.2 Hz, 1H), 5.57 (s, 1H), 2.63 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ: 183.4, 178.2, 149.9, 147.8, 135.5, 135.1, 134.5, 133.4, 131.7, 130.8, 129.9, 129.6, 128.7, 126.7, 126.5, 126.2, 121.1, 120.5, 119.3, 119.0, 116.9, 113.5, 111.4, 29.1, 12.1; IR (KBr): v 3379, 1679, 1660, 1635 cm−1; HRMS-ESI (m/z): calcd for C26H16ClNNaO3 [M + Na]+: 448.0716, found: 448.0707.
2-Methyl-12-(2-methyl-1H-indol-3-yl)-12H-benzo[b]xanthene-6,11-dione (4o): Reddish-brown powder, m.p. 269–270 °C, 1H NMR (400 MHz, DMSO-d6) δ: 10.92 (s, 1H), 8.01 (dd, J = 4.0, 7.2 Hz, 1H), 7.85–7.75 (m, 3H), 7.20–7.17 (m, 2H), 7.09–7.02 (m, 2H), 6.90–6.85 (m, 2H), 6.77 (t, J = 7.6 Hz, 1H), 5.44 (s, 1H), 2.59 (s, 3H), 2.15 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ: 183.5, 178.5, 150.2, 146.9, 135.4, 135.3, 135.0, 134.3, 132.9, 131.7, 130.8, 130.4, 129.3, 126.8, 126.4, 126.1, 123.6, 121.4, 120.4, 119.1, 117.1, 116.8, 114.1, 111.2, 29.1, 20.7, 12.1; IR (KBr): v 3375, 1673, 1632 cm−1; HRMS-ESI (m/z): calcd for C27H19NNaO3 [M + Na]+: 428.1263, found: 428.1263.
12-(2-Methyl-1H-indol-3-yl)-12H-benzo[b]xanthene-6,11-dione (4p): Yellow-brown powder, m.p. 350–352 °C, 1H NMR (400 MHz, DMSO-d6) δ: 10.94 (s, 1H), 8.05 (d, J = 4.4Hz, 1H), 7.86–7.80 (m, 3H), 7.35–7.24 (m, 2H), 7.18–7.07 (m, 4H), 6.87 (t, J = 7.2 Hz, 1H), 6.77 (t, J = 7.2 Hz, 1H) 5.55 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ: 183.5, 178.5, 150.2, 148.9, 135.5, 135.1, 134.4, 132.9, 131.7, 130.8, 130.5, 128.6, 126.8, 126.5, 126.2, 126.1, 124.0, 121.6, 120.4, 119.1, 117.1, 117.0, 114.1, 111.2, 29.0, 12.1; IR (KBr): v 3401, 2919, 1631, 1649 cm−1; HRMS-ESI (m/z): calcd for C26H17NNaO3 [M + Na]+: 414.1106, found: 414.1102.
12-(5-Methoxy-1H-indol-3-yl)-12H-benzo[b]xanthene-6,11-dione (4q): Yellow-brown powder, m.p. 260–261 °C, 1H NMR (400 MHz, DMSO-d6) δ: 10.82 (s, 1H), 8.10–8.08 (m, 1H), 7.93–7.83 (m, 3H), 7.42 (d, J = 7.2 Hz, 1H), 7.35 (d, J = 7.6Hz, 1H), 7.30–7.24 (m, 2H), 7.19–7.12 (m, 2H), 7.06 (d, J = 2.0 Hz, 1H), 6.70–6.67 (m, 1H), 5.61 (s, 1H), 3.72 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ: 183.5, 178.5, 153.7, 150.7, 149.0, 135.0, 134.4, 131.9, 131.1, 130.2, 128.6, 126.8, 126.5, 126.2, 126.0, 125.0, 124.9, 122.2, 118.7, 117.2, 112.8, 111.3, 100.7, 55.7, 29.5; IR (KBr): v 3442, 2926, 1631, 1654 cm−1; HRMS-ESI (m/z): calcd for C26H17NNaO4 [M + Na]+: 430.1055, found: 430.1062.
12-(4-Methoxy-1H-indol-3-yl)-12H-benzo[b]xanthene-6,11-dione (4r): Yellow-brown powder, m.p. 190–192 °C, 1H NMR (400 MHz, DMSO-d6) δ: 8.12–8.10 (m, 1H), 7.87–7.81 (m, 3H), 7.47 (d, J = 7.6 Hz, 1H), 7.27–7.16 (m, 3H), 7.09 (t, J = 6.4 Hz, 1H), 6.97 (t, J = 7.6 Hz, 1H), 6.89 (d, J = 8.0Hz, 1H), 6.51 (d, J = 7.6Hz, 1H), 5.91 (s, 1H), 3.94 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ: 183.6, 178.7, 154.2, 151.0, 148.4, 138.3, 135.0 (2C), 133.7, 132.4, 131.1, 130.1, 128.0, 126.4, 125.9, 123.4, 122.7, 120.1, 117.2, 115.7, 111.5, 105.5, 99.9, 55.4, 29.8; IR(KBr): v 3441, 2930, 1633, 1644 cm−1; HRMS-ESI (m/z): calcd for C26H17NNaO4 [M + Na]+: 430.1055, found: 430.1054.
12-(5-Chloro-1H-indol-3-yl)-12H-benzo[b]xanthene-6,11-dione (4s): Yellow-brown powder, m.p. 256–257 °C, 1H NMR (400 MHz, DMSO-d6) δ: 11.19 (s, 1H), 8.10–8.08 (m, 1H), 7.93–7.83 (m, 3H), 7.72 (d, J = 1.6 Hz, 1H), 7.16–7.12 (m, 5H), 7.06 (t, J = 2.0 Hz, 1H), 7.03 (d, J = 2.0 Hz, 1H), 5.66 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ: 183.5, 178.4, 150.8, 148.8, 135.1, 134.9, 134.4, 131.8, 131.2, 130.2, 128.7, 126.9, 126.5, 126.3, 126.2, 126.1, 124.9, 124.1, 122.1, 121.6, 119.3, 118.1, 117.3, 113.7, 29.1; IR(KBr): 3407, 2922, 1633, 1685 cm−1; HRMS-ESI (m/z): calcd for C25H14ClNNaO3 [M + Na]+: 434.0560, found: 434.0557.